Bone Apatite Nanocrystal: Crystalline Structure, Chemical Composition, and Architecture
Abstract
:1. Introduction
2. Bone-Related, Ideal Hydroxylapatite: A Mineralogical Account
3. A Brief Historical Overview of Bone Mineral Research
4. Bone Apatite Nanocrystal: A Material-Approach Account
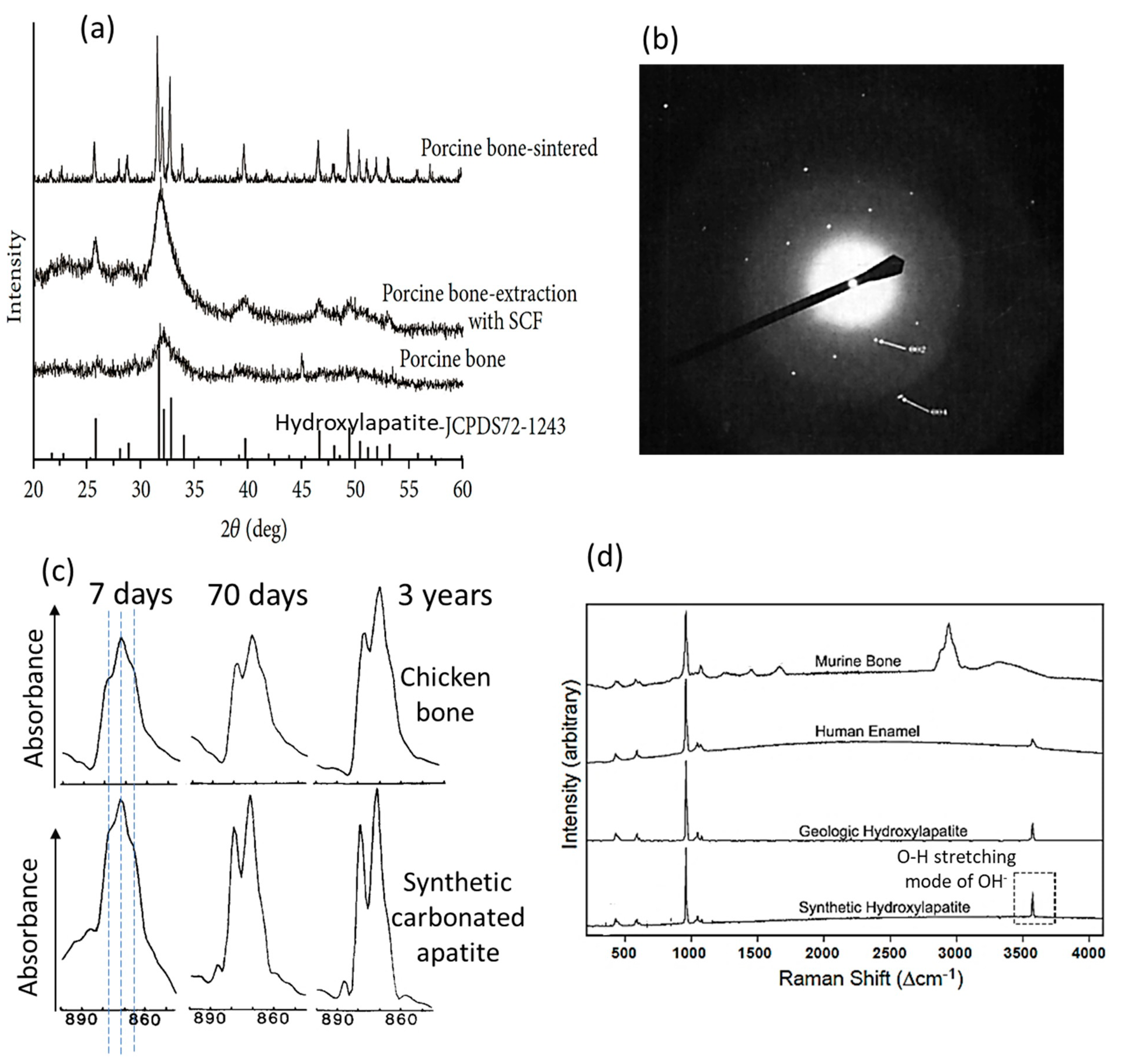
4.1. Crystalline Structure
4.2. Chemical Composition
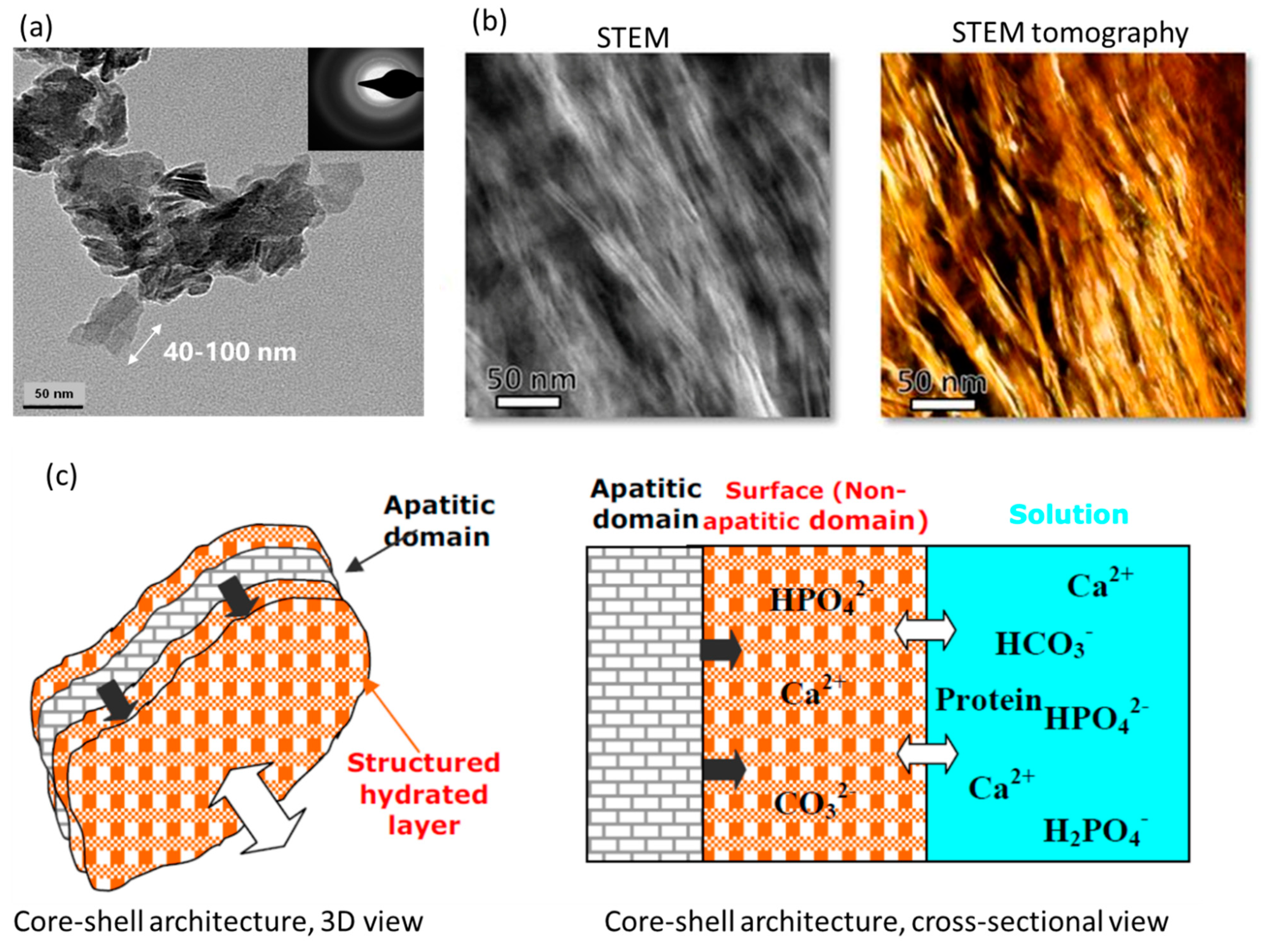
4.3. Geometry: Shape, Size, Architecture
4.4. Mechanical Properties
5. Conclusions and Outlook
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skinner, H. Biominerals. Mineral. Mag. 2005, 69, 621–641. [Google Scholar] [CrossRef]
- Glimcher, M.J. Bone: Nature of the calcium phosphate crystals and cellular, structural, and physical chemical mechanisms in their formation. Rev. Mineral. Geochem. 2006, 64, 223–282. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Wopenka, B.; Valsami-Jones, E. Bone and tooth mineralization: Why apatite? Elements 2008, 4, 97–104. [Google Scholar] [CrossRef]
- Ellies, L.; Carter, J.; Natiella, J.; Featherstone, J.; Nelson, D. Quantitative analysis of early in vivo tissue response to synthetic apatite implants. J. Biomed. Mater. Res. 1988, 22, 137–148. [Google Scholar] [CrossRef]
- Rupani, A.; Hidalgo-Bastida, L.A.; Rutten, F.; Dent, A.; Turner, I.; Cartmell, S. Osteoblast activity on carbonated hydroxyapatite. J. Biomed. Mater. Res. Part. A 2012, 100, 1089–1096. [Google Scholar] [CrossRef]
- Nakamura, M.; Hiratai, R.; Hentunen, T.; Salonen, J.; Yamashita, K. Hydroxyapatite with high carbonate substitutions promotes osteoclast resorption through osteocyte-like cells. ACS Biomater. Sci. Eng. 2016, 2, 259–267. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Tsuchiya, A.; Hayashi, K.; Tsuru, K.; Ohe, G. Physical and histological comparison of hydroxyapatite, carbonate apatite, and β-tricalcium phosphate bone substitutes. Materials 2018, 11, 1993. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Ji, B.; Jäger, I.L.; Arzt, E.; Fratzl, P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc. Natl. Acad. Sci. USA 2003, 100, 5597–5600. [Google Scholar] [CrossRef] [Green Version]
- Reznikov, N.; Bilton, M.; Lari, L.; Stevens, M.M.; Kröger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 2018, 360, eaao2189. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Naleway, S.E.; Wang, B. Biological and bioinspired materials: Structure leading to functional and mechanical performance. Bioact. Mater. 2020, 5, 745–757. [Google Scholar] [CrossRef]
- Bonderer, L.J.; Studart, A.R.; Gauckler, L.J. Bioinspired design and assembly of platelet reinforced polymer films. Science 2008, 319, 1069–1073. [Google Scholar] [CrossRef]
- Liu, Z.; Meyers, M.A.; Zhang, Z.; Ritchie, R.O. Functional gradients and heterogeneities in biological materials: Design principles, functions, and bioinspired applications. Prog. Mater. Sci. 2017, 88, 467–498. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, P.; Zhou, J.; Qi, S.; Yamauchi, Y.; Shi, R.; Fang, R.; Ishida, Y.; Wang, S.; Tomsia, A.P. Layered nanocomposites by shear-flow-induced alignment of nanosheets. Nature 2020, 580, 210–215. [Google Scholar] [CrossRef]
- Von Bibra, E. Chemische Untersuchungen über Die Knochen und Zähne des Menschen und der Wirbeltiere; Kunstverlag: Schweinfurt, Germany, 1844; pp. 63–94. [Google Scholar]
- Aeby, C. Zur Chemie der Knochen. J. Prakt. Chem. 1874, 10, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Wibel, F. Die Constitution des Knochenphosphates, insbesondere die Existenz und Bildung einer basischen Verbindung (Ca3P2O8+ xCaO). J. Prakt. Chem. 1874, 9, 113–132. [Google Scholar] [CrossRef] [Green Version]
- De Jong, W. La substance minerale dans les os. Recl. Trav. Chim. Pays-Bas 1926, 45, 445–448. [Google Scholar] [CrossRef]
