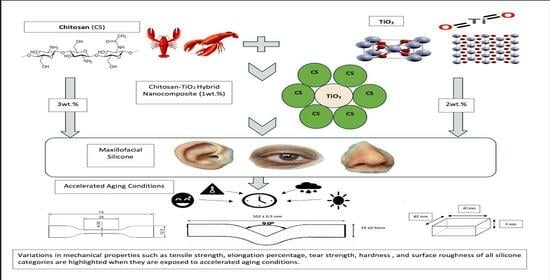
Evaluation of the Mechanical and Physical Properties of Maxillofacial Silicone Type A-2186 Impregnated with a Hybrid Chitosan–TiO2 Nanocomposite Subjected to Different Accelerated Aging Conditions
Abstract
:1. Introduction
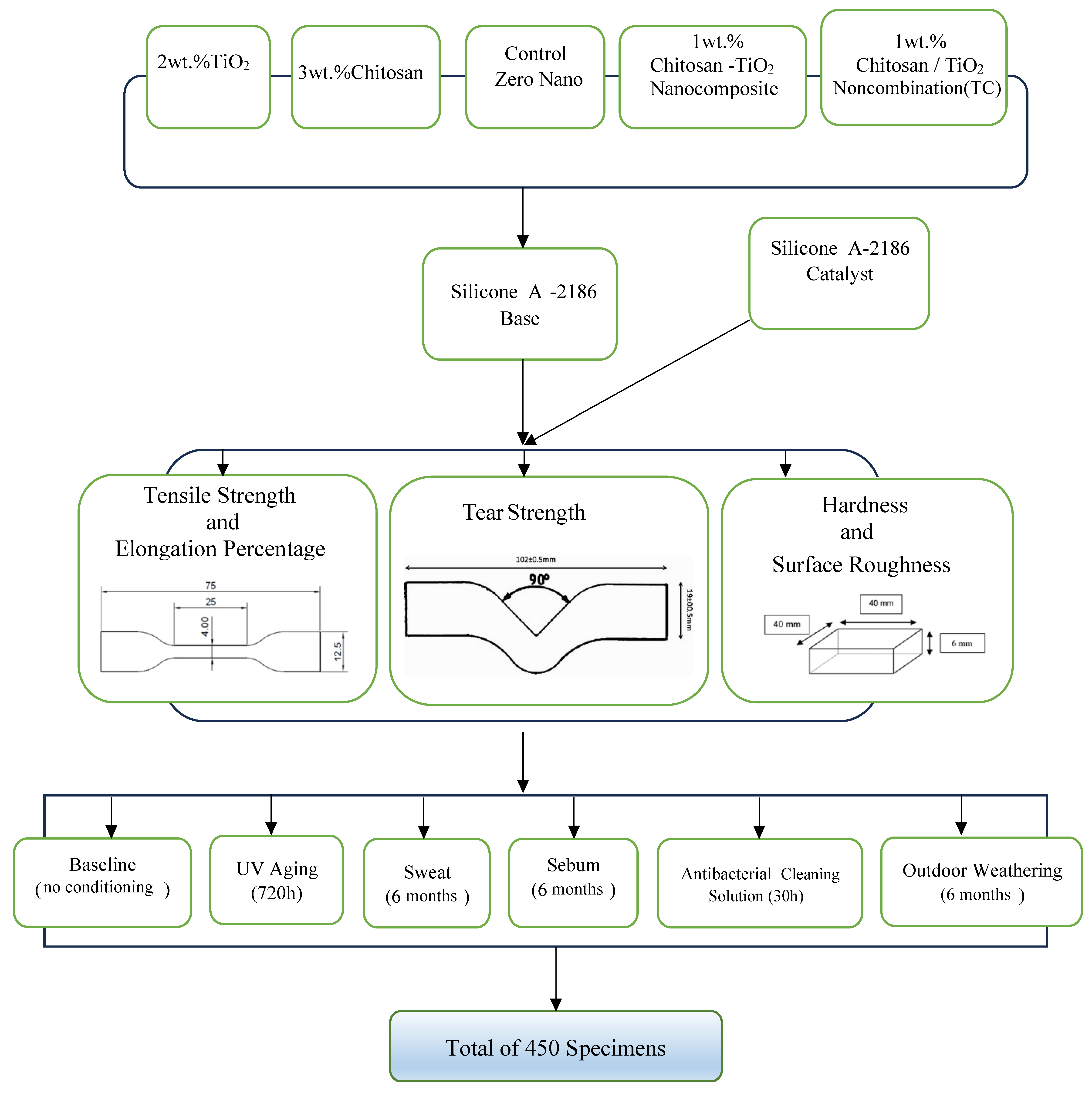
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Nanocomposite
2.2.2. Preparation of Control Group Specimens
2.2.3. Preparation of Experimental Group Specimens Reinforced with the Chitosan–TiO2 Synthesized Nanocomposite
2.2.4. Preparation of the Experimental Group Specimens
2.2.5. Conditioning Modes
2.2.6. Mechanical Tests
Tensile Strength Test
Elongation Percentage
Tear Strength Test
Hardness Test
Surface Roughness
2.3. Statistical Analysis
3. Results
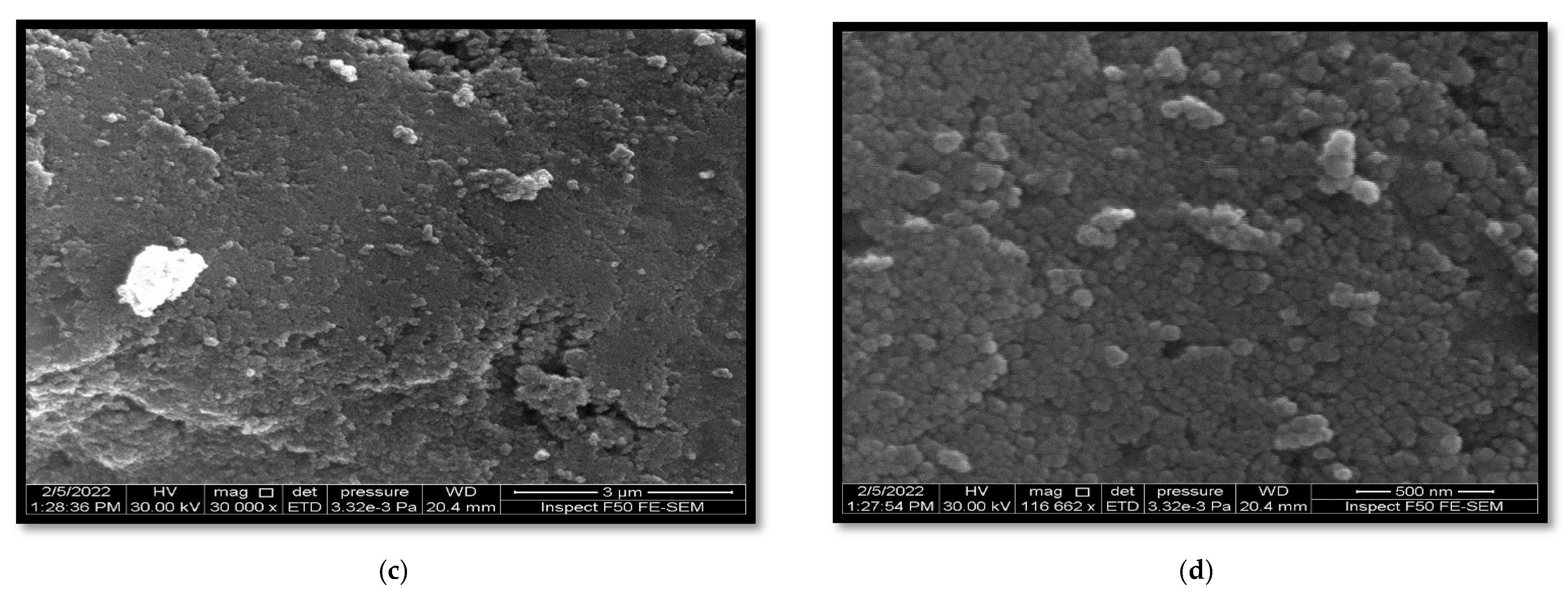
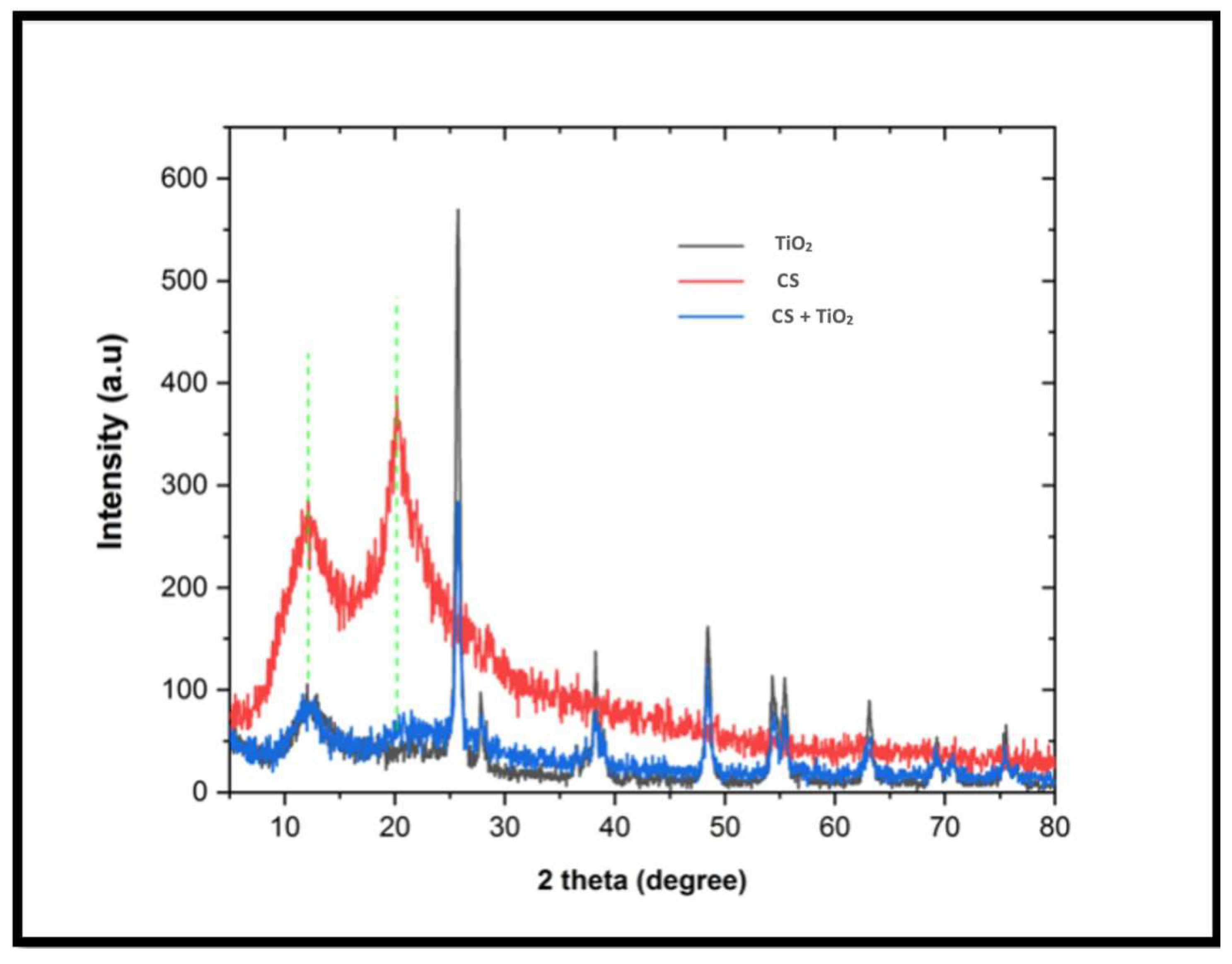
3.1. Morphological Characteristics
3.1.1. Field Emission Scanning Electron Microscopy
3.1.2. X-ray Diffraction (XRD)
3.1.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Results of the Mechanical Tests
