Use of Chitosan from Southern King Crab to Develop Films Functionalized with RGD Peptides for Potential Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Materials
2.3. Isolation and Characterization of CS
2.4. Synthesis of SKC-CS Films
2.5. Thermal Properties of SKC-CS Films
2.6. Measurement of Swelling Percentage
2.7. Synthesis and Characterization of RGD Peptides
2.8. Functionalization of SKC-CS Films with RGD Peptides
2.9. Cell Culture Studies
2.10. Statistical Analysis
3. Results
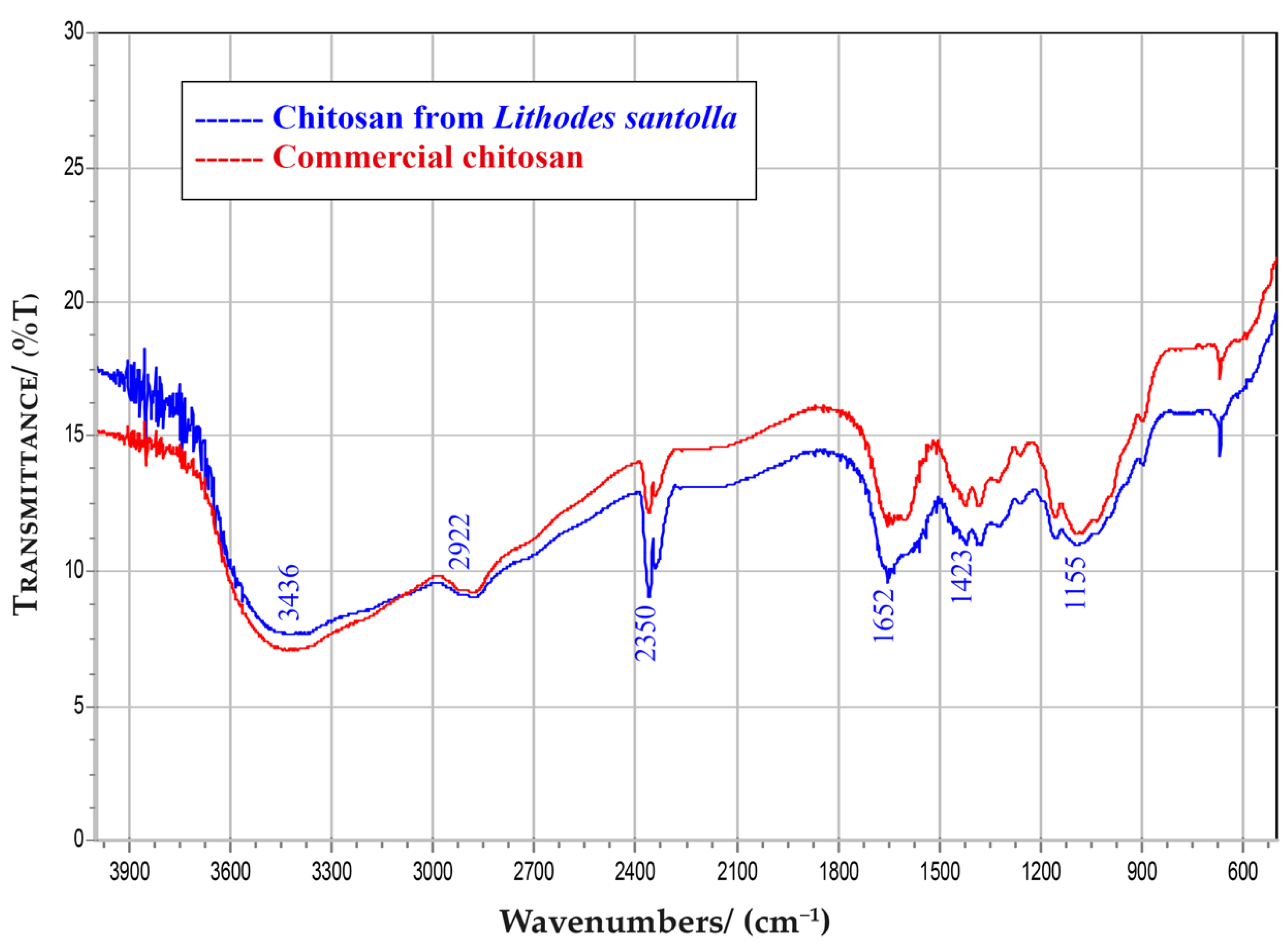
3.1. Isolation and CS Characterization
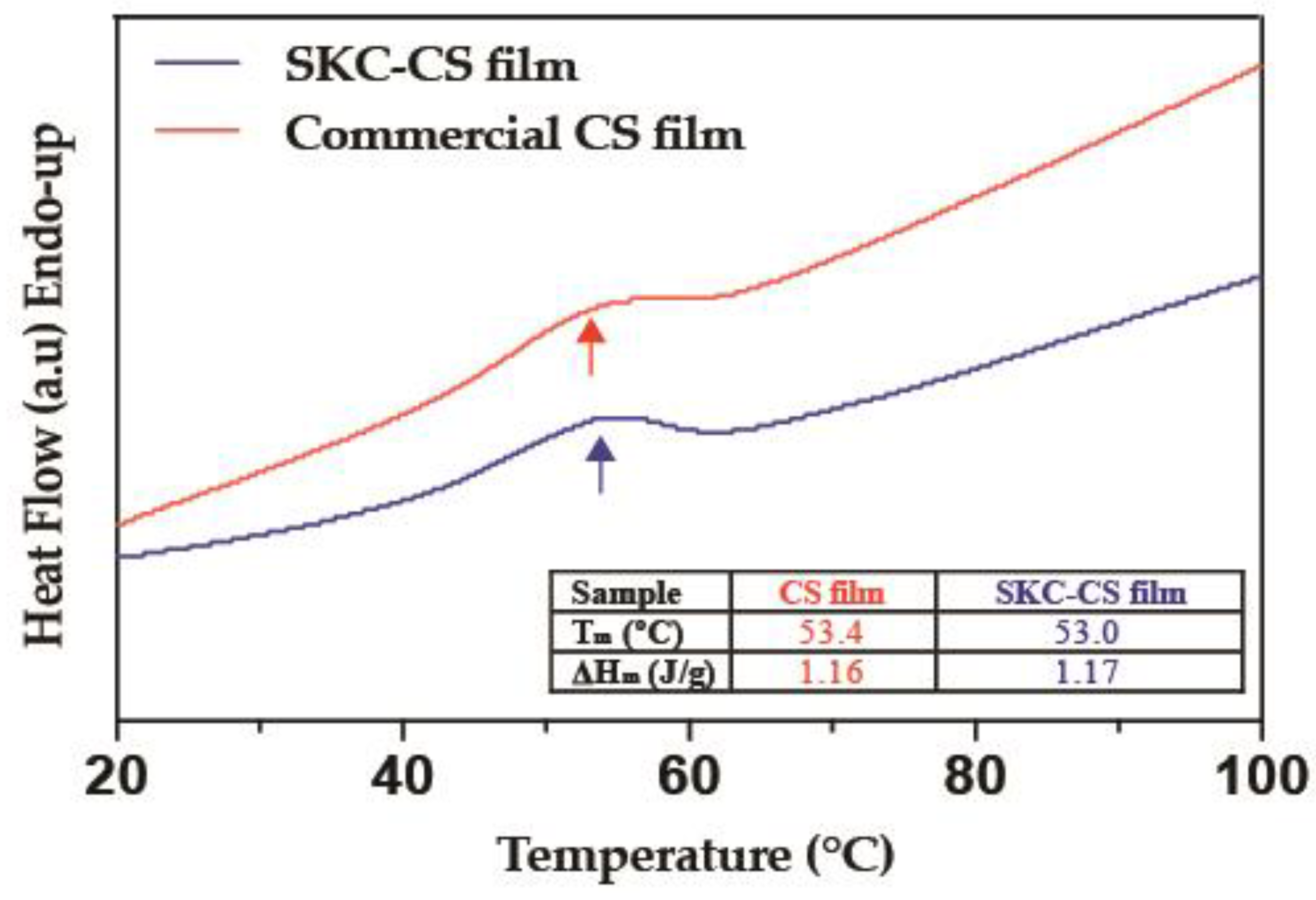
3.2. DSC Analysis
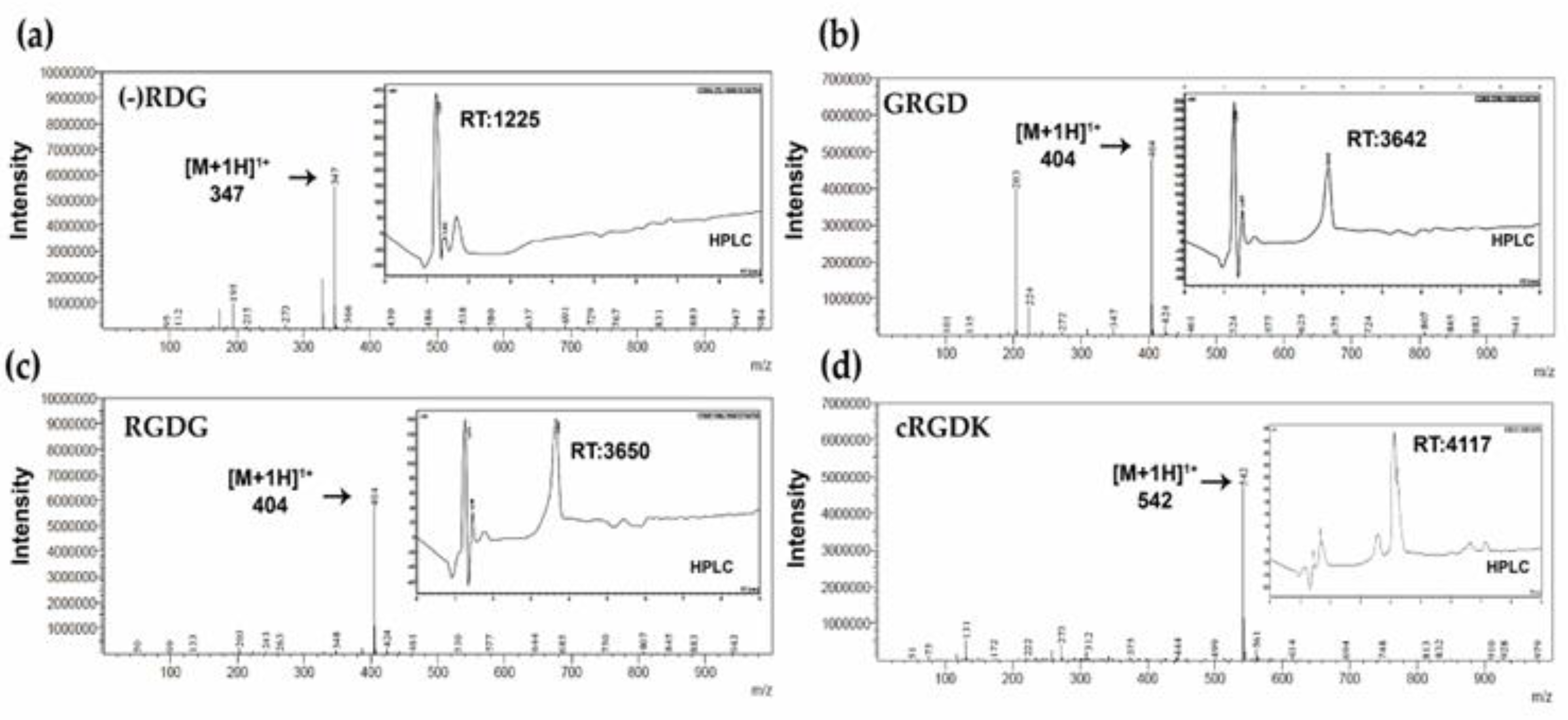
3.3. Characterization of RGD Peptides
3.4. Swelling Properties of SKC-CS and Peptide Conjugation
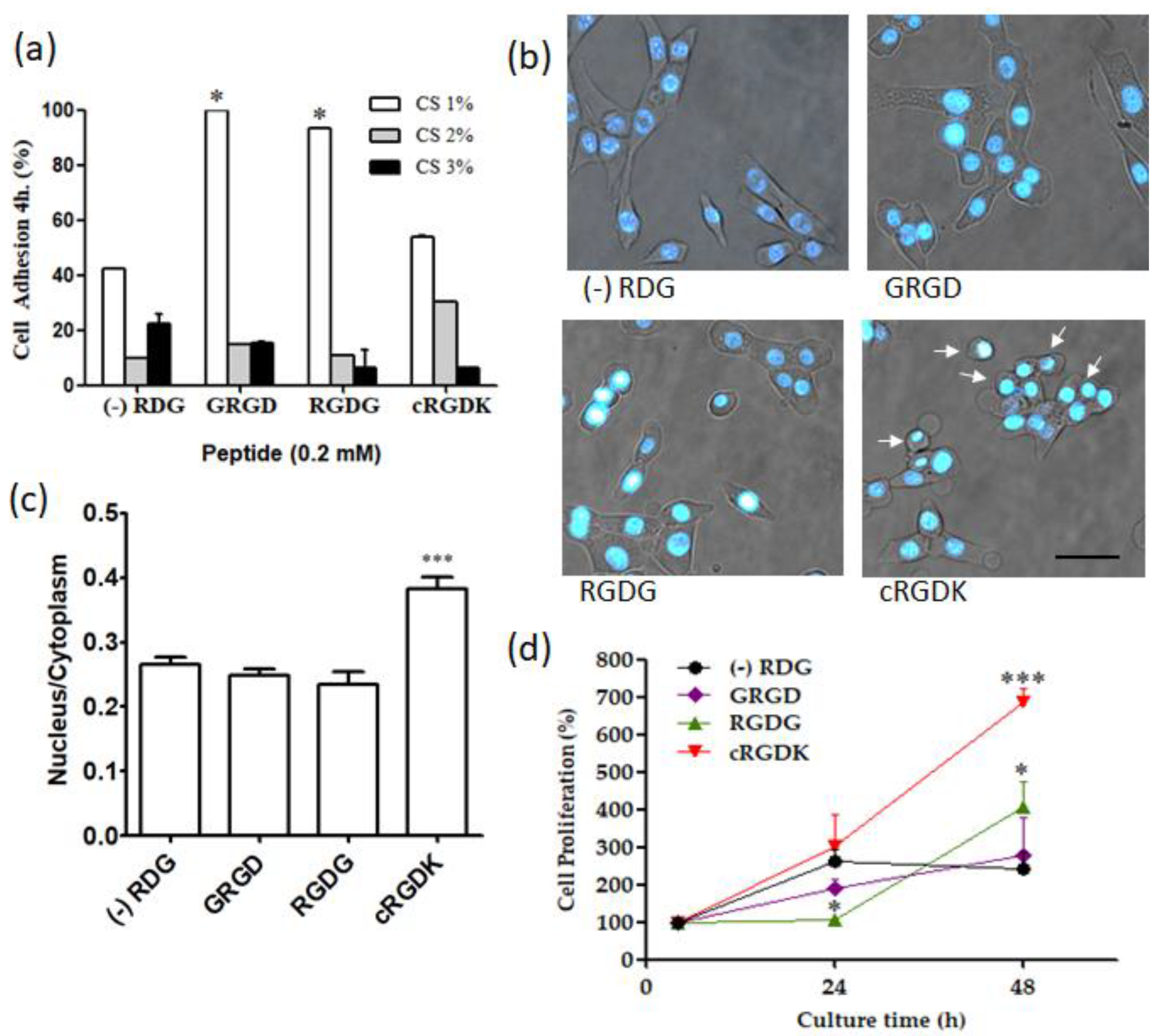
3.5. Influence of RGD Peptides on Cellular Adhesion and Proliferation in SKC-CS Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, S.; Chen, X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K.; Wong, T. Chitosan and Its Application as Tissue Engineering Scaffolds. In Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 133–147. [Google Scholar]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Molinet, C.; Olguín, A.; Gebauer, P.; Díaz, P.A.; Díaz, M.; Matamala, T.; Mora, P.; Paschke, K. Upswing and expansion of the southern king crab (Lithodes santolla) fishery in Northwest Patagonia: Drivers, trends and opportunities for management. Reg. Stud. Mar. Sci. 2020, 34, 101073. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Comparative study of chitosan and chitosan–gelatin scaffold for tissue engineering. Int. Nano Lett. 2017, 7, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Green, D.W. Tissue bionics: Examples in biomimetic tissue engineering. Biomed. Mater. 2008, 3, 034010. [Google Scholar] [CrossRef]
- Wan, K.; Cong, M.; Teng, X.; Feng, M.; Ren, L.; Wang, L. Effects of Pine Bark Extract on Physicochemical Properties and Biological Activity of Active Chitosan Film by Bionic Structure of Dragonfly Wing. Coatings 2021, 11, 1077. [Google Scholar] [CrossRef]
- Brun, P.; Zamuner, A.; Cassari, L.; D’Auria, G.; Falcigno, L.; Franchi, S.; Contini, G.; Marsotto, M.; Battocchio, C.; Iucci, G.; et al. Chitosan Covalently Functionalized with Peptides Mapped on Vitronectin and BMP-2 for Bone Tissue Engineering. Nanomaterials 2021, 11, 2784. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Yang, C.; Huang, R.; Wang, H.; Zhao, Y. Microfluidic 3D printing polyhydroxyalkanoates-based bionic skin for wound healing. Mater. Futures 2022, 1, 015401. [Google Scholar] [CrossRef]