- Taylor, N.W.; Sheard, C. Microscopic and X-ray investigations on the calcification of tissue. J. Biol. Chem. 1929, 81, 479–493. [Google Scholar] [CrossRef]
- Hendricks, S.; Hill, W.; Jacob, K.; Jefferson, M. Structural characteristics of apatite-like substances and composition of phosphate rock and bone as determined from microscopical and X-ray diffraction examinations1. Ind. Eng. Chem. 1931, 23, 1413–1418. [Google Scholar] [CrossRef]
- Roseberry, H.H.; Hastings, A.B.; Morse, J. X-ray analysis of bone and teeth. J. Biol. Chem. 1931, 90, 395–407. [Google Scholar] [CrossRef]
- Bale, W. A comparative Röntgen-ray diffraction study of several natural apatites and the apatite-like constituent of bone and tooth substance. Am. J. Roentgenol. 1940, 43, 735–747. [Google Scholar]
- Hendricks, S.; Hill, W. The nature of bone and phosphate rock. Proc. Natl. Acad. Sci. USA 1950, 36, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Biltz, R.M.; Pellegrino, E.D. The nature of bone carbonate. Clin. Orthop. Relat. Res. 1977, 129, 279–292. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Apatites in biological systems. Prog. Cryst. Growth Charact. 1981, 4, 1–45. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphates in enamel, dentin and bone. In Calcium Phosphates in Oral Biology and Medicine; Karger Publishers: Basel, Switzerland, 1991; Volume 15, pp. 108–129. [Google Scholar]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Rey, C.; Hina, A.; Togfighi, A.; Glimcher, M. Maturation of poorly crystalline apatites: Chemical and structural aspects in vivo and in vitro. Cells Mater. 1995, 5, 345–356. [Google Scholar]
- Elliott, J.C. Calcium phosphate biominerals. Rev. Mineral. Geochem. 2002, 48, 427–453. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Ito, A.; Ishikawa, K.; Sakae, T.; LeGeros, J.P. Fundamentals of hydroxyapatite and related calcium phosphates. Adv. Biomater. Fundam. Process. Appl. 2009, 19–52. [Google Scholar]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Pasteris, J.D. Structurally incorporated water in bone apatite: A cautionary tale. In Calcium Phosphates: Structure, Synthesis, Properties, and Applications; Heimann, R.B., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 63–94. [Google Scholar]
- Rey, C.; Combes, C.; Drouet, C.; Cazalbou, S.; Grossin, D.; Brouillet, F.; Sarda, S. Surface properties of biomimetic nanocrystalline apatites; applications in biomaterials. Prog. Cryst. Growth Charact. Mater. 2014, 60, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.M.; Rakovan, J.F. Structurally robust, chemically diverse: Apatite and apatite supergroup minerals. Elements 2015, 11, 165–170. [Google Scholar] [CrossRef]
- Pasteris, J.D. A mineralogical view of apatitic biomaterials. Am. Mineral. 2016, 101, 2594–2610. [Google Scholar] [CrossRef]
- Deymier, A.C.; Nair, A.K.; Depalle, B.; Qin, Z.; Arcot, K.; Drouet, C.; Yoder, C.H.; Buehler, M.J.; Thomopoulos, S.; Genin, G.M.; et al. Protein-free formation of bone-like apatite: New insights into the key role of carbonation. Biomaterials 2017, 127, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. [Google Scholar] [CrossRef]
- McConnell, D. The problem of the carbonate apatites. IV. Structural substitutions involving CO3 and OH. Bull. De Minéralogie 1952, 75, 428–445. [Google Scholar] [CrossRef]
- Legros, R.; Balmain, N.; Bonel, G. Age-related changes in mineral of rat and bovine cortical bone. Calcif. Tissue Int. 1987, 41, 137–144. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Ripamonti, A.; Roveri, N.; Romanello, M.; Suarez, K.N.; Moro, L. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J. Inorg. Biochem. 1997, 68, 45–51. [Google Scholar] [CrossRef]
