Passing the Wake: Using Multiple Fins to Shape Forces for Swimming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biorobotic Studies
2.1.1. PDAC Body
2.1.2. Fins
2.1.3. Support Structure
2.1.4. Testing Environment
2.1.5. Experimental Methods
2.1.6. Experimental Validation
2.1.7. Data Processing
2.2. Computational Fluid Dynamics Simulation
2.2.1. Geometric Modeling
2.2.2. Numerical Simulations
2.2.3. Data Processing
3. Results
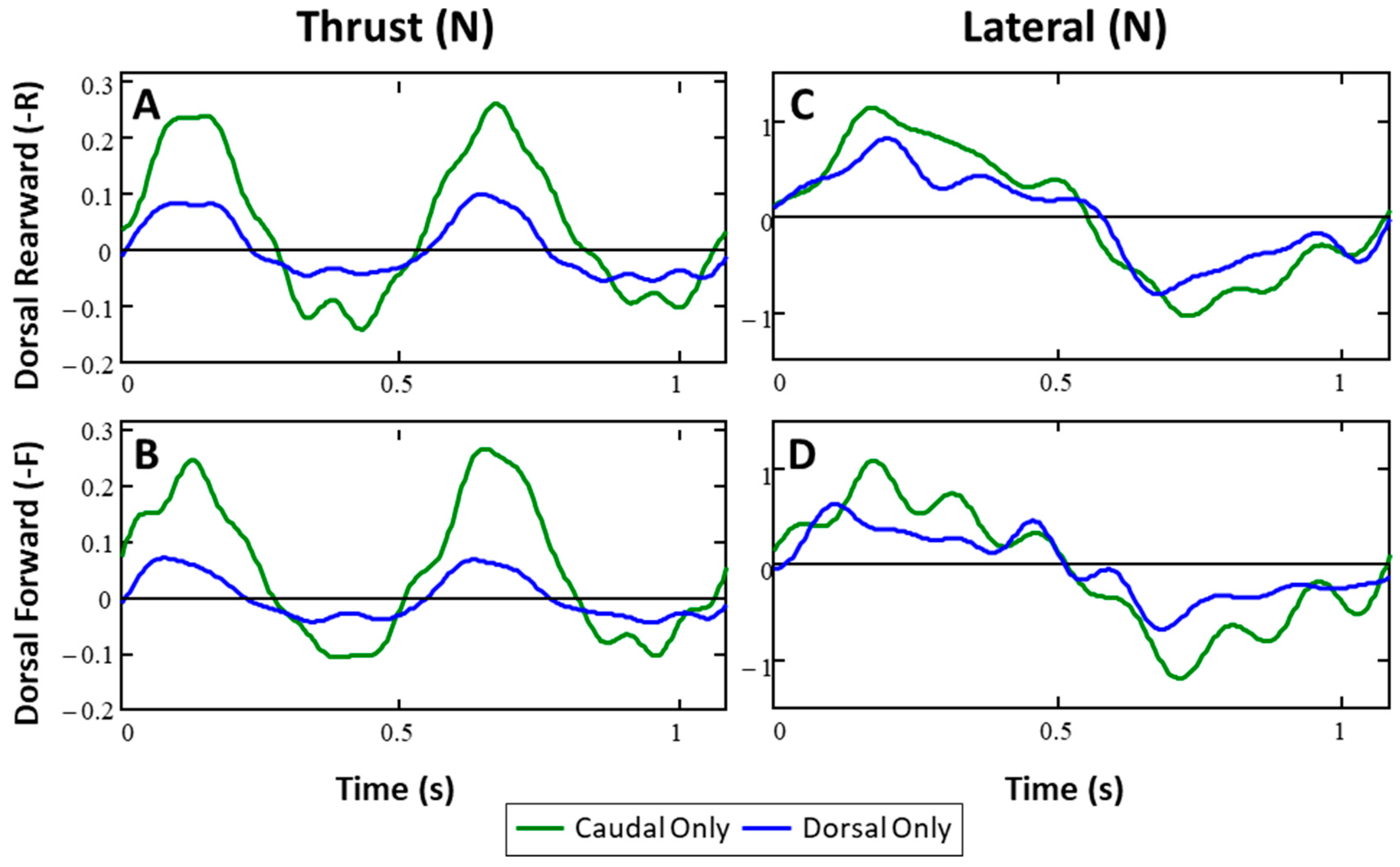
3.1. Forces from Single Fins (Baseline)
3.2. Mean Thrust and Root Mean Square Lateral Forces
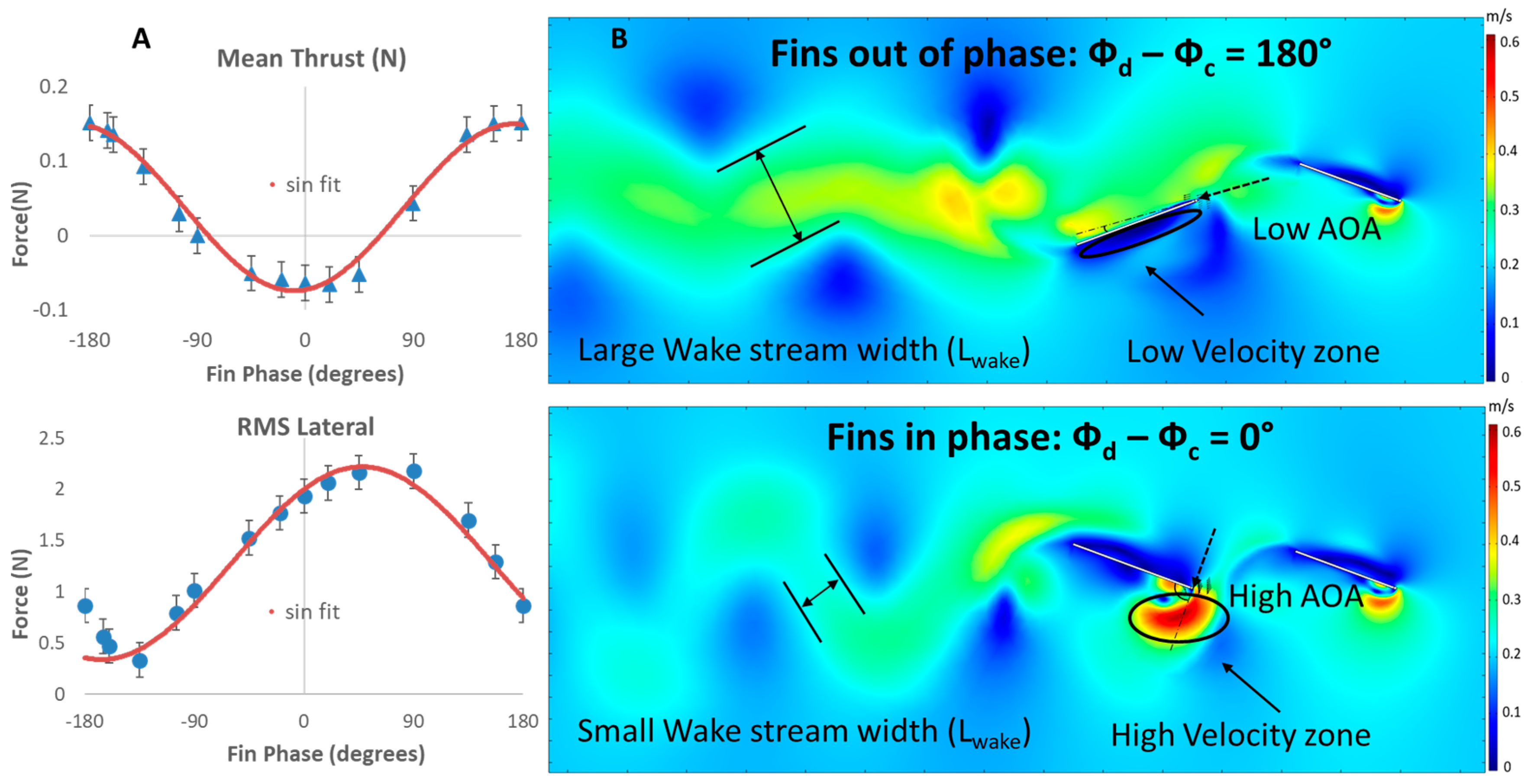
3.2.1. Mean Thrust vs. Phase
3.2.2. Root Mean Square Lateral Forces vs. Phase
3.3. Forces through Time
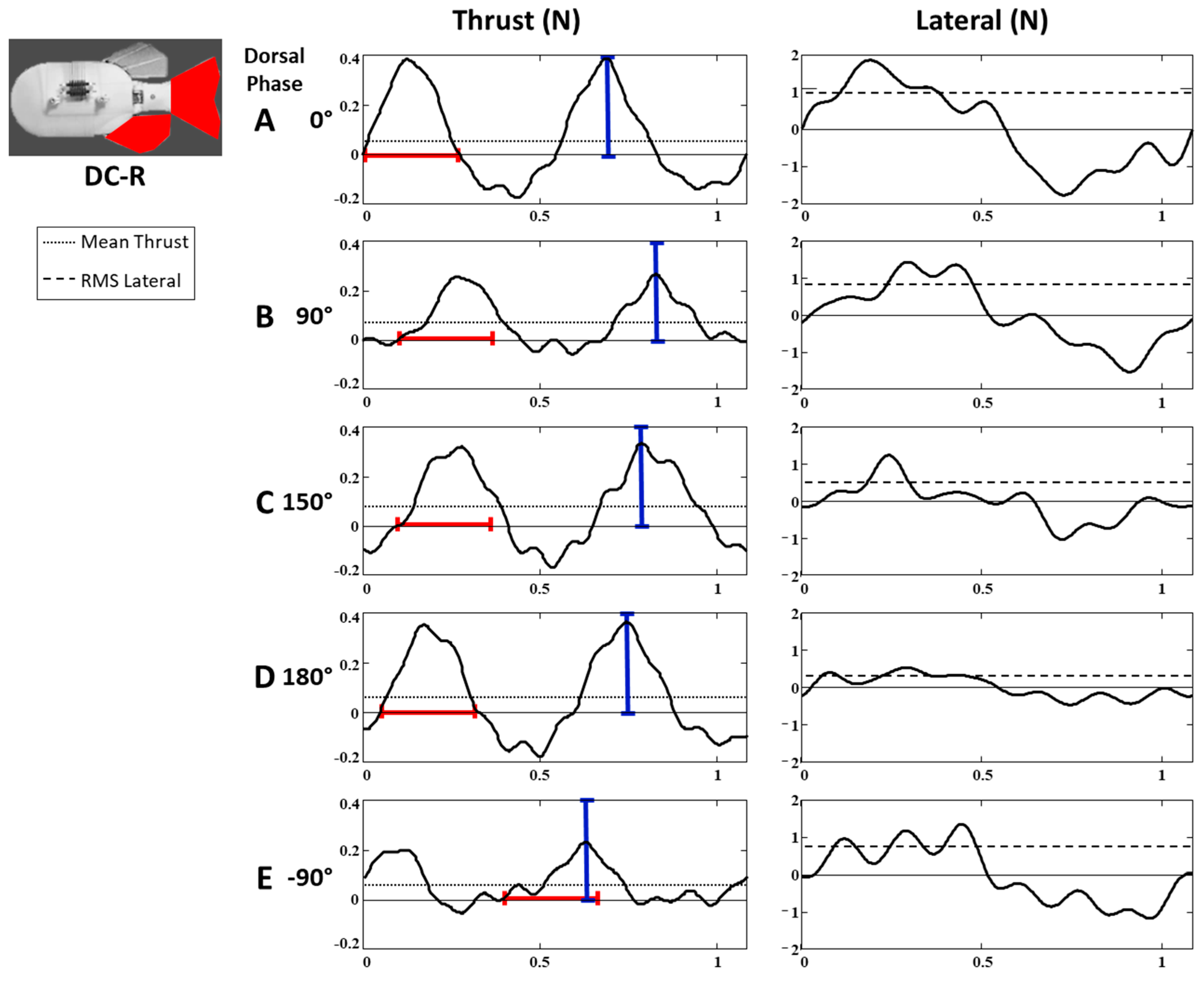
3.3.1. Dorsal and Caudal Forces as Function of Time
Thrust Forces (DC-R Configuration)
Lateral Forces (DC-R Configuration)
3.3.2. Effect of Additional Fins and Fin Configurations
3.3.3. The 2D Forces
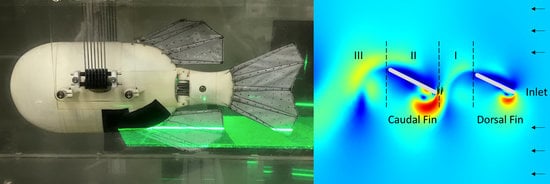
3.4. Wake Flows
3.4.1. Biorobotic Flow
3.4.2. Simulated Flow
4. Discussion
4.1. Forces
4.2. Flows
4.3. Body Motion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Lauder, G.V.; Madden, P.G.A. Learning from fish: Kinematics and experimental hydrodynamics for roboticists. Int. J. Autom. Comput. 2006, 3, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Lauder, G.V. Swimming hydrodynamics: Ten questions and the technical approaches needed to resolve them. Exp. Fluids 2009, 51, 23–35. [Google Scholar] [CrossRef]
- Jayne, B.C.; Lozada, A.F.; Lauder, G.V. Function of the dorsal fin in bluegill sunfish: Motor patterns during four distinct locomotor behaviors. J. Morphol. 1996, 228, 307–326. [Google Scholar] [CrossRef]