- Shi, J.; Wei, F.; Chouraki, B.; Sun, X.; Wei, J.; Zhu, L. Study on Performance Simulation of Vascular-like Flow Channel Model Based on TPMS Structure. Biomimetics 2023, 8, 69. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Lokai, T.; Albin, B.; Yang, I.H. A Review on the Technological Advances and Future Perspectives of Axon Guidance and Regeneration in Peripheral Nerve Repair. Bioengineering 2022, 9, 562. [Google Scholar] [CrossRef]
- Huettner, N.; Dargaville, T.R.; Forget, A. Discovering Cell-Adhesion Peptides in Tissue Engineering: Beyond RGD. Trends Biotechnol. 2018, 36, 372–383. [Google Scholar] [CrossRef]
- Brun, P.; Zamuner, A.; Battocchio, C.; Cassari, L.; Todesco, M.; Graziani, V.; Iucci, G.; Marsotto, M.; Tortora, L.; Secchi, V.; et al. Bio-Functionalized Chitosan for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 5916. [Google Scholar] [CrossRef]
- Gémes, B.; Takács, E.; Székács, I.; Horvath, R.; Székács, A. Comparative Assessment of the Inhibitory Potential of the Herbicide Glyphosate and Its Structural Analogs on RGD-Specific Integrins Using Enzyme-Linked Immunosorbent Assays. Int. J. Mol. Sci. 2022, 23, 12425. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, M.; Escobar-Barrios, V.A.; Pozos-Guillén, A.; Escobar-García, D.M. RGD-functionalization of PLA/starch scaffolds obtained by electrospinning and evaluated in vitro for potential bone regeneration. Mater. Sci. Eng. C 2019, 96, 798–806. [Google Scholar] [CrossRef]
- Shin, Y.C.; Kim, J.; Kim, S.E.; Song, S.-J.; Hong, S.W.; Oh, J.-W.; Lee, J.; Park, J.-C.; Hyon, S.-H.; Han, D.-W. RGD peptide and graphene oxide co-functionalized PLGA nanofiber scaffolds for vascular tissue engineering. Regen. Biomater. 2017, 4, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Le Saux, G.; Magenau, A.; Böcking, T.; Gaus, K.; Gooding, J.J. The Relative Importance of Topography and RGD Ligand Density for Endothelial Cell Adhesion. PLoS ONE 2011, 6, e21869. [Google Scholar] [CrossRef] [Green Version]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef] [Green Version]
- Shahin, A.; Ramazani, S.A.A.; Mehraji, S.; Eslami, H. Synthesis and characterization of a chitosan/gelatin transparent film crosslinked with a combination of EDC/NHS for corneal epithelial cell culture scaffold with potential application in cornea implantation. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 568–578. [Google Scholar] [CrossRef]