- Mkukuma, L.; Skakle, J.M.S.; Gibson, I.R.; Imrie, C.T.; Aspden, R.M.; Hukins, D.W.L. Effect of the proportion of organic material in bone on thermal decomposition of bone mineral: An investigation of a variety of bones from different species using thermogravimetric analysis coupled to mass spectrometry, high-temperature X-ray diffraction, and Fourier transform infrared spectroscopy. Calcif. Tissue Int. 2004, 75, 321–328. [Google Scholar]
- Li, Z.; Pasteris, J.D. Tracing the pathway of compositional changes in bone mineral with age: Preliminary study of bioapatite aging in hypermineralized dolphin’s bulla. Biochim. Biophys. Acta BBA-Gen. Subj. 2014, 1840, 2331–2339. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Zhang, X.; Ai, J.; Ji, T.; Nagai, M.; Duan, Y.; Che, S.; Han, L. Chiral hierarchical structure of bone minerals. Nano Res. 2022, 15, 1295–1302. [Google Scholar] [CrossRef]
- Lundberg, Y.W.; Xu, Y.; Thiessen, K.D.; Kramer, K.L. Mechanisms of otoconia and otolith development. Dev. Dyn. 2015, 244, 239–253. [Google Scholar] [CrossRef] [Green Version]
- Landis, W.J.; Glimcher, M.J. Electron diffraction and electron probe microanalysis of the mineral phase of bone tissue prepared by anhydrous techniques. J. Ultrastruct. Res. 1978, 63, 188–223. [Google Scholar] [CrossRef]
- Burke, E.A. Tidying up mineral names: An IMA-CNMNC scheme for suffixes, hyphens and diacritical marks. Mineral. Rec. 2008, 39, 131. [Google Scholar]
- Pasero, M.; Kampf, A.R.; Ferraris, C.; Pekov, I.V.; Rakovan, J.; White, T.J. Nomenclature of the apatite supergroup minerals. Eur. J. Mineral. 2010, 22, 163–179. [Google Scholar] [CrossRef]
- Fleet, M.E. Carbonated Hydroxyapatite: Materials, Synthesis, and Applications; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Werner, A.G. Kurze Klassifikation und Beschreibung der verschiedenen Gebirgsarten (Dresden: Waltherischen Hofbuchhandlung, 1787). First Publ. Artic. 1786, 5, 1. [Google Scholar]
- Pan, Y.; Fleet, M.E. Compositions of the apatite-group minerals: Substitution mechanisms and controlling factors. Rev. Mineral. Geochem. 2002, 48, 13–49. [Google Scholar] [CrossRef]
- Pasteris, J.D. Thermodynamic approach provides insights into the aging process of biological apatite. Am. Mineral. 2014, 99, 562–563. [Google Scholar] [CrossRef]
- Elliott, J.C.; Mackie, P.; Young, R. Monoclinic hydroxyapatite. Science 1973, 180, 1055–1057. [Google Scholar] [CrossRef]
- Náray-Szabo, S. The structure of apatite (CaF) Ca4 (PO4) 3. Z. Krist. 1930, 75, 387–398. [Google Scholar]
- Posner, A.S.; Perloff, A.; Diorio, A.F. Refinement of the hydroxyapatite structure. Acta Crystallogr. 1958, 11, 308–309. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.; Posner, A. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef]
- Beevers, C.A.; McIntyre, D.B. The atomic structure of fluor-apatite and its relation to that of tooth and bone material (with plates XVI-XVIII). Mineral. Mag. J. Mineral. Soc. 1946, 27, 254–257. [Google Scholar] [CrossRef]
- Elliott, J. The problems of the composition and structure of the mineral components of the hard tissues. Clin. Orthop. Relat. Res. 1973, 93, 313–345. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [Green Version]
- Labarthe, J.; Bonel, G.; Montel, G. Structure and properties of B-type phosphocalcium carbonated apatites. In Proceedings of the Annales De Chimie France, Paris, France, 14 September 1973; pp. 289–301. [Google Scholar]
- Jeanjean, J.; McGrellis, S.; Rouchaud, J.; Fedoroff, M.; Rondeau, A.; Perocheau, S.; Dubis, A. A crystallographic study of the sorption of cadmium on calcium hydroxyapatites: Incidence of cationic vacancies. J. Solid State Chem. 1996, 126, 195–201. [Google Scholar] [CrossRef]
- Wilson, R.; Elliott, J.; Dowker, S. Rietveld refinement of the crystallographic structure of human dental enamel apatites. Am. Mineral. 1999, 84, 1406–1414. [Google Scholar] [CrossRef]