3.2.1. Tensile Strength Test Results
3.2.2. Elongation Percentage Results
3.2.3. Tear Strength Test Results
3.2.4. Hardness Test Results
3.2.5. Surface Roughness Test Results
4. Discussion
4.1. Aging Conditions
4.2. Tensile Strength
4.3. Elongation Percentage
4.4. Tear Strength
4.5. Hardness
4.6. Surface Roughness
4.7. Limitations and Recommendations
5. Conclusions
- Exposure to sweat, UV artificial weathering, and natural outdoor weathering significantly impacts the tensile strength across all silicone categories. Despite the significant differences, the silicone samples containing 1 wt.% chitosan–TiO2 demonstrated the highest tensile strength after being subjected to various aging conditions.
- Upon being subjected to accelerated aging conditions, silicone variants containing 1 wt.% TC and 2 wt.% TiO2 consistently exhibited superior elongation percentages relative to the other silicone categories that were assessed.
- All silicone categories demonstrated an increase in tear strength values after being subjected to diverse conditions compared to their baseline conditions. Remarkably, the silicone samples with 1 wt.% chitosan–TiO2 showed a significant increase in tear strength, especially after exposure to antibacterial and outdoor conditions.
- There are highlighted variations in the hardness properties among silicone categories for all conditions, except their baseline conditions. Upon six months of sustained exposure to sebum, the 1 wt.% TC specimen had the lowest hardness value. Concurrently, when exposed to sweat and outdoor weathering conditions, the 3 wt.% chitosan variant presented a pronounced increase in the hardness metrics compared to the 1 wt.% TC and 1 wt.% chitosan–TiO2 specimens.
- All specimens across the silicone categories showed decreased surface roughness values when exposed to different conditions. Specifically, the 1 wt.% TC specimen showed the most pronounced reduction after UV artificial weathering exposure. However, an exception was noted for the 1 wt.% chitosan–TiO2 specimen, which exhibited an increase in surface roughness in most conditions, contrary to the general trend.