- Gan, D.; Liu, M.; Xu, T.; Wang, K.; Tan, H.; Lu, X. Chitosan/biphasic calcium phosphate scaffolds functionalized with BMP-2-encapsulated nanoparticles and RGD for bone regeneration. J. Biomed. Mater. Res. Part A 2018, 106, 2613–2624. [Google Scholar] [CrossRef]
- Patrulea, V.; Hirt-Burri, N.; Jeannerat, A.; Applegate, L.A.; Ostafe, V.; Jordan, O.; Borchard, G. Peptide-decorated chitosan derivatives enhance fibroblast adhesion and proliferation in wound healing. Carbohydr. Polym. 2016, 142, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Tangpasuthadol, V.; Pongchaisirikul, N.; Hoven, V.P. Surface modification of chitosan films. Effects of hydrophobicity on protein adsorption. Carbohydr. Res. 2003, 338, 937–942. [Google Scholar] [CrossRef]
- Ross, A.; Sauce-Guevara, M.A.; Alarcon, E.I.; Mendez-Rojas, M.A. Peptide Biomaterials for Tissue Regeneration. Front. Bioeng. Biotechnol. 2022, 1, 1116. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Sandanamsamy, S.; Kamaruzaman, S.F.; Ahmad, F. Chitosan extracted from mud crab (Scylla olivicea) shells: Physicochemical and antioxidant properties. J. Food Sci. Technol. 2015, 52, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- Lago, M.A.; Rodríguez Bernaldo de Quirós, A.; Sendón, R.; Sanches-Silva, A.; Costa, H.S.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H.; Aurrekoetxea, G.P.; Angulo, I.; et al. Compilation of analytical methods to characterize and determine chitosan, and main applications of the polymer in food active packaging Recopilación de métodos analíticos para la caracterización y determinación del quitosano y las principales aplicaciones del polímero en los envases activos alimentarios. CyTA J. Food 2011, 9, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Shakeel, M.; Mehmood, K. Extraction and characterization of high purity chitosan by rapid and simple techniques from mud crabs taken from Abbottabad. Pak. J. Pharm. Sci. 2019, 32, 171–175. [Google Scholar]
- Acevedo, C.A.; Olguín, Y.; Briceño, M.; Forero, J.C.; Osses, N.; Díaz-Calderón, P.; Jaques, A.; Ortiz, R. Design of a biodegradable UV-irradiated gelatin-chitosan/nanocomposed membrane with osteogenic ability for application in bone regeneration. Mater. Sci. Eng. C 2019, 99, 875–886. [Google Scholar] [CrossRef]