- Matsunaga, K. Theoretical defect energetics in calcium phosphate bioceramics. J. Am. Ceram. Soc. 2010, 93, 1–14. [Google Scholar] [CrossRef]
- Gabriel, S. Chemische Untersuchungen über die Mineralstoffe der Knochen und Zähne. Biol. Chem. 1894, 18, 257–303. [Google Scholar] [CrossRef]
- Eisenberger, S.; Lehrman, A.; Turner, W.D. The Basic Calcium Phosphates and Related Systems. Some Theoretical and Practical Aspects. Chem. Rev. 1940, 26, 257–296. [Google Scholar] [CrossRef]
- Heimann, R.B.; Lehmann, H.D. Bioceramic Coatings for Medical Implants: Trends and Techniques; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Dorozhkin, S.V. A detailed history of calcium orthophosphates from 1770s till 1950. Mater. Sci. Eng. C 2013, 33, 3085–3110. [Google Scholar] [CrossRef]
- Roscoe, H.; Schorlemmer, C. A Treatise on Chemistry; The Non-metallic Elements, Macmillan and Co.: London, UK, 1881; Volume I, p. 751. [Google Scholar]
- Aeby, C. Ueber die nähern Bestandtheile des Knochenphosphates. J. Prakt. Chem. 1873, 6, 169–171. [Google Scholar] [CrossRef] [Green Version]
- Brande, W.T.; Taylor, A.S. Chemistry; Blanchard and Lea: Philadelphia, PA, USA, 1863. [Google Scholar]
- Warington, R. XXVII.—Researches on the phosphates of calcium, and upon the solubility of tricalcic phosphate. J. Chem. Soc. 1866, 19, 296–318. [Google Scholar] [CrossRef] [Green Version]
- Werner, A.G. Geschichte, Karakteristik, und kurze chymische Untersuchung des Apatits. Bergmännisches J. 1788, 1, 76–96. [Google Scholar]
- Röntgen, W.C. Über eine neue Art von Strahlen (Vorläufige Mittheilung). Sitzungsber. Würzb. Phys. Med. Ges. 1895, 137, 132–141. [Google Scholar]
- Bragg, W. Application of the Ionisation Spectrometer to the Determination of the Structure of Minute Crystals. Proc. Phys. Soc. Lond. 1920, 33, 222. [Google Scholar] [CrossRef]
- Klement, R.; Trömel, G. Hydroxylapatit, der Hauptbestandteil der anorganischen Knochen- und Zahnsubstanz. Ztschr. Physiol. Chem. 1932, 213, 263–269. [Google Scholar] [CrossRef]
- Henschen, C.; Straumann, R.; Bucher, R. Ergebnisse röntgenspektrographischer Untersuchungen am Knochen: I. Mitteilung. Krystallitbau des anorganischen und des organischen Knochens. Dtsch. Z. Chir. 1932, 236, 485–514. [Google Scholar] [CrossRef]
- Bredig, M. The apatite structure of inorganic bone and tooth substance. H.-SZ Physiol. Chem. 1933, 216, 239–243. [Google Scholar] [CrossRef]
- Mehmel, M. Beziehungen zwischen Kristallstruktur und chemischer Formel des Apatits. Z. Phys. Chem. 1932, 15, 223–241. [Google Scholar] [CrossRef]
- Trömel, G.; Möller, H. Die Bildung schwer löslicher Calciumphosphate aus wäßriger Lösung und die Beziehungen dieser Phosphate zur Apatitgruppe. Z. Anorg. Allg. Chem. 1932, 206, 227–240. [Google Scholar] [CrossRef]
- Brasseur, H.; Dallemagne, M.; Melon, J. Chemical nature of salts from bones and teeth and of tricalcium phosphate precipitates. Nature 1946, 157, 453. [Google Scholar] [CrossRef]
- Hendricks, S.B.; Hill, W.L. The inorganic constitution of Bone. Science 1942, 96, 255–257. [Google Scholar] [CrossRef]
- Brown, W.E. Crystal growth of bone mineral. Clin. Orthop. Relat. Res. 1966, 44, 205–220. [Google Scholar] [CrossRef]
- Robinson, R.; Watson, M. Collagen-crystal relationships in bone as seen in the electron microscope. Anat. Rec. 1952, 114, 383–409. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Trautz, O.R.; Legeros, J.P.; Klein, E.; Shirra, W.P. Apatite crystallites: Effects of carbonate on morphology. Science 1967, 155, 1409–1411. [Google Scholar] [CrossRef]
- Glimcher, M.J. The nature of the mineral phase in bone: Biological and clinical implications. In Metabolic Bone Disease; Avioli, L.V., Krane, S.M., Eds.; Academic Press: New York, NY, USA; pp. 23–50.
