Analysis of Plastic-Derived Fuel Oil Produced from High- and Low-Density Polyethylene
Abstract
:1. Introduction
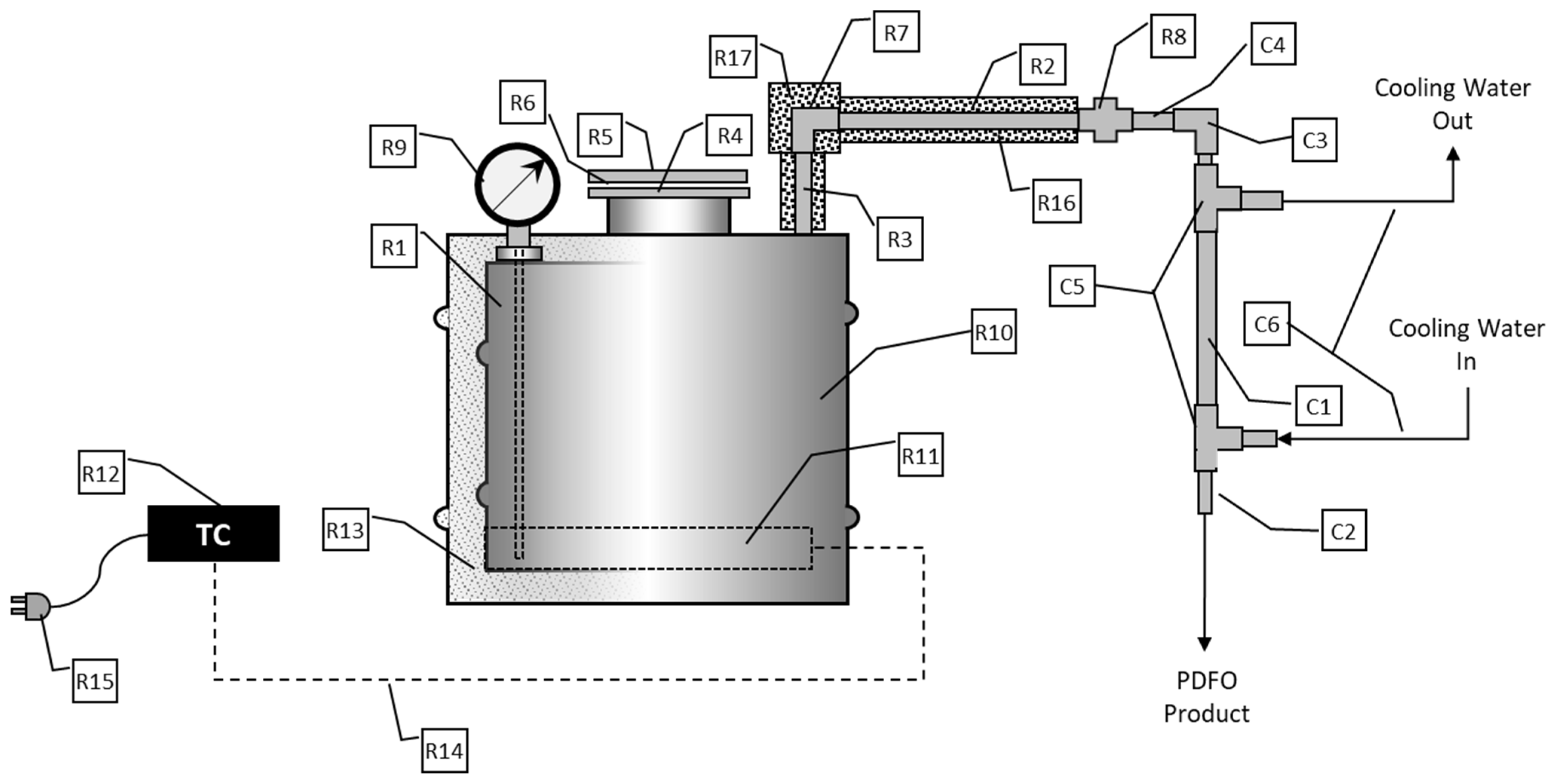
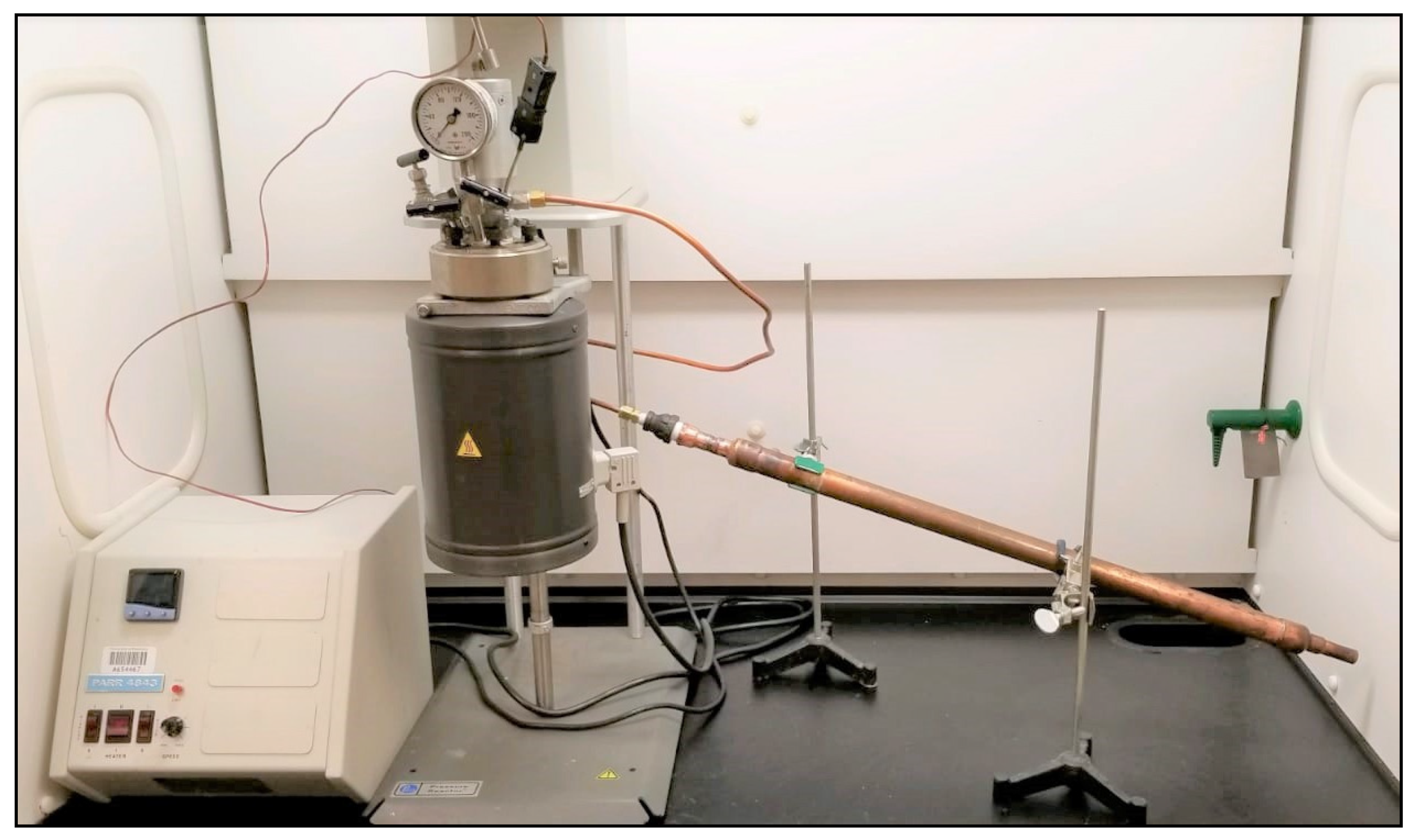
2. Materials and Methods
2.1. Collecting PDFO Samples
2.2. Analyzing PDFO Samples
3. Results and Discussion
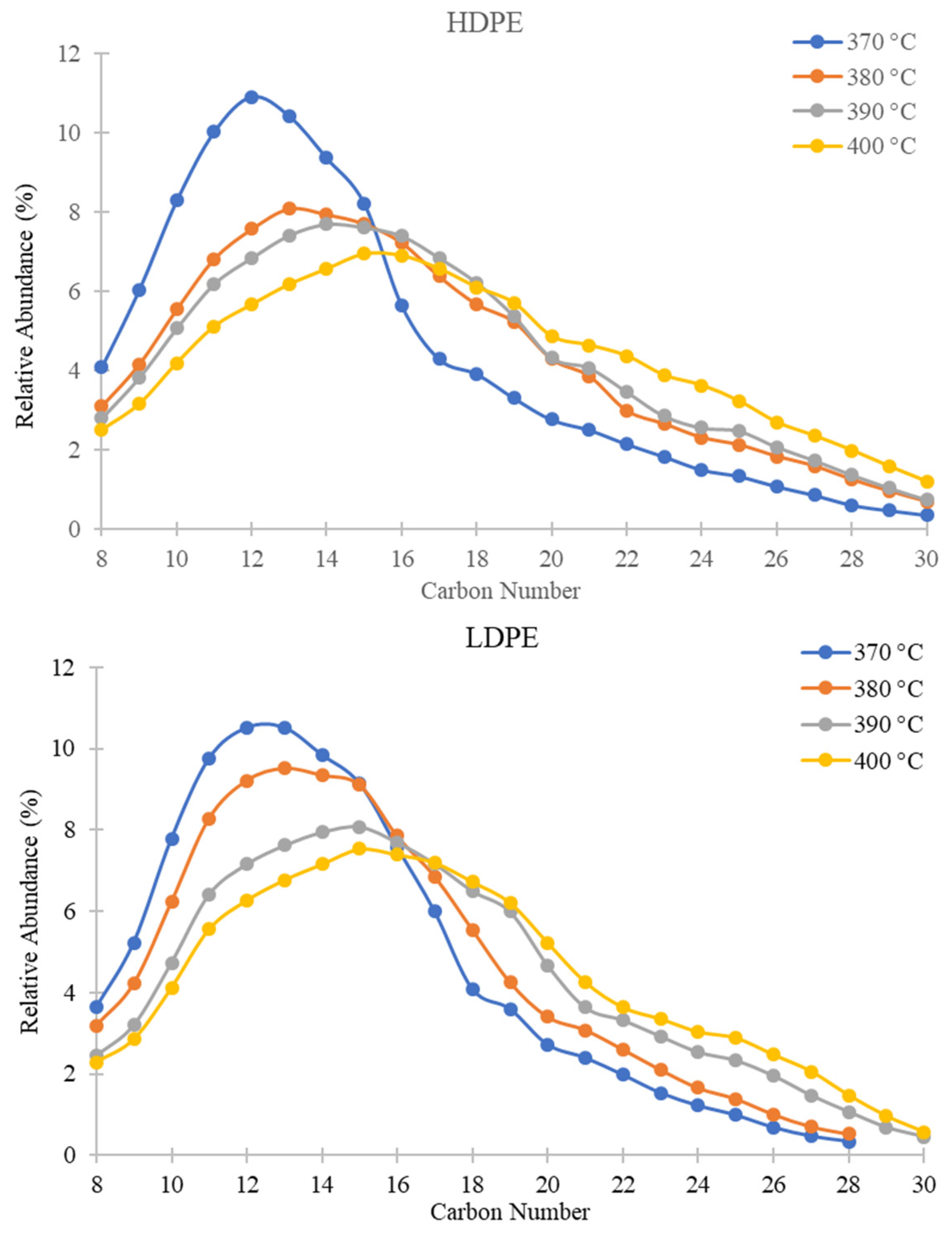
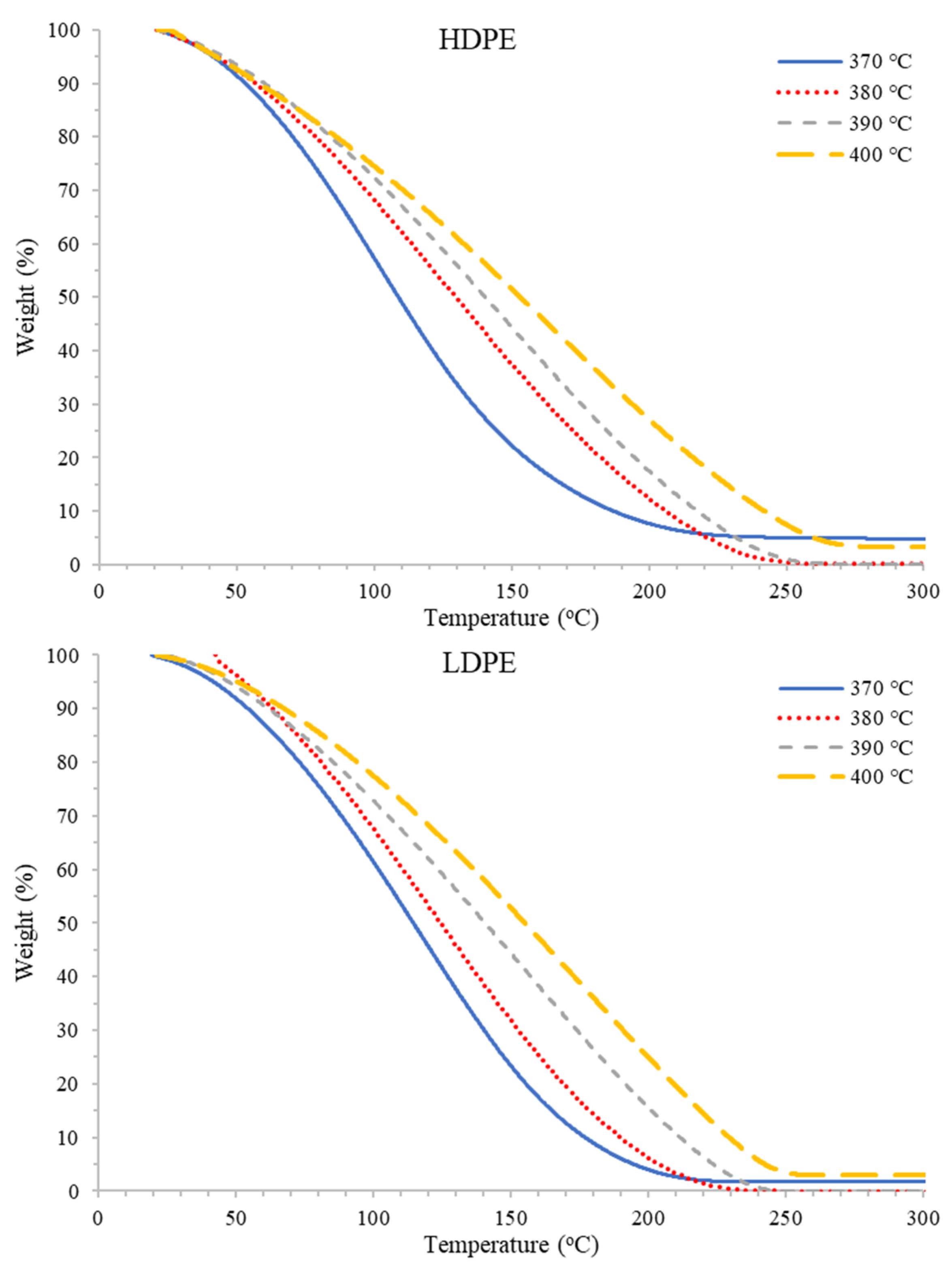
3.1. Effect of Temperature
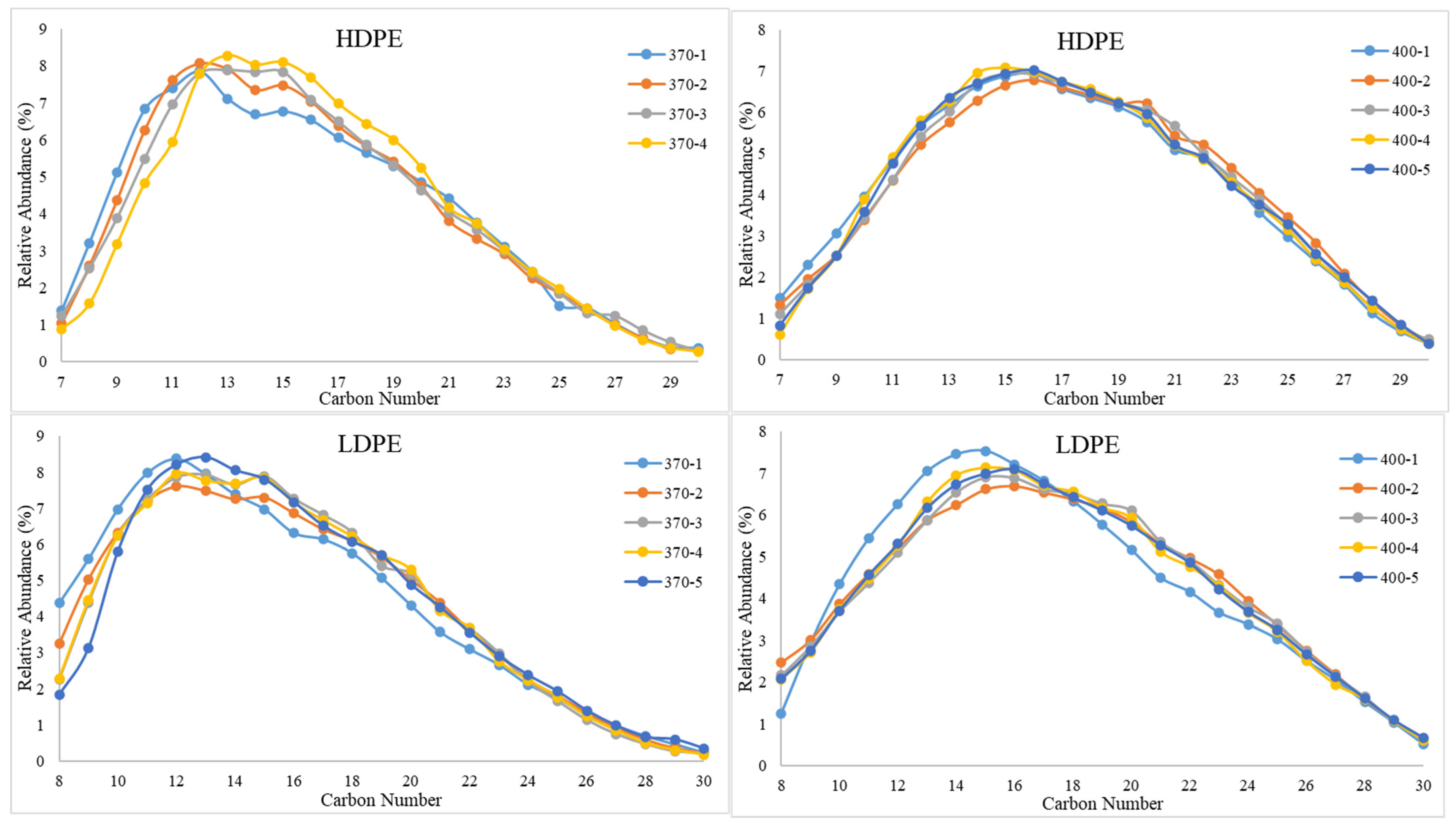
3.1.1. GC-MS Results
3.1.2. TGA Results
3.2. Effect of Time