- Acevedo, C.A.; Sánchez, E.; Díaz-Calderón, P.; Blaker, J.J.; Enrione, J.; Quero, F. Synergistic effects of crosslinking and chitosan molecular weight on the microstructure, molecular mobility, thermal and sorption properties of porous chitosan/gelatin/hyaluronic acid scaffolds. J. Appl. Polym. Sci. 2017, 134, 44772. [Google Scholar] [CrossRef]
- Forero, J.C.; Roa, E.; Reyes, J.G.; Acevedo, C.; Osses, N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold. Materials 2017, 10, 1177. [Google Scholar] [CrossRef] [Green Version]
- Pomari, A.; Montanheiro, T.; Siqueira, C.; Silva, R.; Tada, D.; Lemes, A. Chitosan Hydrogels Crosslinked by Genipin and Reinforced with Cellulose Nanocrystals: Production and Characterization. J. Compos. Sci. 2019, 3, 84. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, F.; Gauna, A.; Luna, O.; Román, T.; Álvarez, C.; Albericio, F.; Cárdenas, C. The tea-bag protocol for comparison of Fmoc removal reagents in solid-phase peptide synthesis. Amino Acids 2020, 52, 1201–1205. [Google Scholar] [CrossRef]
- Luna, O.F.; Gomez, J.; Cárdenas, C.; Albericio, F.; Marshall, S.H.; Guzmán, F. Deprotection Reagents in Fmoc Solid Phase Peptide Synthesis: Moving Away from Piperidine? Molecules 2016, 21, 1542. [Google Scholar] [CrossRef] [Green Version]
- Sarin, V.K.; Kent, S.B.; Tam, J.P.; Merrifield, R.B. Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal. Biochem. 1981, 117, 147–157. [Google Scholar] [CrossRef]
- Ho, M.-H.; Wang, D.-M.; Hsieh, H.-J.; Liu, H.-C.; Hsien, T.-Y.; Lai, J.-Y.; Hou, L.-T. Preparation and characterization of RGD-immobilized chitosan scaffolds. Biomaterials 2005, 26, 3197–3206. [Google Scholar] [CrossRef]
- Riss, T.; Niles, A.; Minor, L. Cell Viability Assays Assay Guidance Manual. In Assay Guidance Manual; National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004; pp. 1–23. [Google Scholar]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Weinhold, M.X.; Sauvageau, J.C.M.; Keddig, N.; Matzke, M.; Tartsch, B.; Grunwald, I.; Kübel, C.; Jastorff, B.; Thöming, J. Strategy to improve the characterization of chitosan for sustainable biomedical applications: SAR guided multi-dimensional analysis. Green Chem. 2009, 11, 498–509. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Dhakshanamurthy, T.; Kim, K.J.; Hasan, N.; Kwon, S.J.; Chun, S. Sustainable ecofriendly phytoextract mediated one pot green recovery of chitosan. Sci. Rep. 2019, 9, 13832. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.; Mello, J.; Dalcanton, F.; Macuvele, D.L.; Padoin, N.; Fiori, M.; Soares, C.; Riella, H. Mechanical, Thermal and Antimicrobial Properties of Chitosan-Based-Nanocomposite with Potential Applications for Food Packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głąb, M.; Kędzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe vera Designed for Biomedical Use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chen, Y.T.; Ke, C.J.; Chen, C.Y.; Lin, F.H. The Synthesis and Evaluation of RGD-Conjugated Chitosan Gel as Daily Supplement for Body Weight Control. Materials 2021, 14, 4467. [Google Scholar] [CrossRef]
- Ahyat, N.M.; Mohamad, F.; Ahmad, A.; Azmi, A.A. Chitin and chitosan extraction from Portunus pelagicus. Malays. J. Anal. Sci. 2017, 21, 770–777. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, D.; Regenstein, J.M.; Xia, W.; Dong, J. A comprehensive review on natural bioactive films with controlled release characteristics and their applications in foods and pharmaceuticals. Trends Food Sci. Technol. 2021, 112, 690–707. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.K.; Fan, J.; Fartash, A.; Aghaloo, T.L.; Lee, M. Photocrosslinkable chitosan hydrogels functionalized with the RGD peptide and phosphoserine to enhance osteogenesis. J. Mater. Chem. B 2016, 4, 5289–5298. [Google Scholar] [CrossRef] [Green Version]
- Safi, C.; Solano, A.G.; Liberelle, B.; Therriault, H.; Delattre, L.; Abdelkhalek, M.; Wang, C.; Bergeron-Fortier, S.; Moreau, V.; De Crescenzo, G.; et al. Effect of Chitosan on Alginate-Based Macroporous Hydrogels for the Capture of Glioblastoma Cancer Cells. ACS Appl. Bio Mater. 2022, 5, 4531–4540. [Google Scholar] [CrossRef]
- Bobu, E.; Nicu, R.; Lupei, M.; Ciolacu, F.; Desbrieres, J. Synthesis and characterization of n-alkyl chitosan for papermaking applications. Cellul. Chem. Technol. 2011, 45, 619–625. [Google Scholar]
- Hao, G.; Hu, Y.; Shi, L.; Chen, J.; Cui, A.; Weng, W.; Osako, K. Physicochemical characteristics of chitosan from swimming crab (Portunus trituberculatus) shells prepared by subcritical water pretreatment. Sci. Rep. 2021, 11, 3893. [Google Scholar] [CrossRef]
- Pereira, F.S.; da Silva Agostini, D.L.; Job, A.E.; González, E.R.P. Thermal studies of chitin–chitosan derivatives. J. Therm. Anal. Calorim. 2013, 114, 321–327. [Google Scholar] [CrossRef]