- Pasteris, J.D.; Yoder, C.H.; Wopenka, B. Molecular water in nominally unhydrated carbonated hydroxylapatite: The key to a better understanding of bone mineral. Am. Mineral. 2014, 99, 16–27. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite Biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K. Bone substitute fabrication based on dissolution-precipitation reactions. Materials 2010, 3, 1138–1155. [Google Scholar] [CrossRef] [Green Version]
- Aoki, H.; Kato, K. Application of apatite to biomaterials. Ceramics 1975, 10, 469–479. [Google Scholar]
- Jarcho, M.; Bolen, C.; Thomas, M.; Bobick, J.; Kay, J.; Doremus, R.H. Hydroxylapatite synthesis and characterization in dense polycrystalline form. J. Mater. Sci. 1976, 11, 2027–2035. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Effect of carbonate on the lattice parameters of apatite. Nature 1965, 206, 403–404. [Google Scholar] [CrossRef]
- Baig, A.; Fox, J.; Young, R.; Wang, Z.; Hsu, J.; Higuchi, W.; Chhettry, A.; Zhuang, H.; Otsuka, M. Relationships among carbonated apatite solubility, crystallite size, and microstrain parameters. Calcif. Tissue Int. 1999, 64, 437–449. [Google Scholar] [CrossRef]
- Katz, J.; Ukraincik, K. On the anisotropic elastic properties of hydroxyapatite. J. Biomech. 1971, 4, 221–227. [Google Scholar] [CrossRef]
- Grenoble, D.E.; Katz, J.L.; Dunn, K.L.; Gilmore, R.S.; Murty, K.L. The elastic properties of hard tissues and apatites. J. Biomed. Mater. Res. 1972, 6, 221–233. [Google Scholar] [CrossRef]
- Deymier, A.C.; Almer, J.D.; Stock, S.R.; Haeffner, D.R.; Dunand, D.C. High Energy X-ray Diffraction Measurement of Load Transfer Between Hydroxyapatite and Collagen in Bovine Dentin. MRS Online Proc. Libr. OPL 2009, 1, 1187. [Google Scholar] [CrossRef]
- Ren, F.; Lu, X.; Leng, Y. Ab initio simulation on the crystal structure and elastic properties of carbonated apatite. J. Mech. Behav. Biomed. Mater. 2013, 26, 59–67. [Google Scholar] [CrossRef]
- Wingender, B.; Azuma, M.; Krywka, C.; Zaslansky, P.; Boyle, J.; Deymier, A. Carbonate substitution significantly affects the structure and mechanics of carbonated apatites. Acta Biomater. 2021, 122, 377–386. [Google Scholar] [CrossRef]
- Smith, C.; Smith, D. An X-ray diffraction investigation of age-related changes in the crystal structure of bone apatite. Calcif. Tissue Res. 1976, 22, 219–226. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into biological apatite: Physiochemical properties and preparation approaches. BioMed Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef] [Green Version]
- Boskey, A.L. Natural and synthetic hydroxyapatites. In Biomaterials Science an Introduction to Materials in Medicine, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 151–161. [Google Scholar]
- Kim, H.M.; Rey, C.; Glimcher, M.J. Isolation of calcium-phosphate crystals of bone by non-aqueous methods at low temperature. J. Bone Miner. Res. 1995, 10, 1589–1601. [Google Scholar] [CrossRef]
- Bang, S.; Baud, C.A.; Boivin, G.; Demeurisse, C.; Gossi, M.; Tochon-Danguy, H.J.; Very, J.M. Morphometric and biophysical study of bone tissue in industrial fluorosis. In Fluoride and Bone; Second Symposium, CEMO; Courvoisier, B., Donatth, A., Baud, C., Eds.; Hans Huber Publishers: Bern, Switzerland; Stuttgart, Germany, 1978; pp. 168–175. [Google Scholar]
- Baud, C.A.; Very, J.M. Ionic substitutions in vivo in bone and tooth apatite crystals. Colloq. Int. CNRS 1975, 405. [Google Scholar]
- Termine, J.D.; Posner, A.S. Infrared analysis of rat bone: Age dependency of amorphous and crystalline mineral fractions. Science 1966, 153, 1523–1525. [Google Scholar] [CrossRef]
- Bonar, L.; Roufosse, A.; Sabine, W.; Grynpas, M.; Glimcher, M. X-ray diffraction studies of the crystallinity of bone mineral in newly synthesized and density fractionated bone. Calcif. Tissue Int. 1983, 35, 202–209. [Google Scholar] [CrossRef]
- Brés, E.; Steuer, P.; Voegel, J.C.; Frank, R.; Cuisinier, F. Observation of the loss of the hydroxyapatite sixfold symmetry in a human fetal tooth enamel crystal. J. Microsc. 1993, 170, 147–154. [Google Scholar] [CrossRef]
- Rey, C.; Renugopalakrishman, V.; Collins, B.; Glimcher, M.J. Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif. Tissue Int. 1991, 49, 251–258. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X.; King, P.L. Accommodation of the carbonate ion in apatite: An FTIR and X-ray structure study of crystals synthesized at 2–4 GPa. Am. Mineral. 2004, 89, 1422–1432. [Google Scholar] [CrossRef]
- Yoder, C.H.; Bollmeyer, M.M.; Stepien, K.R.; Dudrick, R.N. The effect of incorporated carbonate and sodium on the IR spectra of A-and AB-type carbonated apatites. Am. Mineral. J. Earth Planet. Mater. 2019, 104, 869–877. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V. Structure, chemistry and synthesis of carbonate apatites—The main components of dental and bone tissues. In Minerals as Advanced Materials I; Springer: Berlin/Heidelberg, Germany, 2008; pp. 241–252. [Google Scholar]
