Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair
Abstract
:1. Introduction
2. Materials and Method
2.1. Chitosan
2.2. Cellulose Nanofibers
2.3. Preparation of the CNF-Filled Chitosan Composite Hydrogels
2.4. Microtensile Testing of CNF-Filled Chitosan Composite Hydrogels
2.5. Suitability of the CNF/CHI Composite Hydrogels for IVD Repair and the Re-Establishment of IVD Biomechanics
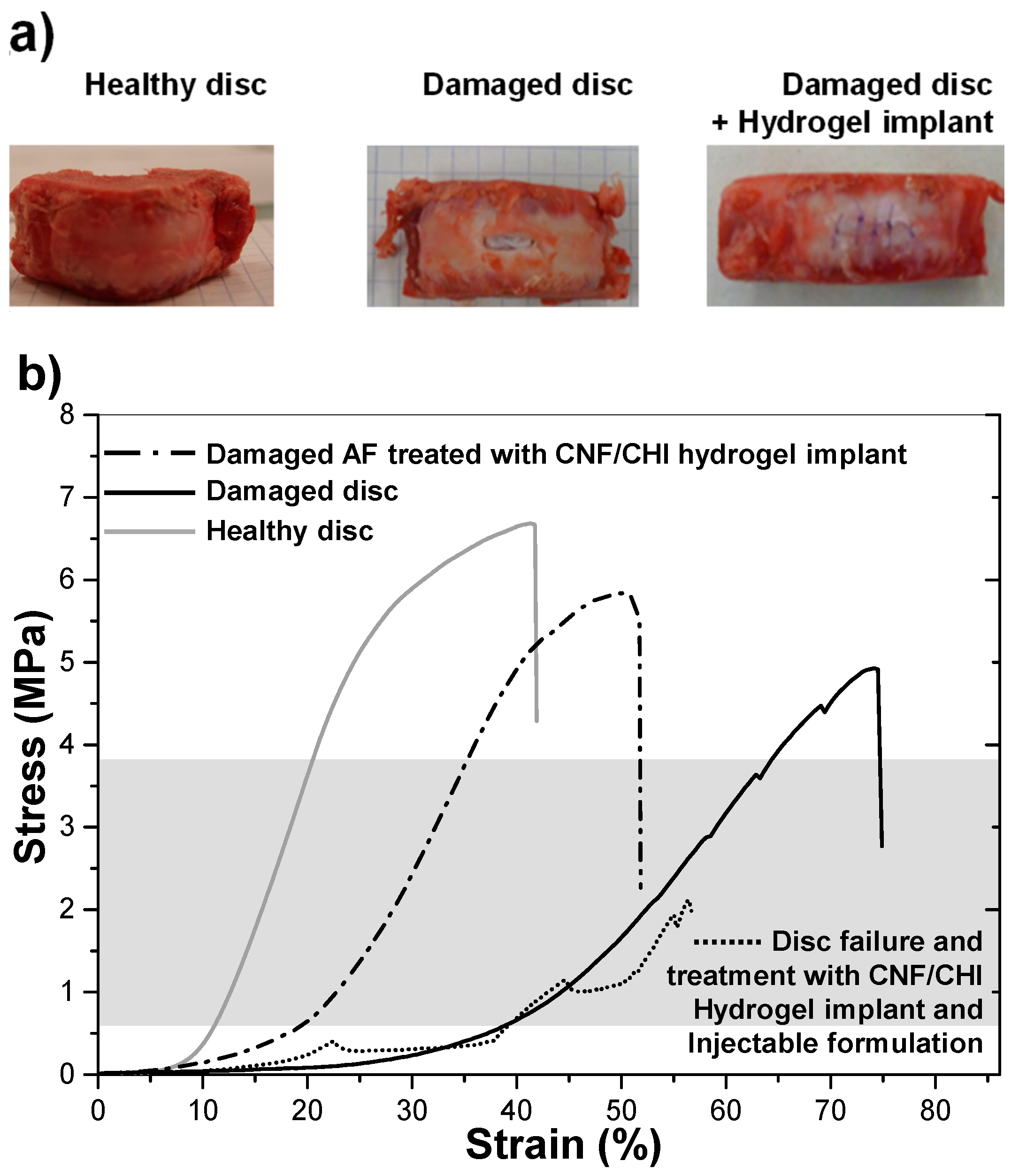
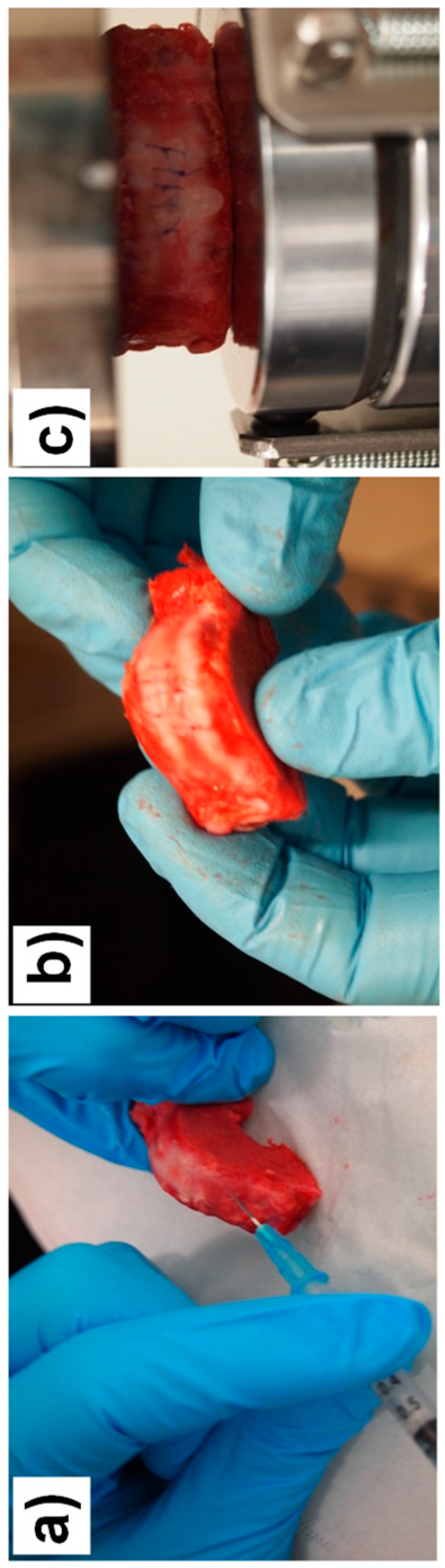
2.5.1. Implant of CNF/CHI Composite Hydrogels in the Annulus Fibrosus Region of the IVD. Biomechanical Studies
2.5.2. Cell Culture of NIH/3T3 Fibroblasts on CNF/CHI Hydrogels
3. Results and Discussion
3.1. Structure of the CNF/CHI Hydrogels
3.2. Micromechanical Properties of the CNF/CHI Composite Hydrogels
3.3. Suitability of the CNF/CHI Composite Hydrogels for IVD Repair and Biomechanics Re-Establishment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urban, J.P.G.; Smith, S.; Fairbank, J.C.T. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.W.; Gantenbein-Ritter, B. Intervertebral disc regeneration or repair with biomaterials and stem cell therapy—Feasible or fiction? Swiss Med. Wkly. 2012, 142, w13598. [Google Scholar] [CrossRef] [PubMed]
- Whatley, B.R.; Wen, X. Intervertebral disc (IVD): Structure, degeneration, repair and regeneration. Mater. Sci. Eng. C 2012, 32, 61–77. [Google Scholar] [CrossRef]
- Rapoff, A.J.; Zdeblick, T.A. Biomechanical models of the cervical spine. In Frontiers in Head and Neck Trauma: Clinical and Biomechanical; Yoganandan, N., Pintar, F.A., Larson, S.J., Sances, A.J., Eds.; IOS Press: Amsterdam, The Netherlands, 1998; p. 743. [Google Scholar]
- Sato, K.; Kikuchi, S.; Yonezawa, T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine 1999, 24, 2468. [Google Scholar] [CrossRef] [PubMed]
- Hukins, D.W.L.; Meakin, J.R. Relationship between structure and mechanical function of the tissues of the intervertebral joint. Am. Zool. 2000, 40, 42–52. [Google Scholar] [CrossRef]
- Adams, M.A.; McNally, D.S.; Dolan, P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. The Journal of bone and joint surgery. J. Bone Joint Surg. Br. 1996, 78, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Bulstra, S.K.; Grijpma, D.W.; Kuije, R. Treatment of the degenerated intervertebral disc; closure, repair and regeneration of the annulus fibrosus. J. Tissue Eng. Regen. Med. 2015, 9, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.; Sadosky, A.; Stacey, B.R.; Tai, K.-S.; Leslie, D. The burden of chronic low back pain: Clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012, 37, E668–E677. [Google Scholar] [CrossRef] [PubMed]