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalanatarifard, A.; Yang, G.S. Identification of the Municipal Solid Waste Characteristics and Potential of Plastic Recovery at Bakri Landfill, Muar, Malaysia. J. Sust. Dev. 2012, 5, 11. [Google Scholar] [CrossRef]
- Minghua, Z.; Xiumin, F.; Rovettac, A.; Qichang, H.; LiuBingkai, F.V.; Giustic, A.; Yi, L. Municipal solid waste management in Pudong New Area, China. Waste Manag. 2009, 29, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.R.A.; Mokhtarani, N.; Mokhtarani, B. Municipal solid waste management in Rasht City, Iran. Waste Manag. 2009, 29, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Mrayyan, B.; Hamdi, M.R. Management approaches to integrated solid waste in industrialized zones in Jordan: A case of Zarqa City. Waste Manag. 2006, 26, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Seng, B.; Kaneko, H.; Hirayama, K.; Katayama-Hirayama, K. Municipal solid waste management in Phnom Penh, capital city of Cambodia. Waste Manag. Res. 2010, 29, 491–500. [Google Scholar] [CrossRef]
- Sujauddin, M.; Huda, M.S.; Hoque, R.A.T.M. Household solid waste characteristics and management in Chittagong, Bangladesh. Waste Manag. 2008, 28, 1688–1695. [Google Scholar] [CrossRef]
- Troschinetz, A.M.; Mihelcic, J.R. Sustainable recycling of municipal solid waste in developing countries. Waste Manag. 2009, 29, 915–923. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos.Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Barnes, D.K.; Walters, A.; Goncalves, L. Macroplastics at sea around Antarctica. Mar. Environ. Res. 2011, 70, 250–252. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [Green Version]
- Geyer, R.; Jambeck, J.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Jayasiri, H.B.; Purushothaman, C.S.; Vennila, A. Quantitative analysis of plastic debris on recreational beaches in Mumbai, India. Mar. Poll. Bull. 2013, 77, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, J. Plastic Not-So-Fantastic: How the Versatile Material Harms the Environment and Human Health; Scientific American: New York, NY, USA, 2019; Available online: https://www.scientificamerican.com/article/plastic-not-so-fantastic/ (accessed on 15 May 2021).
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Parker, L. In a First, Microplastics Found in Human Poop; National Geographic Magazine: Washington, DC, USA, 2018; Available online: www.nationalgeographic.com (accessed on 15 May 2021).