- Ishikawa, K. Carbonate apatite bone replacement: Learn from the bone. J. Ceram. Soc. Jpn. 2019, 127, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef]
- Rey, C.; Shimizu, M.; Collins, B.; Glimcher, M.J. Resolution-enhanced fourier transform infrared spectroscopy study of the environment of phosphate ions in the early deposits of a solid phase of calcium-phosphate in bone and enamel, and their evolution with age. I: Investigations in the v 4 PO 4 domain. Calcif. Tissue Int. 1990, 46, 384–394. [Google Scholar] [CrossRef]
- Yoder, C.; Landes, N.; Tran, L.; Smith, A.; Pasteris, J. The relative stabilities of A-and B-type carbonate substitution in apatites synthesized in aqueous solution. Mineral. Mag. 2016, 80, 977–983. [Google Scholar] [CrossRef]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.J. The carbonate environment in bone mineral: A resolution-enhanced Fourier transform in-frared spectroscopy study. Calcif. Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef]
- Barralet, J.; Best, S.; Bonfield, W. Carbonate substitution in precipitated hydroxyapatite: An investigation into the effects of reaction temperature and bicarbonate ion concentration. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 41, 79–86. [Google Scholar] [CrossRef]
- Marković, S.; Veselinović, L.; Lukić, M.J.; Karanović, L.; Bračko, I.; Ignjatović, N.; Uskoković, D. Synthetical bone-like and biological hydroxyapatites: A comparative study of crystal structure and morphology. Biomed. Mater. 2011, 6, 045005. [Google Scholar] [CrossRef]
- Wang, Y.; Von Euw, S.; Laurent, G.; Crevant, C.; Bonhomme-Coury, L.; Giraud-Guille, M.-M.; Babonneau, F.; Nassif, N.; Azaïs, T. Impact of collagen confinement vs. ionic substitutions on the local disorder in bone and biomimetic apatites. Mater. Horiz. 2014, 1, 224–231. [Google Scholar] [CrossRef] [Green Version]
- LeGeros, R.; Trautz, O.; Klein, E.; LeGeros, J. Two types of carbonate substitution in the apatite structure. Experientia 1969, 25, 5–7. [Google Scholar] [CrossRef]
- LeGeros, R.; Trautz, O.; LeGeros, J.; Klein, E. Carbonate substitution in apatite structure. In Proceedings of the Bulletin de la Societe Chimique de France, Toulouse, Paris, France, 14 September 1968; pp. 1712–1717. [Google Scholar]
- Rogers, K.; Daniels, P. An X-ray diffraction study of the effects of heat treatment on bone mineral microstructure. Biomaterials 2002, 23, 2577–2585. [Google Scholar] [CrossRef]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Okazaki, M.; Moriwaki, Y.; Aoba, T.; Doi, Y.; Takahashi, J. Solubility behavior of CO3 apatites in relation to crystallinity. Caries Res. 1981, 15, 477–483. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Honeycomb blocks composed of carbonate apatite, β-tricalcium phosphate, and hydroxyapatite for bone regeneration: Effects of composition on biological responses. Mater. Today Bio 2019, 4, 100031. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hayashi, K. Carbonate apatite artificial bone. Sci. Technol. Adv. Mater. 2021, 22, 683–694. [Google Scholar] [CrossRef]
- Gardner, T.; Elliott, J.; Sklar, Z.; Briggs, G. Acoustic microscope study of the elastic properties of fluorapatite and hydroxyapatite, tooth enamel and bone. J. Biomech. 1992, 25, 1265–1277. [Google Scholar] [CrossRef]
- Forien, J.-B.; Fleck, C.; Krywka, C.; Zolotoyabko, E.; Zaslansky, P. In situ compressibility of carbonated hydroxyapatite in tooth dentine measured under hydrostatic pressure by high energy X-ray diffraction. J. Mech. Behav. Biomed. Mater. 2015, 50, 171–179. [Google Scholar] [CrossRef]
- de Leeuw, N.H.; Bowe, J.R.; Rabone, J.A. A computational investigation of stoichiometric and calcium-deficient oxy-and hydroxy-apatites. Faraday Discuss. 2007, 134, 195–214. [Google Scholar] [CrossRef]
- Snyders, R.; Music, D.; Sigumonrong, D.; Schelnberger, B.; Jensen, J.; Schneider, J. Experimental and ab initio study of the mechanical properties of hydroxyapatite. Appl. Phys. Lett. 2007, 90, 193902. [Google Scholar] [CrossRef]
- Bhat, S.S.; Waghmare, U.V.; Ramamurty, U. First-principles study of structure, vibrational, and elastic properties of stoichiometric and calcium-deficient hydroxyapatite. Cryst. Growth Des. 2014, 14, 3131–3141. [Google Scholar] [CrossRef]
- Rey, C.; Dickson, R.; Shapiro, F.; Shimizu, M.; Glimcher, M. Spectroscopic evidence for a labile environment of carbonate and phosphate ions in mineral deposits of bone and enamel. Proceed Biomat 1987, 87, 31–46. [Google Scholar]
- Wu, Y.; Glimcher, M.J.; Rey, C.; Ackerman, J.L. A unique protonated phosphate group in bone mineral not present in synthetic calcium phosphates: Identification by phosphorus-31 solid state NMR spectroscopy. J. Mol. Biol. 1994, 244, 423–435. [Google Scholar] [CrossRef]