- The findings of this study emphasize that integrating two unique nanoparticles (which formed a nanocomposite) into the A-2168 silicone enhances its mechanical properties more effectively than its counterparts and preserves the silicone’s properties against various accelerated aging conditions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dings, J.P.J.; Merkx, M.A.W.; de Clonie Maclennan-Naphausen, M.T.P.; van de Pol, P.; Maal, T.J.J.; Meijer, G.J. Maxillofacial Prosthetic Rehabilitation: A Survey on the Quality of Life. J. Prosthet. Dent. 2018, 120, 780–786. [Google Scholar] [CrossRef]
- Goiato, M.C.; Pesqueira, A.A.; Ramos da Silva, C.; Gennari Filho, H.; Micheline Dos Santos, D. Patient Satisfaction with Maxillofacial Prosthesis. Literature Review. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 175–180. [Google Scholar] [CrossRef]
- Kumar, S.; Rajtilak, G.; Rajasekar, V.; Kumar, M. Nasal Prosthesis for a Patient with Xeroderma Pigmentosum. J. Pharm. Bioallied Sci. 2013, 5, S176–S178. [Google Scholar] [CrossRef]
- Aziz, T.; Waters, M.; Jagger, R. Analysis of the Properties of Silicone Rubber Maxillofacial Prosthetic Materials. J. Dent. 2003, 31, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Polyzois, G.L.; Nuseir, A.; Hatamleh, K.; Alnazzawi, A. Mechanical Properties and Simulated Aging of Silicone Maxillofacial Elastomers: Advancements in the Past 45 Years: Advancements in Maxillofacial Silicone Mechanical Properties. J. Prosthodont. 2016, 25, 418–426. [Google Scholar] [CrossRef]
- Huber, H.; Studer, S.P. Materials and Techniques in Maxillofacial Prosthodontic Rehabilitation. Oral Maxillofac. Surg. Clin. N. Am. 2002, 14, 73–93. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Goiato, M.C.; Moreno, A.; Pesqueira, A.A.; Haddad, M.F. Influence of Pigments and Opacifiers on Color Stability of an Artificially Aged Facial Silicone. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2011, 20, 205–208. [Google Scholar] [CrossRef]
- Haddad, M.F.; Goiato, M.C.; Dos Santos, D.M.; Pesqueira, A.A.; Moreno, A.; Pellizzer, E.P. Influence of Pigment and Opacifier on Dimensional Stability and Detail Reproduction of Maxillofacial Silicone Elastomer. J. Craniofac. Surg. 2011, 22, 1612–1616. [Google Scholar] [CrossRef]
- Bangera, B.S.; Guttal, S.S. Evaluation of Varying Concentrations of Nano-Oxides as Ultraviolet Protective Agents When Incorporated in Maxillofacial Silicones: An in Vitro Study. J. Prosthet. Dent. 2014, 112, 1567–1572. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Jing, D.; Zhou, S.; Shao, L. Biomechanical Properties of Nano-TiO(2) Addition to a Medical Silicone Elastomer: The Effect of Artificial Ageing. J. Dent. 2014, 42, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.N.; Goiato, M.C.; de Carvalho Dekon, S.F.; Gennari-Filho, H. Visual Evaluation of Color Stability after Accelerated Aging of Pigmented and Nonpigmented Silicones to Be Used in Facial Prostheses. Indian J. Dent. Res. 2009, 20, 77–80. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Goiato, M.C.; Sinhoreti, M.A.C.; Fernandes, A.U.R.; do Prado Ribeiro, P.; de Carvalho Dekon, S.F. Color Stability of Polymers for Facial Prosthesis. J. Craniofac. Surg. 2010, 21, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Polyzois, G.L.; Eleni, P.N.; Krokida, M.K. Effect of Time Passage on Some Physical Properties of Silicone Maxillofacial Elastomers. J. Craniofac. Surg. 2011, 22, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Fernandes, A.U.R.; dos Santos, D.M.; Barão, V.A.R. Positioning Magnets on a Multiple/Sectional Maxillofacial Prosthesis. J. Contemp. Dent. Pract. 2007, 8, 101–107. [Google Scholar] [CrossRef]
- Goiato, M.C.; Pesqueira, A.A.; dos Santos, D.M.; Antenucci, R.M.F.; Ribeiro, P. do P. Evaluation of Dimensional Change and Detail Reproduction in Silicones for Facial Prostheses. Acta Odontol. Latinoam. 2008, 21, 85–88. [Google Scholar]