- Parker, L. Plastics. Planet or Plastic? Natl. Geogr. 2018, 40–91. [Google Scholar]
- Rochman, C.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Swee Teh, S.; Thompson, R.C. Policy: Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef]
- Wieczorek, A.M.; Morrison, L.; Croot, P.L.; Allcock, A.L.; MacLoughlin, E.; Savard, O.; Brownlow, H.; Doyle, T.K. Frequency of microplastics in mesopelagic fishes from the Northwest Atlantic. Front. Mar. Sci. 2018, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, C.; Sebille, E.V.; Hardesty, B.D. Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proc. Natl. Acad. Sci. USA 2015, 112, 11899–11904. [Google Scholar] [CrossRef] [Green Version]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and Recovery Routes of Plastic Solid Waste (PSW): A Review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- DeNeve, D.; Joshi, C.; Higgins, J.; Seay, J. Optimization of an Appropriate Technology Based Process for Converting Waste Plastic in to Liquid Fuel via Thermal Decomposition. J. Sust. Dev. 2017, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, R.K. Recovery of Hydrocarbon Liquid from Waste High Density Polyethylene by Thermal Pyrolysis. Braz. J. Chem. Eng. 2011, 28, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Miskolczi, N.; Bartha, L.; Deak, G.; Jover, B. Thermal Degradation of Municipal Plastic Waste for Production of Fuel-Like Hydrocarbons. Polym. Degrad. Stab. 2004, 86, 357–366. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.K.; Mishra, D.K. Thermolysis of Waste Plastics to Liquid Fuel. A Suitable Method for Plastic Waste Management and Manufacture of Value Added Products—A World Prospective. Renew. Sustain. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Patil, L.; Varma, A.K.; Singh, G.; Mondal, P. Thermocatalytic Degradation of High Density Polyethylene into Liquid Product. J. Polym. Environ. 2017, 26, 1920–1929. [Google Scholar] [CrossRef]
- Pinto, F.; Costa, P.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of plastic wastes. 1. Effect of plastic waste composition on product yield. J. Anal. Appl. Pyrolysis 1999, 51, 39–55. [Google Scholar] [CrossRef]
- Santaweesuk, C.; Janyalertadun, A. The Production of Fuel Oil by Conventional Slow Pyrolysis Using Plastic Waste from a Municipal Landfill. Int. J. Environ. Sci. Dev. 2017, 8, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M. Converting waste plastic to hydrocarbon fuel materials. Energy Eng. 2011, 108, 35–43. [Google Scholar] [CrossRef]
- Sarker, M.; Molla, M.; Rashid, M.M.; Rahman, M.S. Production of Valuable Heavy Hydrocarbon Fuel Oil by Thermal Degradation Process of Post-Consumer Municipal Polystyrene (PS) Waste Plastic in Steel Reactor. Energy Power 2012, 2, 89–95. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B. Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel 2016, 174, 164–171. [Google Scholar] [CrossRef]
- Wong, S.I.; Ngadia, N.; Abdullahb, T.A.T.; Inuwac, I.M. Current State and Future Prospects of Plastic Waste as Source of Fuel: A Review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Joshi, C.A.; Seay, J.R. An appropriate technology based solution to convert waste plastic into fuel oil in underdeveloped re-gions. J. Sustain. Dev. 2016, 9, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Joshi, C.; Seay, J. Building momentum for sustainable behaviors in developing regions using Locally Managed Decentralized Circular Economy. Chin. J. Chem. Eng. 2019, 27, 1566–1571. [Google Scholar] [CrossRef]
- Joshi, C.A.; Seay, J.R. Total generation and combustion emissions of plastic derived fuels: A trash to tank approach. Environ. Prog. Sustain. Energy 2019, 39. [Google Scholar] [CrossRef]
- Joshi, C.; Seay, J.; Banadda, N. A Perspective on a Locally Managed Decentralized Circular Economy for Waste Plastic in Developing Countries. Environ. Prog. Sustain. Energy 2019, 38, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Joshi, C.; Browning, S.; Seay, J. Combating plastic waste via Trash to Tank. Nat. Rev. Earth Environ. 2020, 1, 142. [Google Scholar] [CrossRef]
- Schumacher, E.F. Small Is Beautiful: Economics as If People Mattered; Harper & Row: New York, NY, USA, 1973. [Google Scholar]
- Budsaereechai, S.; Hunt, A.J.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv. 2019, 9, 5844–5857. [Google Scholar] [CrossRef] [Green Version]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, Ε.V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef]