- Hove, J.R.; O’Bryan, L.M.; Gordon, M.S.; Webb, P.W.; Weihs, D. Boxfishes (Teleostei: Ostraciidae) as a model system for fishes swimming with many fins: Kinematics. J. Exp. Biol. 2001, 204, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Standen, E.M.; Lauder, G.V. Hydrodynamic function of dorsal and anal fins in brook trout (Salvelinus fontinalis). J. Exp. Biol. 2007, 210, 325–339. [Google Scholar] [CrossRef]
- Standen, E.M.; Lauder, G.V. Dorsal and anal fin function in bluegill sunfish Lepomis macrochirus: Three-dimensional kinematics during propulsion and maneuvering. J. Exp. Biol. 2005, 208, 2753–2763. [Google Scholar] [CrossRef]
- Drucker, E.G.; Lauder, G.V. Locomotor function of the dorsal fin in teleost fishes: Experimental analysis of wake forces in sunfish. J. Exp. Biol. 2001, 204, 2943–2958. [Google Scholar]
- Thorsen, D.H.; Cassidy, J.J.; Hale, M.E. Swimming of larval zebrafish: Fin–axis coordination and implications for function and neural control. J. Exp. Biol. 2004, 207, 4175–4183. [Google Scholar] [CrossRef]
- Hale, M.E.; Day, R.D.; Thorsen, D.H.; Westneat, M.W. Pectoral fin coordination and gait transitions in steadily swimming juvenile reef fishes. J. Exp. Biol. 2006, 209, 3708–3718. [Google Scholar] [CrossRef]
- Lauder, G.V.; Tytell, E.D. Hydrodynamics of undulatory propulsion. In Fish Biomechanics; Shadwick, R.E., Lauder, G.V., Eds.; Academic Press: San Diego, CA, USA, 2006; Volume 23, pp. 425–468. [Google Scholar]
- Higham, T.E. Feeding, fins and braking maneuvers: Locomotion during prey capture in centrarchid fishes. J. Exp. Biol. 2007, 210, 107–117. [Google Scholar] [CrossRef]
- Chadwell, B.a.; Standen, E.M.; Lauder, G.V.; Ashley-Ross, M.a. Median fin function during the escape response of bluegill sunfish (Lepomis macrochirus). I: Fin-ray orientation and movement. J. Exp. Biol. 2012, 215, 2869–2880. [Google Scholar] [CrossRef]
- Flammang, B.E.; Lauder, G.V. Pectoral fins aid in navigation of a complex environment by bluegill sunfish under sensory deprivation conditions. J. Exp. Biol. 2013, 216, 3084–3089. [Google Scholar] [CrossRef] [Green Version]