- Polyzois, G.L. Color Stability of Facial Silicone Prosthetic Polymers after Outdoor Weathering. J. Prosthet. Dent. 1999, 82, 447–450. [Google Scholar] [CrossRef]
- Eleni, P.N.; Katsavou, I.; Krokida, M.K.; Polyzois, G.L. Color Stability of Facial Silicon Prosthetic Elastomer after Artificial Weathering. Dent. Res. J. 2008, 5, 71–79. [Google Scholar]
- Sethi, T.; Kheur, M.; Coward, T.; Patel, N. Change in Color of a Maxillofacial Prosthetic Silicone Elastomer, Following Investment in Molds of Different Materials. J. Indian Prosthodont. Soc. 2015, 15, 153–157. [Google Scholar] [CrossRef]
- Chotprasert, N.; Shrestha, B.; Sipiyaruk, K. Effects of Disinfection Methods on the Color Stability of Precolored and Hand-Colored Maxillofacial Silicone: An In Vitro Study. Int. J. Biomater. 2022, 2022, 7744744. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, D.S.; Sethuraman, R. Comparative Evaluation of Tensile Strength, Tear Strength, Color Stability and Hardness of Conventional and 1% Trisnorbornenylisobutyl Polyhedralsilsesquioxane Modified Room Temperature Vulcanizing Maxillofacial Silicone after a Six Month Artificial Aging Period. J. Indian Prosthodont. Soc. 2022, 22, 328–337. [Google Scholar] [CrossRef]
- Akay, C.; Cevik, P.; Karakis, D.; Sevim, H. In Vitro Cytotoxicity of Maxillofacial Silicone Elastomers: Effect of Nano-Particles. J. Prosthodont. 2018, 27, 584–587. [Google Scholar] [CrossRef]
- Sonnahalli, N.K.; Chowdhary, R. Effect of Nanoparticles on Color Stability and Mechanical and Biological Properties of Maxillofacial Silicone Elastomer: A Systematic Review. J. Indian Prosthodont. Soc. 2020, 20, 244–254. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Y.; Xie, C.; Powers, J.M.; Kiat-amnuay, S. Color Stability of Pigmented Maxillofacial Silicone Elastomer: Effects of Nano-Oxides as Opacifiers. J. Dent. 2010, 38 (Suppl. S2), e100–e105. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.; Ibadurrohman, M. Slamet Synthesis of Chitosan/TiO2 Nanocomposite for Antibacterial Sunscreen Application; AIP Publishing: Coimbatore, India, 2020; p. 060020. [Google Scholar]
- Shakir, D.A.; Abdul-Ameer, F.M. Effect of Nano-Titanium Oxide Addition on Some Mechanical Properties of Silicone Elastomers for Maxillofacial Prostheses. J. Taibah Univ. Med. Sci. 2018, 13, 281–290. [Google Scholar] [CrossRef]
- Prameshwari, F.R.; Karlina, E.; Hasratiningsih, Z. Synthesis of Ca-Psz Nanoparticles Using Sol-Gel Technique with Chitosan as a Dispersant for Raw Materials Restoration and Dental Rehabilitation Equipment. Padjadjaran J. Dent. 2013, 25, 3. [Google Scholar] [CrossRef]
- Mattu, C.; Li, R.; Ciardelli, G. Chitosan Nanoparticles as Therapeutic Protein Nanocarriers: The Effect of Ph on Particle Formation and Encapsulation Efficiency. Polym. Compos. 2013, 34, 1538–1545. [Google Scholar] [CrossRef]
- Shahabi, M.; Movahedi Fazel, S.; Rangrazi, A. Incorporation of Chitosan Nanoparticles into a Cold-Cure Ortho-Dontic Acrylic Resin: Effects on Mechanical Properties. Biomimetics 2021, 6, 7. [Google Scholar] [CrossRef]
- Remanan, S.; Ghosh, S.; Das, T.K.; Sharma, M.; Bose, M.; Bose, S.; Das, A.K.; Das, N.C. Gradient Crystallinity and Its Influence on the Poly(Vinylidene Fluoride)/Poly(Methyl Methacrylate) Membrane-derived by Immersion Precipitation Method. J. Appl. Polym. Sci. 2020, 137, 48677. [Google Scholar] [CrossRef]
- Han, Y.; Kiat-Amnuay, S.; Powers, J.M.; Zhao, Y. Effect of Nano-Oxide Concentration on the Mechanical Properties of a Maxillofacial Silicone Elastomer. J. Prosthet. Dent. 2008, 100, 465–473. [Google Scholar] [CrossRef]
- Ibrahim, A.H.J.; Al-Judy, H.J. Mechanical Properties of Chitosan Incorporated in Maxillofacial Silicone and Its Anti Candidal Activity In Vitro. J. Res. Med. Dent. Sci. 2018, 6, 101–107. [Google Scholar]