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H. Isolation and characterization of chitosan from different local insects in Egypt. Int. J. Biol. Macromol. 2016, 82, 871–877. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Y.; Yu, L.; Zhang, C.; Xu, X.; Xue, Y.; Li, Z.; Xue, C. Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba). Carbohydr. Polym. 2013, 92 Pt 1, 90–97. [Google Scholar] [CrossRef]
- Suneeta, K.; Rath, P.; Annamareddy, S. Chitosan from shrimp shell (Crangon crangon) and fish scales (Labeorohita): Extraction and characterization. Afr. J. Biotechnol. 2016, 15, 1258–1268. [Google Scholar] [CrossRef] [Green Version]
- Jahan, M.; Nessa, F.; Masum, S.; Asaduzzaman, M.; Roy, S.K.; Hossain, M. A process of preparation of chitin and chitosan from prawn shell waste. Bangladesh J. Sci. Ind. Res. 2010, 45, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.Q. DSC Analysis of Thermophysical Properties for Biomaterials and Formulations. Methods Mol. Biol. 2021, 2180, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Kittur, F.S.; Harish Prashanth, K.V.; Udaya Sankar, K.; Tharanathan, R.N. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Valenzuela, L.D.; Sammalkorpi, M.; Lutkenhaus, J.L. Effect of Water on the Thermal Transition Observed in Poly(allylamine hydrochloride)–Poly(acrylic acid) Complexes. Macromolecules 2016, 49, 7563–7570. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Heller, M.; Brieger, J.; Klein, M.O.; Al-Nawas, B.; Gabriel, M. Immobilisation of linear and cyclic RGD-peptides on titanium surfaces and their impact on endothelial cell adhesion and proliferation. Eur. Cells Mater. 2011, 21, 364–372. [Google Scholar] [CrossRef]
- Troeira Henriques, S.; Craik, D.J. Cyclotide Structure and Function: The Role of Membrane Binding and Permeation. Biochemistry 2017, 56, 669–682. [Google Scholar] [CrossRef]
- Yadav, A.S.; Radharani, N.N.V.; Gorain, M.; Bulbule, A.; Shetti, D.; Roy, G.; Baby, T.; Kundu, G.C. RGD functionalized chitosan nanoparticle mediated targeted delivery of raloxifene selectively suppresses angiogenesis and tumor growth in breast cancer. Nanoscale 2020, 12, 10664–10684. [Google Scholar] [CrossRef]
- Tığlı Aydın, R.S.; Gümüşderelioğlu, M. Evaluation of RGD- or EGF-immobilized chitosan scaffolds for chondrogenic activity. Int. J. Biol. Macromol. 2008, 43, 121–128. [Google Scholar] [CrossRef]
- Davachi, S.M.; Haramshahi, S.M.A.; Akhavirad, S.A.; Bahrami, N.; Hassanzadeh, S.; Ezzatpour, S.; Hassanzadeh, N.; Kebria, M.M.; Khanmohammadi, M.; Bagher, Z. Development of chitosan/hyaluronic acid hydrogel scaffolds via enzymatic reaction for cartilage tissue engineering. Mater. Today Commun. 2022, 30, 103230. [Google Scholar] [CrossRef]