- Drucker, E.G. The use of gait transition speed in comparative studies of fish locomotion. Am. Zool. 1996, 36, 555–566. [Google Scholar] [CrossRef]
- Drucker, E.G.; Lauder, G.V. A hydrodynamic analysis of fish swimming speed: Wake structure and locomotor force in slow and fast labriform swimmers. J. Exp. Biol. 2000, 203, 2379–2393. [Google Scholar] [PubMed]
- Korsmeyer, K.E.; Steffensen, J.F.; Herskin, J. Energetics of median and paired fin swimming, body and caudal fin swimming, and gait transition in parrotfish (Scarus schlegeli) and triggerfish (Rhinecanthus aculeatus). J. Exp. Biol. 2002, 205, 1253–1263. [Google Scholar]
- Tytell, E.D. Median fin function in bluegill sunfish Lepomis macrochirus: Streamwise vortex structure during steady swimming. J. Exp. Biol. 2006, 209, 1516–1534. [Google Scholar] [CrossRef] [PubMed]
- Flammang, B.E.; Lauder, G.V.; Troolin, D.R.; Strand, T.E. Volumetric imaging of fish locomotion. Biol. Lett. 2011, 7, 695–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauder, G.V. Fish locomotion: Recent advances and new directions. Annu. Rev. Mar. Sci. 2015, 7, 521–545. [Google Scholar] [CrossRef]
- Lauder, G.V.; Di Santo, V. Swimming mechanics and energetics of elasmobranch fishes. In Fish Physiology; Shadwick, R.E., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: San Diego, CA, USA, 2015; Volume 34, pp. 219–253. [Google Scholar]
- Birch, J.M.; Dickinson, M.H. The influence of wing–wake interactions on the production of aerodynamic forces in flapping flight. J. Exp. Biol. 2003, 206, 2257–2272. [Google Scholar] [CrossRef]
- Lehmann, F.-O.; Sane, S.P.; Dickinson, M. The aerodynamic effects of wing–wing interaction in flapping insect wings. J. Exp. Biol. 2005, 208, 3075–3092. [Google Scholar] [CrossRef]