- Nobrega, A.S.; Andreotti, A.M.; Moreno, A.; Sinhoreti, M.A.C.; Dos Santos, D.M.; Goiato, M.C. Influence of Adding Nanoparticles on the Hardness, Tear Strength, and Permanent Deformation of Facial Silicone Subjected to Accelerated Aging. J. Prosthet. Dent. 2016, 116, 623–629.e1. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Maqableh, A.M.; Al-Wahadni, A.; Al-Rabab’ah, M.A. Mechanical Properties and Bonding of Maxillofacial Silicone Elastomer Mixed with Nano-Sized Anti-Microbials. Dent. Mater. 2023, 39, 677–681. [Google Scholar] [CrossRef]
- Radey, N.; Al Shimy, A.; Ahmed, D. Effect of extraoral aging conditions on mechanical properties of facial silicone elastomer reinforced with titanium-oxide nanoparticles (in vitro study). Alex. Dent. J. 2020, 45, 29–36. [Google Scholar] [CrossRef]
- Al-Kadi, F.K.; Abdulkareem, J.F.; Azhdar, B.A. Hybrid Chitosan-TiO2 Nanocomposite Impregnated in Type A-2186 Maxillofacial Silicone Subjected to Different Accelerated Aging Conditions: An Evaluation of Color Stability. Nanomaterials 2023, 13, 2379. [Google Scholar] [CrossRef] [PubMed]
- Haldorai, Y.; Shim, J.-J. Novel Chitosan-TiO 2 Nanohybrid: Preparation, Characterization, Antibacterial, and Photocatalytic Properties. Polym. Compos. 2014, 35, 327–333. [Google Scholar] [CrossRef]
- Hatamleh, M.M.; Polyzois, G.L.; Silikas, N.; Watts, D.C. Effect of Extraoral Aging Conditions on Mechanical Properties of Maxillofacial Silicone Elastomer. J. Prosthodont. 2011, 20, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Polyzois, G.L.; Tarantili, P.A.; Frangou, M.J.; Andreopoulos, A.G. Physical Properties of a Silicone Prosthetic Elastomer Stored in Simulated Skin Secretions. J. Prosthet. Dent. 2000, 83, 572–577. [Google Scholar] [CrossRef]
- Hatamleh, M.M.; Watts, D.C. Effect of Extraoral Aging Conditions on Color Stability of Maxillofacial Silicone Elastomer. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2010, 19, 536–543. [Google Scholar] [CrossRef]
- ISO 105-E04:1996; Textiles–Tests for Color Fastness. Part E04: Color Fastness to Perspiration. ISO: Geneva, Switzerland, 1996.
- Nair, A.; Saratchandran, S. Comparative Evaluation of Color Stability of Maxillofacial Silicones following Accelerated Aging Conditions. FACE 2022, 3, 362–368. [Google Scholar] [CrossRef]
- ASTM G7-97; Standard Practice for Atmospheric Environmental Exposure Testing of Nonmetallic Materials. ASTM International: West Conshohocken, PA, USA, 1997.
- Shihab, N.M.; Abdul-Ameer, F.M. Evaluation of Tensile Strength and Percentage of Elongation for Pigmented and Non-Pigmented Maxillofacial Silicone Before and After Aging. Int. J. Sci. Res. 2018, 7, 310–317. [Google Scholar]
- ISO 37; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 34-1; Rubber, Vulcanized or Thermoplastic—Determination of Tear Strength—Part 1. International Organization for Standardization: Geneva, Switzerland, 2010.
- ASTM D2240-15; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2012. [CrossRef]
- Anaya-Esparza, L.M.; Ruvalcaba-Gómez, J.M.; Maytorena-Verdugo, C.I.; González-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Pérez-Larios, A.; Montalvo-González, A.E. Chitosan-TiO2: A Versatile Hybrid Composite. Materials 2020, 13, 811. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Sarkar, S.; Jagajjanani Rao, K.; Paria, S. Core/Shell Nanoparticles in Biomedical Applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef]
- Hood, M.; Mari, M.; Muñoz-Espí, R. Synthetic Strategies in the Preparation of Polymer/Inorganic Hybrid Nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, P.C.; Kiat-Amnuay, S. Survey of Currently Used Materials for Fabrication of Extraoral Maxillofacial Prostheses in North America, Europe, Asia, and Australia: Survey of Maxillofacial Prostheses Materials Used. J. Prosthodont. 2009, 19, 482–490. [Google Scholar] [CrossRef]
- Al-Dharrab, A.A.; Tayel, S.B.; Abodaya, M.H. The Effect of Different Storage Conditions on the Physical Properties of Pigmented Medical Grade I Silicone Maxillofacial Material. ISRN Dent. 2013, 2013, 582051. [Google Scholar] [CrossRef]