- Dilruba Öznur, K.G.; Ayşe Pınar, T.D. Statistical evaluation of biocompatibility and biodegradability of chitosan/gelatin hydrogels for wound-dressing applications. Polym. Bull. 2023, 1–34. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Gui, Q.; Yang, H. Drug-loaded chitosan film prepared via facile solution casting and air-drying of plain water-based chitosan solution for ocular drug delivery. Bioact. Mater. 2020, 5, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Runge, T.; Wang, L.; Li, R.; Feng, J.; Shu, X.-L.; Shi, Q.-S. Hydrogen bonding impact on chitosan plasticization. Carbohydr. Polym. 2018, 200, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Saheb, M.; Fereydouni, N.; Nemati, S.; Barreto, G.E.; Johnston, T.P.; Sahebkar, A. Chitosan-based delivery systems for curcumin: A review of pharmacodynamic and pharmacokinetic aspects. J. Cell. Physiol. 2019, 234, 12325–12340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, P.; Jiang, Q.; Xia, W. Green fabrication of lignin nanoparticles/chitosan films for refrigerated fish preservation application. Food Hydrocoll. 2023, 139, 108548. [Google Scholar] [CrossRef]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications-A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.R.; López-Cebral, R.; Silva-Correia, J.; Silva, J.M.; Mano, J.F.; Silva, T.H.; Freier, T.; Reis, R.L.; Oliveira, J.M. Investigation of cell adhesion in chitosan membranes for peripheral nerve regeneration. Mater. Sci. Eng. C 2017, 71, 1122–1134. [Google Scholar] [CrossRef] [Green Version]
- Pavinatto, F.J.; Pavinatto, A.; Caseli, L.; Santos, D.S., Jr.; Nobre, T.M.; Zaniquelli, M.E.; Oliveira, O.N., Jr. Interaction of chitosan with cell membrane models at the air-water interface. Biomacromolecules 2007, 8, 1633–1640. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, C.; Kottke-Marchant, K.; Marchant, R.E. Design and synthesis of biomimetic hydrogel scaffolds with controlled organization of cyclic RGD peptides. Bioconjug. Chem. 2009, 20, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Dix, C.L.; Matthews, H.K.; Uroz, M.; McLaren, S.; Wolf, L.; Heatley, N.; Win, Z.; Almada, P.; Henriques, R.; Boutros, M.; et al. The Role of Mitotic Cell-Substrate Adhesion Re-modeling in Animal Cell Division. Dev. Cell 2018, 45, 132–145.e133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Javdan, B.; Cowan, A.; Smith, K. More than skin deep: Cyclic peptides as wound healing and cytoprotective compounds. Front. Cell Dev. Biol. 2023, 11, 1195600. [Google Scholar] [CrossRef] [PubMed]