- Lehmann, F.-O. When wings touch wakes: Understanding locomotor force control by wake–wing interference in insect wings. J. Exp. Biol. 2008, 211, 224–233. [Google Scholar] [CrossRef]
- Usherwood, J.R.; Lehmann, F.-O. Phasing of dragonfly wings can improve aerodynamic efficiency by removing swirl. J. R. Soc. Interface 2008, 5, 1303–1307. [Google Scholar] [CrossRef] [Green Version]
- Broering, T.M.; Lian, Y.-S. The effect of phase angle and wing spacing on tandem flapping wings. Acta Mech. Sin. 2012, 28, 1557–1571. [Google Scholar] [CrossRef]
- Liang, Z.; Dong, H. Computational Study of Wing-Wake Interactions Between Ipsilateral Wings of Dragonfly in Flight; American Institute of Aeronautics and Astronautics Paper; American Institute of Aeronautics and Astronautics: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Flammang, B.E.; Lauder, G.V. Caudal fin shape modulation and control during acceleration, braking and backing maneuvers in bluegill sunfish, Lepomis macrochirus. J. Exp. Biol. 2009, 212, 277–286. [Google Scholar] [CrossRef]
- Low, K.H.; Chong, C.W.; Zhou, C. Performance Study of a Fish Robot Propelled by a Flexible Caudal Fin. In Proceedings of the IEEE International Conference on Robotics and Automation, Anchorage, AK, USA, 3–7 May 2010; pp. 90–95. [Google Scholar]
- Esposito, C.J.; Tangorra, J.L.; Flammang, B.E.; Lauder, G.V. A robotic fish caudal fin: Effects of stiffness and motor program on locomotor performance. J. Exp. Biol. 2012, 215, 56–67. [Google Scholar] [CrossRef]
- Tangorra, J.; Phelan, C.; Esposito, C.; Lauder, G. Use of biorobotic models of highly deformable fins for studying the mechanics and control of fin forces in fishes. Integr. Comp. Biol. 2011, 51, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, I.; Mittal, R.; Lauder, G.V.; Drucker, E. Hydrodynamics of a biologically inspired tandem flapping foil configuration. Theor. Comput. Fluid Dyn. 2007, 21, 155–170. [Google Scholar] [CrossRef]