- Chandrasekaran, S.R.; Kunwar, B.; Moser, B.R.; Rajagopalan, N.; Sharma, B.K. Catalytic thermal cracking of postconsumer waste plastics to fuels. 1. Kinetics and optimization. Energy Fuels 2015, 29, 6068–6077. [Google Scholar] [CrossRef]
- Kunwar, B.; Chandrasekaran, S.R.; Moser, B.R.; Deluhery, J.; Kim, P.; Rajagopalan, N.; Sharma, B.K. Catalytic thermal cracking of postconsumer waste plastics to fuels. 2. Pilot-scale thermochemical conversion. Energy Fuels 2017, 31, 2705–2715. [Google Scholar] [CrossRef]
- Liu, S.; Kots, P.A.; Vance, B.C.; Danielson, A.; Vlachos, D.G. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 2021, 7, eabf8283. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Ismail, I.M.I.; Nizami, A.S. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag. 2017, 69, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic pyrolysis of plastic waste: Moving toward pyrolysis based biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Aboulkas, A.; Nadifiyine, M. Investigation on pyrolysis of Moroccan oil shale/plastic mixtures by thermogravimetric analysis. Fuel Process. Technol. 2008, 89, 1000–1006. [Google Scholar] [CrossRef]
- Rahman, S. Pyrolysis of Waste Plastic Fish Bags to Useable Fuel Oil; Harris Centre, Memorial University: St. John’s, NL, Canada, 2018. [Google Scholar]
- Zhou, L.; Wang, Y.; Huang, Q.; Cai, J. Thermogravimetric characteristics and kinetic of plastic and biomass blends co-pyrolysis. Fuel Process. Technol. 2006, 87, 963–969. [Google Scholar] [CrossRef]
- Phetyim, N.; Pivsa-Art, S. Prototype co-Pyrolysis of used lubricant oil and mixed plastic waste to produce a diesel-like fuel. Energies 2018, 11, 2973. [Google Scholar] [CrossRef] [Green Version]
- Breyer, S.; Mekhitarian, L.; Rimez, B.; Haut, B. Production of an alternative fuel by the co-pyrolysis of landfill recovered plastic wastes and used lubrication oils. Waste Manag. 2017, 60, 363–374. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Zhou, L.; Huang, Q. Thermogravimetric analysis and kinetics of coal/plastic blends during co-pyrolysis in nitrogen atmosphere. Fuel Process. Technol. 2008, 89, 21–27. [Google Scholar] [CrossRef]
- Xing, X.; Wang, S.; Zhang, Q. Thermogravimetric analysis and kinetics of mixed combustion of waste plastics and semicoke. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Boadu, E.K.; Dapaah, S. Plastic waste to fuel via pyrolysis: A key way to solving the severe plastic waste problem in Ghana. Therm. Sci. Eng. Prog. 2019, 11, 417–424. [Google Scholar] [CrossRef]
- Akoueson, F.; Chbib, C.; Monchy, S.; Paul-Pont, I.; Doyen, P.; Dehaut, A.; Duflos, G. Identification and quantification of plastic additives using Pyrolysis-GC/MS: A review. Sci. Total Environ. 2021, 773, 145073. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Himber, C.; Boricaud, B.; Kazour, M.; Amara, R.; Cassone, A.-L.; Laurentie, M.; Paul-Pont, I.; Soudant, P.; Dehaut, A.; et al. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal. Bioanal. Chem. 2018, 410, 6663–6676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agboola, O.; Sadiku, R.; Mokrani, T.; Amer, I.; Imoru, O. Polyolefins and the environment. In Fibres; Woodhead Publishing: Cambridge, UK, 2017; pp. 89–133. [Google Scholar]
- CROW. Depolymerization, Polymer Properties Database. Crow Polymer Science. 2021. Available online: http://polymerdatabase.com/home.html (accessed on 17 June 2021).
- Zeus®. Technical Whitepaper: Thermal Degradation of Plastics; Zeus Industrial Products, Inc.: Orangeburg, SC, USA, 2005. [Google Scholar]
- U.S. Department of Health and Human Services. Toxicological Profile for Fuel Oil, Chapter 3, Chemical and Physical Information; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1995; pp. 105–109. [Google Scholar]
- Rentar Fuel Catalyst. What Are Hydrocarbons and What Hydrocarbons Are in Diesel? 19 April 2018. Available online: https://rentar.com/hydrocarbons-hydrocarbons-diesel/#:%7E:text=%E2%80%9CPetroleum-derived (accessed on 15 May 2021).
- Gad, S.C. Kerosene. Encycl. Toxicol. 2005, 2, 664–665. [Google Scholar]
- International Energy Association—Advanced Motor Fuels (IEA-AMF). Diesel and Gasoline. Available online: https://www.iea-amf.org/content/fuel_information/diesel_gasoline (accessed on 15 May 2021).
- ALS Global. Petroleum Fractions by Carbon Range. 2021. Available online: www.alsglobal.com (accessed on 15 May 2021).
- Gad, S.C. Diesel Fuel. Encycl. Toxicol. 2014, 3, 115–118. [Google Scholar]
- CorrosionpediaTM. Fuel Stability. Janatta Interactive. January 2018. Available online: https://www.corrosionpedia.com/definition/1644/fuel-stability (accessed on 15 May 2021).