- Wilson, R.M.; Elliott, J.C.; Dowker, S.E.; Rodriguez-Lorenzo, L.M. Rietveld refinements and spectroscopic studies of the structure of Ca-deficient apatite. Biomaterials 2005, 26, 1317–1327. [Google Scholar] [CrossRef]
- Von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azaïs, T. Bone mineral: New insights into its chemical composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef] [Green Version]
- Biltz, R.M.; Pellegrino, E.D. The hydroxyl content of calcified tissue mineral: A letter to the editors and readers of calcified tissue research. Calcif. Tissue Res. 1971, 7, 259–263. [Google Scholar] [CrossRef]
- Termine, J.; Lundy, D. Hydroxide and carbonate in rat bone mineral and its synthetic analogues. Calcif. Tissue Res. 1973, 13, 73–82. [Google Scholar] [CrossRef]
- Cho, G.; Wu, Y.; Ackerman, J.L. Detection of hydroxyl ions in bone mineral by solid-state NMR spectroscopy. Science 2003, 300, 1123–1127. [Google Scholar] [CrossRef]
- Kolmas, J.; Kolodziejski, W. Concentration of hydroxyl groups in dental apatites: A solid-state 1 H MAS NMR study using inverse 31 P→ 1 H cross-polarization. Chem. Commun. 2007, 42, 4390–4392. [Google Scholar] [CrossRef]
- Rey, C.; Miquel, J.; Facchini, L.; Legrand, A.; Glimcher, M. Hydroxyl groups in bone mineral. Bone 1995, 16, 583–586. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Wopenka, B.; Freeman, J.J.; Rogers, K.; Valsami-Jones, E.; Van der Houwen, J.A.; Silva, M.J. Lack of OH in nanocrystalline apatite as a function of degree of atomic order: Implications for bone and biomaterials. Biomaterials 2004, 25, 229–238. [Google Scholar] [CrossRef]
- Loong, C.-K.; Rey, C.; Kuhn, L.; Combes, C.; Wu, Y.; Chen, S.-H.; Glimcher, M. Evidence of hydroxyl-ion deficiency in bone apatites: An inelastic neutron-scattering study. Bone 2000, 26, 599–602. [Google Scholar] [CrossRef]
- Bonar, L.C.; Shimizu, M.; Roberts, J.E.; Griffin, R.G.; Glimcher, M.J. Structural and composition studies on the mineral of newly formed dental enamel: A chemical, x-ray diffraction, and 31P and proton nuclear magnetic resonance study. J. Bone Mineral. Res. 1991, 6, 1167–1176. [Google Scholar] [CrossRef]
- Ivanova, T.; Frank-Kamenetskaya, O.; Kol’tsov, A.; Ugolkov, V. Crystal structure of calcium-deficient carbonated hydroxyapatite. Thermal decomposition. J. Solid State Chem. 2001, 160, 340–349. [Google Scholar] [CrossRef]
- Yoder, C.H.; Pasteris, J.D.; Worcester, K.N.; Schermerhorn, D.V. Structural water in carbonated hydroxylapatite and fluorapatite: Confirmation by solid state 2 H NMR. Calcif. Tissue Int. 2012, 90, 60–67. [Google Scholar] [CrossRef]
- Watts, H. A Dictionary of Chemistry and the Allied Branches of Other Sciences; Longmans, Green, and Co.: London, UK, 1875. [Google Scholar]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Lebugle, A.; Sfihi, H.; Barroug, A. Nanocrystalline apatites in biological systems: Characterisation, structure and properties. Mater. Werkst. Entwickl. Fert. Prüfung Eig. Anwend. Tech. Werkst. 2007, 38, 996–1002. [Google Scholar] [CrossRef]
- Brown, W.; Eidelman, N.; Tomazic, B. Octacalcium phosphate as a precursor in biomineral formation. Adv. Dent. Res. 1987, 1, 306–313. [Google Scholar] [CrossRef]
- Chow, L.C.; Eanes, E. Solubility of calcium phosphates. Monogr. Oral Sci. 2001, 18, 94–111. [Google Scholar]
- Roufosse, A.; Landis, W.; Sabine, W.; Glimcher, M. Identification of brushite in newly deposited bone mineral from embryonic chicks. J. Ultrastruct. Res. 1979, 68, 235–255. [Google Scholar] [CrossRef]
- Grynpas, M.D.; Omelon, S. Transient precursor strategy or very small biological apatite crystals? Bone 2007, 41, 162–164. [Google Scholar] [CrossRef]
- Suzuki, O.; Hamai, R.; Sakai, S. The material design of octacalcium phosphate bone substitute: Increased dissolution and osteogenecity. Acta Biomater. 2022; in press. [Google Scholar] [CrossRef]
- Crane, N.J.; Popescu, V.; Morris, M.D.; Steenhuis, P.; Ignelzi Jr, M.A. Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone 2006, 39, 434–442. [Google Scholar] [CrossRef]
- Bonar, L.C.; Grynpas, M.D.; Glimcher, M.J. Failure to detect crystalline brushite in embryonic chick and bovine bone by X-ray diffraction. J. Ultrastruct. Res. 1984, 86, 93–99. [Google Scholar] [CrossRef]
- Robinson, R.; Bishop, F. Methods of preparing bone and tooth samples for viewing in the electron microscope. Science 1950, 111, 655–657. [Google Scholar] [CrossRef]
- Robinson, R.A. An electron-microscopic study of the crystalline inorganic component of bone and its relationship to the organic matrix. J. Bone Jt. Surg. 1952, 34, 389–476. [Google Scholar] [CrossRef]
- Glimcher, M.J. Molecular biology of mineralized tissues with particular reference to bone. Rev. Mod. Phys. 1959, 31, 359. [Google Scholar] [CrossRef]
- Glimcher, M.J. A basic architectural principle in the organization of mineralized tissues. Clin. Orthop. Relat. Res. 1968, 61, 16–36. [Google Scholar] [CrossRef]
- Weiner, S.; Traub, W. Crystal size and organization in bone. Connect. Tissue Res. 1989, 21, 259–265. [Google Scholar] [CrossRef]