- van Ooij, A.; Oner, F.C.; Verbout, A.J. Complications of artificial disc replacement: A report of 27 patients with the SB Charité disc. J. Spinal Disord. Tech. 2003, 16, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.I.; Nicoll, S.B. Characterization of photocrosslinked alginate hydrogels for nucleus pulposus cell encapsulation. J. Biomed. Mater. Res. A 2009, 91, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Pöppelmann, B.; Pappelbaum, K.; Moerschbacher, B.M.; Schneider, S.W. Human macrophage activation triggered by chitotriosidase-mediated chitin and chitosan degradation. Biomaterials 2010, 31, 8556–8563. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Mathews, S.; Gupta, P.K.; Bhonde, R.; Totey, S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011, 44, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.G.; Tahiri, K.; Montembault, A.S.; Domard, A.J.; Corvol, M.T.M. Multi-membrane chitosan hydrogels as chondrocytic cell bioreactors. Biomaterials 2011, 32, 5354–5364. [Google Scholar] [CrossRef] [PubMed]
- Montembault, A.; Tahiri, K.; Korwin-Zmijowska, C.; Chevalier, X.; Corvol, M.-T.; Domard, A. A material decoy of biological media based on chitosan physical hydrogels: Application to cartilage tissue engineering. Biochimie 2006, 88, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; Eder, M.; Rueggeberg, M.; Pandey, J.K.; Harrington, M.J.; Nishiyama, Y.; Putaux, J.-L.; Rochas, C.; Burgert, I. Reorientation of cellulose nanowhiskers in agarose hydrogels under tensile loading. Biomacromolecules 2012, 13, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; David, L.; Peniche-Covas, C.; Rochas, C.; Putaux, J.-L.; Trombotto, S.; Alcouffe, P.; Domard, A. Fine microstructure of processed chitosan nanofibril networks preserving directional packing and high molecular weight. Carbohydr. Polym. 2015, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Highly crystalline chitosan produced by multi-steps acid hydrolysis in the solid-state. Carbohydr. Polym. 2011, 83, 1730–1739. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; Laborie, M.-P. Morphological and thermal investigations of cellulosic bionanocomposites. In Biopolymer Nanocomposites; Dufresne, A., Thomas, S., Pothen, L.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 411–436. [Google Scholar]