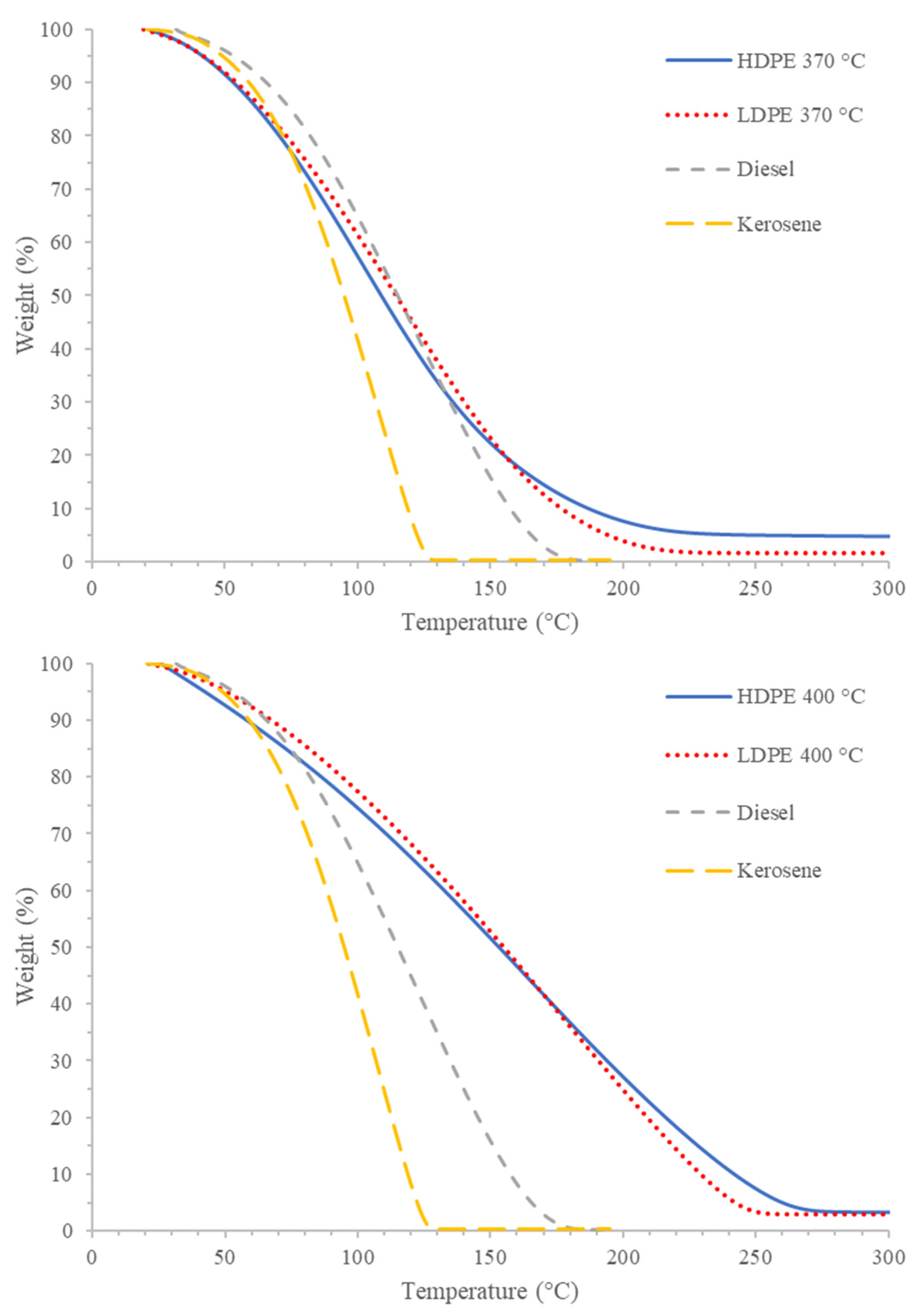
| Diesel (C11–C20) | ||||
|---|---|---|---|---|
| Kerosene (C9–C16) | ||||
| PDFO Type and Temperature | PDFO Temperature (°C) | Relative Abundance (%) | Standard Deviation | Hydrocarbon Range |
| HDPE | 370 | 81.17 | 2.67 | C8–C18 |
| 400 | 80.82 | 1.18 | C9–C23 | |
| LDPE | 370 | 80.45 | 2.30 | C9–C18 |
| 400 | 80.90 | 1.53 | C9–C22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jangid, C.J.; Miller, K.M.; Seay, J.R. Analysis of Plastic-Derived Fuel Oil Produced from High- and Low-Density Polyethylene. Recycling 2022, 7, 29. https://doi.org/10.3390/recycling7030029
Jangid CJ, Miller KM, Seay JR. Analysis of Plastic-Derived Fuel Oil Produced from High- and Low-Density Polyethylene. Recycling. 2022; 7(3):29. https://doi.org/10.3390/recycling7030029
Chicago/Turabian StyleJangid, Chandni Joshi, Kevin M. Miller, and Jeffrey R. Seay. 2022. "Analysis of Plastic-Derived Fuel Oil Produced from High- and Low-Density Polyethylene" Recycling 7, no. 3: 29. https://doi.org/10.3390/recycling7030029