- Gunay, Y.; Kurtoglu, C.; Atay, A.; Karayazgan, B.; Gurbuz, C.C. Effect of Tulle on the Mechanical Properties of a Maxillofacial Silicone Elastomer. Dent. Mater. J. 2008, 27, 775–779. [Google Scholar] [CrossRef]
- Lai, J.H.; Wang, L.L.; Ko, C.C.; DeLong, R.L.; Hodges, J.S. New Organosilicon Maxillofacial Prosthetic Materials. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2002, 18, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Moore, D.J.; Cruz, D.L.; Chappell, R. Comparison of the Physical Properties of Two Types of Polydimethyl Siloxane for Fabrication of Facial Prostheses. J. Prosthet. Dent. 1992, 67, 679–682. [Google Scholar] [CrossRef]
- Lai, J.H.; Hodges, J.S. Effects of Processing Parameters on Physical Properties of the Silicone Maxillofacial Prosthetic Materials. Dent. Mater. 1999, 15, 450–455. [Google Scholar] [CrossRef]
- Dootz, E.R.; Koran, A.; Craig, R.G. Physical Properties of Three Maxillofacial Materials as a Function of Accelerated Aging. J. Prosthet. Dent. 1994, 71, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Chambers, M.S.; Powers, J.M.; Kiat-Amnuay, S. Effect of Opacifiers and UV Absorbers on Pigmented Maxillofacial Silicone Elastomer, Part 2: Mechanical Properties after Artificial Aging. J. Prosthet. Dent. 2013, 109, 402–410. [Google Scholar] [CrossRef]
- Kanakaraj, S.; Kumar, H.K.; Ravichandran, R.; Nair, V.V.; Janardhanan, K.; Vs, D. Effect of Extraoral Aging Conditions on the Color Stability of High Temperature Vulcanizing and Room Temperature Vulcanizing Maxillofacial Silicone Elastomers: An in Vitro Study. Int. J. Appl. Dent. Sci. 2022, 8, 556–560. [Google Scholar] [CrossRef]
- Eleni, P.N.; Krokida, M.K.; Polyzois, G.L. The Effect of Artificial Accelerated Weathering on the Mechanical Properties of Maxillofacial Polymers PDMS and CPE. Biomed. Mater. 2009, 4, 035001. [Google Scholar] [CrossRef]
- Nóbrega, A.S.; Neto, C.L.M.M.; Dos Santos, D.M.; Bertoz, A.P.M.; de MeloMoreno, A.L.; Goiato, M.C. Effect of Accelerated Aging on the Sorption and Solubility Percentages of Silicone Facial Prostheses. Eur. J. Dent. 2022, 16, 223–226. [Google Scholar] [CrossRef]
- Marrega Malavazi, E.; Dos Santos, D.M.; de Moraes Melo Neto, C.L.; Pereira de Caxias, F.; Freitas da Silva, E.V.; Bannwart, L.C.; Pesqueira, A.A.; de Melo Moreno, A.L.; de Magalhães Bertoz, A.P.; Goiato, M.C. Influence of Different Pigmentations and Accelerated Aging on the Hardness and Tear Strength of the A-2186 and MDX4-4210 Silicones. Int. J. Dent. 2020, 2020, 8492091. [Google Scholar] [CrossRef]
- Goiato, M.C.; Haddad, M.F.; dos Santos, D.M.; Pesqueira, A.A.; Moreno, A. Hardness Evaluation of Prosthetic Silicones Containing Opacifiers Following Chemical Disinfection and Accelerated Aging. Braz. Oral Res. 2010, 24, 303–308. [Google Scholar] [CrossRef]
- Goiato, M.C.; Haddad, M.F.; Sinhoreti, M.A.C.; dos Santos, D.M.; Pesqueira, A.A.; Moreno, A. Influence of Opacifiers on Dimensional Stability and Detail Reproduction of Maxillofacial Silicone Elastomer. Biomed. Eng. Online 2010, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Paulini, M.B.; Micheline Dos Santos, D.; De Moraes Melo Neto, C.L.; Bitencourt, S.B.; Freitas Da Silva, E.V.; Pereira De Caxias, F.; Ribeiro, R.P.; Rangel, E.C.; Sônego, M.V.; Goiato, M.C. Analysis of Physical Properties of Facial Silicones with Different Pigmentations Submitted to Nonthermal Plasma Treatment and Accelerated Aging. J. Prosthet. Dent. 2020, 124, e1–e815. [Google Scholar] [CrossRef]
- Mouzakis, D.E.; Kandilioti, G.; Elenis, A.; Gregoriou, V.G. Ultraviolet Radiation Induced Cold Chemi-Crystallization in Syndiotactic Polypropylene Clay-Nanocomposites. J. Macromol. Sci. Part A 2006, 43, 259–267. [Google Scholar] [CrossRef]