- Vedaraman, S.; Bernhagen, D.; Haraszti, T.; Licht, C.; Castro Nava, A.; Omidinia Anarkoli, A.; Timmerman, P.; De Laporte, L. Bicyclic RGD peptides enhance nerve growth in synthetic PEG-based Anisogels. Biomater. Sci. 2021, 9, 4329–4342. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowich-Knipp, S.J.; Chakrabarti, S.; Williams, T.D.; Dillman, R.K.; Siahaan, T.J. Solution stability of linear vs. cyclic RGD peptides. J. Pept. Res. Off. J. Am. Pept. Soc. 1999, 53, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Kiser, R.C.; Lu, Y.Y.; Fong, E.; Ho, W.C.; Tirrell, D.A.; Grubbs, R.H. Synthesis and Cell Adhesive Properties of Linear and Cyclic RGD Functionalized Polynorbornene Thin Films. Biomacromolecules 2012, 13, 2546–2553. [Google Scholar] [CrossRef] [Green Version]
- Civera, M.; Arosio, D.; Bonato, F.; Manzoni, L.; Pignataro, L.; Zanella, S.; Gennari, C.; Piarulli, U.; Belvisi, L. Investigating the Interaction of Cyclic RGD Peptidomimetics with α(V)β6 Integrin by Biochemical and Molecular Docking Studies. Cancers 2017, 9, 128. [Google Scholar] [CrossRef] [Green Version]


| Parameters (%) | SKC-CS | Commercial CS |
|---|---|---|
| Moisture | 14.44 ± 0.04 | 11.67 |
| Ash | 2.0 ± 1 | 0.48 |
| Nitrogen | 54.7 ± 2.4 | 79 |
| DDA | 94.9 | >75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forero, J.C.; Carvajal, K.; Guzmán, F.; Acevedo, C.; Osses, N.; Santana, P. Use of Chitosan from Southern King Crab to Develop Films Functionalized with RGD Peptides for Potential Tissue Engineering Applications. Biomimetics 2023, 8, 323. https://doi.org/10.3390/biomimetics8030323
Forero JC, Carvajal K, Guzmán F, Acevedo C, Osses N, Santana P. Use of Chitosan from Southern King Crab to Develop Films Functionalized with RGD Peptides for Potential Tissue Engineering Applications. Biomimetics. 2023; 8(3):323. https://doi.org/10.3390/biomimetics8030323
Chicago/Turabian StyleForero, Juan Carlos, Karina Carvajal, Fanny Guzmán, Cristian Acevedo, Nelson Osses, and Paula Santana. 2023. "Use of Chitosan from Southern King Crab to Develop Films Functionalized with RGD Peptides for Potential Tissue Engineering Applications" Biomimetics 8, no. 3: 323. https://doi.org/10.3390/biomimetics8030323