- Samyn, P.; Osorio-Madrazo, A. Native crystalline polysaccharide nanofibers: Processing and properties. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–36. [Google Scholar]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation of cellulose I nanowhiskers with a mildly acidic aqueous ionic liquid: Reaction efficiency and whiskers attributes. Cellulose 2013, 20, 1829–1840. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaillé, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed Engl. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Šturcová, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.; Ching, Y.; Chuah, C.; Julai, S.; Liou, N.-S. Preparation and characterization of polyvinyl alcohol–chitosan composite films reinforced with cellulose nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, M.; Ranginkar Jahromi, I.; Moallemi-Oreh, A.; Ebadi-Dehaghani, H.; Akbarzadeh, M. Evaluation of mixing efficiency in elaborating of chitosan/cellulose nanocomposite via statistical analyses. Int. J. Biol. Macromol. 2016, 93, 703–711. [Google Scholar] [CrossRef] [PubMed]
- de Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132, 42004. [Google Scholar] [CrossRef]
- Li, H.-Z.; Chen, S.-C.; Wang, Y.-Z. Preparation and characterization of nanocomposites of polyvinyl alcohol/cellulose nanowhiskers/chitosan. Compos. Sci. Technol. 2015, 115, 60–65. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Adnan, A.S.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Celebi, H.; Kurt, A. Effects of processing on the properties of chitosan/cellulose nanocrystal films. Carbohydr. Polym. 2015, 133, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, H.; Chen, J.; Li, S.; Huang, J.; Zhou, C. Chitosan-chitin nanocrystal composite scaffolds for tissue engineering. Carbohydr. Polym. 2016, 152, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, T.; Naish, V.; O’Connor, B.; Blaise, C.; Gagne, F.; Hall, L.; Trudeau, V.; Martel, P. An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Pértile, R.A.; Moreira, S.; Gil, R.M.; Correia, A.; Guãrdao, L. Bacterial cellulose: Long-term biocompatibility studies. J. Biomater. Sci. Polym. 2012, 23, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, M. Nanofibrillar Cellulose—In Vitro Study of Cytotoxic And Genotoxic Properties. In Proceedings of the 2010 International Conferene on Nanotechnology for the Forest Products Industry, Espoo, Finland, 27–29 September 2010. [Google Scholar]