- Eppell, S.J.; Tong, W.; Katz, J.L.; Kuhn, L.; Glimcher, M.J. Shape and size of isolated bone mineralites measured using atomic force microscopy. J. Orthop. Res. 2001, 19, 1027–1034. [Google Scholar] [CrossRef]
- Finean, J.; Engström, A. The low-angle scatter of X-rays from bone tissue. Biochim. Biophys. Acta 1953, 11, 178–189. [Google Scholar] [CrossRef]
- Carlström, D.; Finean, J. X-ray diffraction studies on the ultrastructure of bone. Biochim. Biophys. Acta 1954, 13, 183–191. [Google Scholar] [CrossRef]
- Fernandez-Moran, H.; Engström, A. Electron microscopy and X-ray diffraction of bone. Biochim. Biophys. Acta 1957, 23, 260–264. [Google Scholar] [CrossRef]
- Johansen, E.; Parks, H.F. Electron Microscopic Observations on the Three-Dimensional Morphology of Apatite Crystallites of Human Dentine and Bone. J. Cell Biol. 1960, 7, 743–746. [Google Scholar] [CrossRef]
- Landis, W.J.; Paine, M.C.; Glimcher, M.J. Electron microscopic observations of bone tissue prepared anhydrously in organic solvents. J. Ultrastruct. Res. 1977, 59, 1–30. [Google Scholar] [CrossRef]
- Landis, W.; Song, M.; Leith, A.; McEwen, L.; McEwen, B. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J. Struct. Biol. 1993, 110, 39–54. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Stokes, A.; McKittrick, J. Comparison of the structure and mechanical properties of bovine femur bone and antler of the North American elk (Cervus elaphus canadensis). Acta Biomater. 2009, 5, 693–706. [Google Scholar] [CrossRef]
- Engström, A.; Finean, J. Low-angle X-ray diffraction of bone. Nature 1953, 171, 564. [Google Scholar] [CrossRef]
- Moradian-Oldak, J.; Weiner, S.; Addadi, L.; Landis, W.; Traub, W. Electron imaging and diffraction study of individual crystals of bone, mineralized tendon and synthetic carbonate apatite. Connect. Tissue Res. 1991, 25, 219–228. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Navarrete, D.A. Biological Apatites in Bone and Teeth. In Nanoceramics in Clinical Use: From Materials to Applications, 2nd ed.; Vallet-Regi, M., Arcos Navarrete, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–29. [Google Scholar]
- Gross, K.A.; Berndt, C.C. Biomedical application of apatites. Rev. Mineral. Geochem. 2002, 48, 631–672. [Google Scholar] [CrossRef]
- Blumenthal, N.; Betts, F.; Posner, A. Effect of carbonate and biological macromolecules on formation and properties of hydroxyapatite. Calcif. Tissue Res. 1975, 18, 81–90. [Google Scholar] [CrossRef]
- Chen, L.; Jacquet, R.; Lowder, E.; Landis, W.J. Refinement of collagen–mineral interaction: A possible role for osteocalcin in apatite crystal nucleation, growth and development. Bone 2015, 71, 7–16. [Google Scholar] [CrossRef]
- Sharma, V.; Srinivasan, A.; Nikolajeff, F.; Kumar, S. Biomineralization process in hard tissues: The interaction complexity within protein and inorganic counterparts. Acta Biomater. 2021, 120, 20–37. [Google Scholar] [CrossRef]
- Hankermeyer, C.R.; Ohashi, K.L.; Delaney, D.C.; Ross, J.; Constantz, B.R. Dissolution rates of carbonated hydroxyapatite in hydrochloric acid. Biomaterials 2002, 23, 743–750. [Google Scholar] [CrossRef]
- Gao, H. Application of fracture mechanics concepts to hierarchical biomechanics of bone and bone-like materials. Int. J. Fract. 2006, 138, 101–137. [Google Scholar] [CrossRef]
- Ji, B.; Gao, H. Mechanical properties of nanostructure of biological materials. J. Mech. Phys. Solids 2004, 52, 1963–1990. [Google Scholar] [CrossRef]
- Ji, B.; Gao, H. Elastic properties of nanocomposite structure of bone. Compos. Sci. Technol. 2006, 66, 1212–1218. [Google Scholar] [CrossRef]
- Gilmore, R.; Katz, J. Elastic properties of apatites. J. Mater. Sci. 1982, 17, 1131–1141. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. Thermodynamic, elastic, and vibrational (IR/Raman) behavior of mixed type-AB carbonated hydroxylapatite by density functional theory. Am. Mineral. J. Earth Planet. Mater. 2021, 106, 1928–1939. [Google Scholar] [CrossRef]
- McConnell, D.; Gruner, J.W. Clinobarrandite and the isodimorphous series, variscite-metavariscite. Am. Mineral. 1940, 25, 157. [Google Scholar]
- McConnell, D. The problem of the carbonate apatites; a carbonate oxy-apatite (dahllite). Am. J. Sci. 1938, 238, 296. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhang, Z.; Pan, H. Bone Apatite Nanocrystal: Crystalline Structure, Chemical Composition, and Architecture. Biomimetics 2023, 8, 90. https://doi.org/10.3390/biomimetics8010090
Wang B, Zhang Z, Pan H. Bone Apatite Nanocrystal: Crystalline Structure, Chemical Composition, and Architecture. Biomimetics. 2023; 8(1):90. https://doi.org/10.3390/biomimetics8010090
Chicago/Turabian StyleWang, Bin, Zuoqi Zhang, and Haobo Pan. 2023. "Bone Apatite Nanocrystal: Crystalline Structure, Chemical Composition, and Architecture" Biomimetics 8, no. 1: 90. https://doi.org/10.3390/biomimetics8010090