- Liu, G.; Ren, Y.; Dong, H.; Akanyeti, O.; Liao, J.C.; Lauder, G.V. Computational analysis of vortex dynamics and performance enhancement due to body–fin and fin–fin interactions in fish-like locomotion. J. Fluid Mech. 2017, 829, 65–88. [Google Scholar] [CrossRef]
- Arreola, V.I.; Westneat, M.W. Mechanics of propulsion by multiple fins: Kinematics of aquatic locomotion in the burrfish (Chilomycterus schoepfi). Proc. Biol. Sci. 1996, 263, 1689–1696. [Google Scholar]
- Drucker, E.G.; Lauder, G.V. Locomotor function of the dorsal fin in rainbow trout: Kinematic patterns and hydrodynamic forces. J. Exp. Biol. 2005, 208, 4479–4494. [Google Scholar] [CrossRef]
- Wen, L.; Ren, Z.; Di Santo, V.; Hu, K.; Yuan, T.; Wang, T.; Lauder, G.V. understanding fish linear acceleration using an undulatory biorobotic model with soft fluidic elastomer actuated morphing median fins. Soft Robot. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tangorra, J.L.; Mignano, A.P.; Carryon, G.N.; Kahn, J.C. Biologically Derived Models of the Sunfish for Experimental Investigations of Multi-Fin Swimming. In Proceedings of the 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Francisco, CA, USA, 25–30 September 2011; pp. 580–587. [Google Scholar]
- Geder, J.D.; Ramamurti, R.; Viswanath, K.; Pruessner, M. Underwater thrust performance of Tandem Flapping Fins: Effects of Stroke Phasing and Fin Spacing. In Proceedings of the OCEANS 2017, Anchorage, AK, USA, 18–21 September 2017; pp. 1–7. [Google Scholar]
- Tytell, E.D.; Standen, E.M.; Lauder, G.V. Escaping Flatland: Three-dimensional kinematics and hydrodynamics of median fins in fishes. J. Exp. Biol. 2008, 211, 187–195. [Google Scholar] [CrossRef]
- Flammang, B.E.; Tangorra, J.L.; Mignano, A.P.; Lauder, G.V. Building a Fish: The Biology and engineering behind a bioinspired autonomous. Mar. Technol. Soc. J. 2017, 51, 15–22. [Google Scholar] [CrossRef]