- Doench, I.; Torres-Ramos, M.E.; Motembault, A.; de Oliveira, P.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.; Osorio-Madrazo, A. Injectable and gelable chitosan formulations filled with cellulose nanofibers for intervertebral disc tissue engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef]
- Eyholzer, C.; Borges de Couraça, A.; Duc, F.; Bourban, P.E.; Tingaut, P.; Zimmermann, T.; Månson, J.A.E.; Oksman, K. Biocomposite hydrogels with carboxymethylated, nanofibrillated cellulose powder for replacement of the nucleus pulposus. Biomacromolecules 2011, 12, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.C.; Eyholzer, C.; Duc, F.; Bourban, P.-E.; Tingaut, P.; Zimmermann, T.; Pioletti, D.P.; Månson, J.-A.E. Nanofibrillated cellulose composite hydrogel for the replacement of the nucleus pulposus. Acta Biomater. 2011, 7, 3412–3421. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Oh, D.X.; Choy, S.; Nguyen, H.-L.; Cha, H.J.; Hwang, D.S. 3D cellulose nanofiber scaffold with homogeneous cell population and long-term proliferation. Cellulose 2018, 25, 7299–7314. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Nguyen, T.H.M.; Abueva, C.; Ho, H.V.; Lee, S.-Y.; Lee, B.-T. In vitro and in vivo acute response towards injectable thermosensitive chitosan/TEMPO-oxidized cellulose nanofiber hydrogel. Carbohydr. Polym. 2018, 180, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Sereni, N.; Enache, A.; Sudre, G.; Montembault, A.; Rochas, C.; Durand, P.; Perrard, M.-H.; Bozga, G.; Puaux, J.-P.; Delair, T.; et al. Dynamic structuration of physical chitosan hydrogels. Langmuir 2017, 33, 12697–12707. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; van Kooten, T.G.; Kranenburg, H.-J.C.; Meij, B.P.; Behl, M.; Lendlein, A.; Grijpma, D.W. An annulus fibrosus closure device based on a biodegradable shape-memory polymer network. Biomaterials 2013, 34, 8105–8113. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed]
- Gharehkhani, S.; Sadeghinezhad, E.; Kazi, S.N.; Yarmand, H.; Badarudin, A.; Safaei, M.R.; Zubir, M.N.M. Basic effects of pulp refining on fiber properties—A review. Carbohydr. Polym. 2015, 115, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Falcoz-Vigne, L.; Ogawa, Y.; Molina-Boisseau, S.; Nishiyama, Y.; Meyer, V.; Petit-Conil, M.; Mazeau, K.; Heux, L. Quantification of a tightly adsorbed monolayer of xylan on cellulose surface. Cellulose 2017, 24, 3725–3739. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kurki-Suonio, S.; Schacher, F.H.; Hietala, S.; Rojas, O.J.; Ikkala, O. Water-resistant, transparent hybrid nanopaper by physical cross-linking with chitosan. Biomacromolecules 2015, 16, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, E.; Winkler, T.; Mielke, G.; Paetzold, H.; Schuettler, D.; Goepfert, C.; Machens, H.-G.; Morlock, M.M.; Schilling, A.F. High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue-engineered cartilage. Tissue Eng. Part A 2011, 17, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Ayturk, U.M.; Garcia, J.J.; Puttlitz, C.M. The micromechanical role of the annulus fibrosus components under physiological loading of the lumbar spine. J. Biomech. Eng. 2010, 132, 061007. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.W.; Garbutt, G.; Adams, M.A. Effect of sustained loading on the water content of intervertebral discs: Implications for disc metabolism. Ann. Rheum. Dis. 1996, 55, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Mauck, R.L.; Elliott, D.M. Modeling interlamellar interactions in angle-ply biologic laminates for annulus fibrosus tissue engineering. Biomech. Model. Mechanobiol. 2011, 10, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]





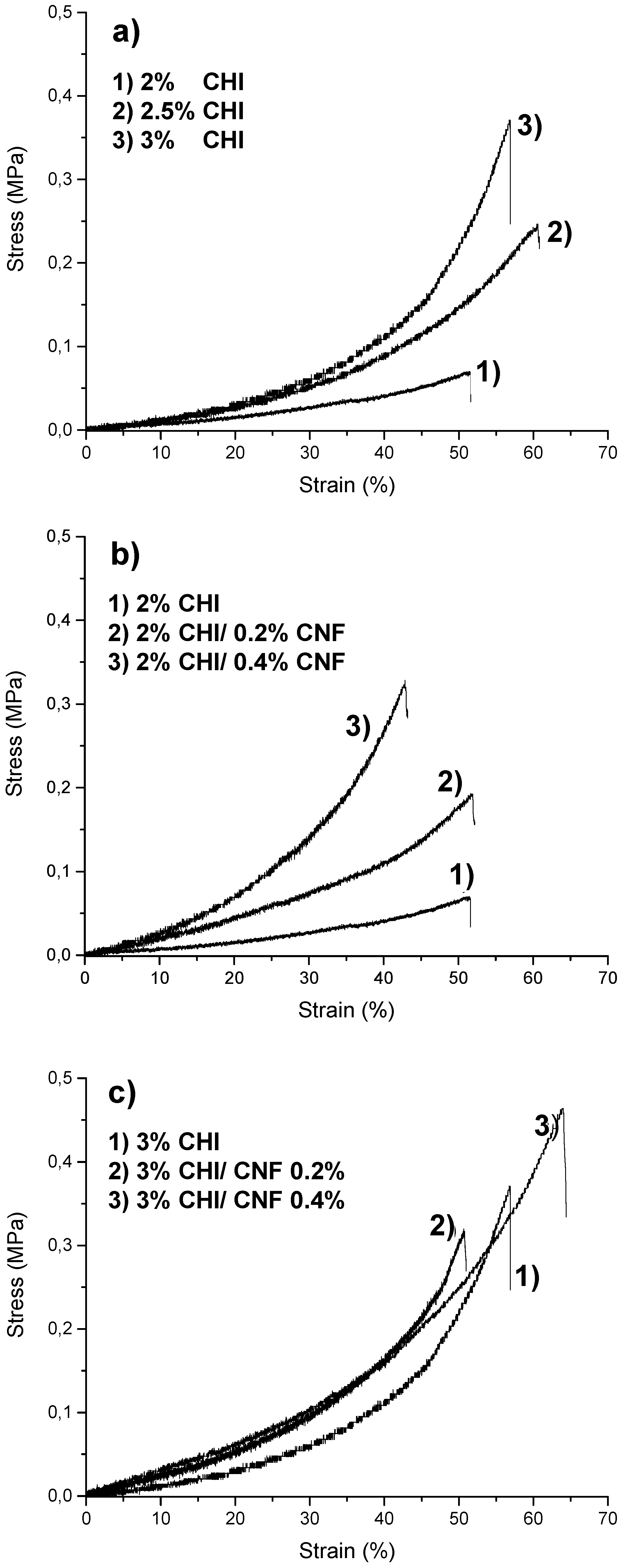

| Hydrogel Composition | E (MPa) 1 |
|---|---|
| 2% CHI | 0.073 ± 0.0024 |
| 3% CHI | 0.13 ± 0.0045 |
| 2% CHI/0.2% CNF | 0.22 ± 0.0013 |
| 3% CHI/0.2% CNF | 0.24 ± 0.0013 |
| 2% CHI/0.4% CNF | 0.28 ± 0.0021 |
| 3% CHI/0.2% CNF | 0.30 ± 0.0011 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doench, I.; Ahn Tran, T.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N.; et al. Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair. Biomimetics 2019, 4, 19. https://doi.org/10.3390/biomimetics4010019
Doench I, Ahn Tran T, David L, Montembault A, Viguier E, Gorzelanny C, Sudre G, Cachon T, Louback-Mohamed M, Horbelt N, et al. Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair. Biomimetics. 2019; 4(1):19. https://doi.org/10.3390/biomimetics4010019
Chicago/Turabian StyleDoench, Ingo, Tuan Ahn Tran, Laurent David, Alexandra Montembault, Eric Viguier, Christian Gorzelanny, Guillaume Sudre, Thibaut Cachon, Malika Louback-Mohamed, Niels Horbelt, and et al. 2019. "Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair" Biomimetics 4, no. 1: 19. https://doi.org/10.3390/biomimetics4010019