- Tangorra, J.L.; Esposito, C.J.; Lauder, G.V. Biorobotic Fins for Investigations of Fish Locomotion. In Proceedings of the 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems, St. Louis, MO, USA, 10–15 October 2009; pp. 2120–2125. [Google Scholar]
- Tangorra, J.L.; Lauder, G.V.; Hunter, I.W.; Mittal, R.; Madden, P.G.A.; Bozkurttas, M. The effect of fin ray flexural rigidity on the propulsive forces generated by a biorobotic fish pectoral fin. J. Exp. Biol. 2010, 213, 4043–4054. [Google Scholar] [CrossRef] [Green Version]
- Drucker, E.G.; Lauder, G.V. Locomotor forces on a swimming fish: Three-dimensional vortex wake dynamics quantified using digital particle image velocimetry. J. Exp. Biol. 1999, 202, 2393–2412. [Google Scholar]
- Taylor, G.K.; Nudds, R.L.; Thomas, A.L.R. Flying and swimming animals cruise at a Strouhal number tuned for high power efficiency. Nature 2003, 425, 707–711. [Google Scholar] [CrossRef]
- Van Buren, T.; Floryan, D.; Wei, N.; Smits, A.J. Flow speed has little impact on propulsive characteristics of oscillating foils. Phys. Rev. Fluids 2018, 3, 013103. [Google Scholar] [CrossRef] [Green Version]
- For all testing conditions of the validations, similar trends in mean force, in time-varying shape of forces, and in flow were either measured (for the PDAC robot) or observed in swimming behavior (for the free-swimming robot). This indicates that the trends presented in this paper (Section 3) are typical of the fin–wake–fin interaction. A detailed reporting of all validation experiments is beyond the scope of this paper, and will be reported in future publications that focus on particular aspects of the force-shaping phenomenon.
- Oppenheim, A.V.; Schafer, R.W.; Buck, J.R. Discrete-Time Signal Processing; Prentice Hall: Englewood Cliffs, NJ, USA, 1999. [Google Scholar]
- Wilcox, D.C. Turbulence Modeling for CFD, 2nd ed.; DCW Industries: La Canada Flintridge, CA, USA, 1998; Volume 2. [Google Scholar]
- Maia, A.; Lauder, G.V.; Wilga, C.D. Hydrodynamic function of dorsal fins in spiny dogfish and bamboo sharks during steady swimming. J. Exp. Biol. 2017. [Google Scholar] [CrossRef]







| PDAC Fish Body | Overall PDAC Robot | |||||
|---|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Height (mm) | Dorsal Fin Translation (mm) | Length (mm) | Width (mm) | Height (mm) |
| 470 | 100 | 225 | 100 | 470 | 235 | 600 |
| DC-R | DAC-R | PDAC-R | DC-F | |||||
|---|---|---|---|---|---|---|---|---|
| DA Phase | (N) | (N) | (N) | (N) | ||||
| In-phase (0°) | 0.052 | 0.000 | 0.039 | 0.000 | 0.108 | 0.000 | 0.046 | 0.000 |
| Max (150°) | 0.077 | +0.025 | 0.087 | +0.048 | 0.146 | +0.038 | 0.065 | +0.019 |
| Min (−90°) | 0.035 | −0.016 | 0.020 | −0.019 | 0.076 | −0.032 | 0.034 | −0.012 |
| Δ (min − max) | - | 0.041 | - | 0.067 | - | 0.070 | - | 0.031 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mignano, A.P.; Kadapa, S.; Tangorra, J.L.; Lauder, G.V. Passing the Wake: Using Multiple Fins to Shape Forces for Swimming. Biomimetics 2019, 4, 23. https://doi.org/10.3390/biomimetics4010023
Mignano AP, Kadapa S, Tangorra JL, Lauder GV. Passing the Wake: Using Multiple Fins to Shape Forces for Swimming. Biomimetics. 2019; 4(1):23. https://doi.org/10.3390/biomimetics4010023
Chicago/Turabian StyleMignano, Anthony P., Shraman Kadapa, James L. Tangorra, and George V. Lauder. 2019. "Passing the Wake: Using Multiple Fins to Shape Forces for Swimming" Biomimetics 4, no. 1: 23. https://doi.org/10.3390/biomimetics4010023
APA StyleMignano, A. P., Kadapa, S., Tangorra, J. L., & Lauder, G. V. (2019). Passing the Wake: Using Multiple Fins to Shape Forces for Swimming. Biomimetics, 4(1), 23. https://doi.org/10.3390/biomimetics4010023