- Rahman, A.M.; Jamayet, N.B.; Nizami, M.M.U.I.; Johari, Y.; Husein, A.; Alam, M.K. Effect of Tropical Outdoor Weathering on the Surface Roughness and Mechanical Properties of Maxillofacial Silicones. J. Prosthet. Dent. 2022, 127, 937–942. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Z.; Chen, X.; Wan, J.; Luo, L.; Zhang, H.; Shu, S.; Tu, Z. Temperature and Humidity Effect on Aging of Silicone Rubbers as Sealing Materials for Proton Exchange Membrane Fuel Cell Applications. Appl. Therm. Eng. 2016, 104, 472–478. [Google Scholar] [CrossRef]
- Tetteh, S.; Bibb, R.; Martin, S. Mechanical and Morphological Effect of Plant Based Antimicrobial Solutions on Maxillofacial Silicone Elastomer. Materials 2018, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Andreopoulos, A.G.; Evangelatou, M. Evaluation of Various Reinforcements for Maxillofacial Silicone Elastomers. J. Biomater. Appl. 1994, 8, 344–360. [Google Scholar] [CrossRef]
- Nada, H.E.; Ahmad, M.A.; Moustafa, N.A. Evaluation of Intrinsic Color Stability of Facial Silicone Elastomer Reinforced with Different Nanoparticles. Alex. Dent. J. 2016, 41, 50–54. [Google Scholar] [CrossRef]
- Eleni, P.N.; Krokida, M.; Polyzois, G.; Gettleman, L.; Bisharat, G.I. Effects of Outdoor Weathering on Facial Prosthetic Elastomers. Odontology 2011, 99, 68–76. [Google Scholar] [CrossRef]
- Azeez, Z.; Tukmachi, M.; Hussein Mohammed, D. Effect of Silver-Zinc Zeolite Addition on Mechanical Properties of Maxillofacial Silicone. Int. J. Med. Res. Health Sci. 2018, 7, 19–29. [Google Scholar]
- Liu, Q.; Shao, L.; Fan, H.; Long, Y.; Zhao, N.; Yang, S.; Zhang, X.; Xu, J. Characterization of Maxillofacial Silicone Elastomer Reinforced with Different Hollow Microspheres. J. Mater. Sci. 2015, 50, 3976–3983. [Google Scholar] [CrossRef]
- Cevik, P.; Eraslan, O. Effects of the Addition of Titanium Dioxide and Silaned Silica Nanoparticles on the Mechanical Properties of Maxillofacial Silicones: Nanoparticle Addition to a Maxillofacial Silicone. J. Prosthodont. 2017, 26, 611–615. [Google Scholar] [CrossRef]
- Eleni, P.N.; Perivoliotis, D.; Dragatogiannis, D.A.; Krokida, M.K.; Polyzois, G.L.; Charitidis, C.A.; Ziomas, I.; Gettleman, L. Tensile and Microindentation Properties of Maxillofacial Elastomers after Different Disinfecting Procedures. J. Mech. Behav. Biomed. Mater. 2013, 28, 147–155. [Google Scholar] [CrossRef]
- Al-Harbi, F.A.; Ayad, N.M.; Saber, M.A.; ArRejaie, A.S.; Morgano, S.M. Mechanical Behavior and Color Change of Facial Prosthetic Elastomers after Outdoor Weathering in a Hot and Humid Climate. J. Prosthet. Dent. 2015, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Jose, S. Understanding the Mechanism of Ionic Gelation for Synthesis of Chitosan Nanoparticles Using Qualitative Techniques. Asian J. Pharm. 2010, 4, 148. [Google Scholar] [CrossRef]
- Liu, Q.; Shao, L.Q.; Xiang, H.F.; Zhen, D.; Zhao, N.; Yang, S.G.; Zhang, X.L.; Xu, J. Biomechanical Characterization of a Low Density Silicone Elastomer Filled with Hollow Microspheres for Maxillofacial Prostheses. J. Biomater. Sci. Polym. Ed. 2013, 24, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Sonnahalli, N.K.; Chowdhary, R. Effect of Adding Silver Nanoparticle on Physical and Mechanical Properties of Maxillofacial Silicone Elastomer Material-an in-Vitro Study. J. Prosthodont. Res. 2020, 64, 431–435. [Google Scholar] [CrossRef]
- Eleni, P.N.; Krokida, M.K.; Polyzois, G.L. Effects of Storage in Simulated Skin Secretions on Mechanical Behavior and Color of Polydimethylsiloxanes Elastomers. J. Craniofac. Surg. 2011, 22, 830–836. [Google Scholar] [CrossRef]
- Alsmael, M.; Ali, M. The Effect of Nano Titanium Silicate Addition on Some Properties of Maxillofacial Silicone Material. J. Res. Med. Dent. Sci. 2018, 6, 127–132. [Google Scholar]
- Zayed, S.M.; Alshimy, A.M.; Fahmy, A.E. Effect of Surface Treated Silicon Dioxide Nanoparticles on Some Mechanical Properties of Maxillofacial Silicone Elastomer. Int. J. Biomater. 2014, 2014, 750398. [Google Scholar] [CrossRef]
- Mousa, M.A. Influence of Weather on Hardness and Surface Roughness of Maxillofacial Elastomeric Materials. J. Contemp. Dent. Pract. 2020, 21, 678–682. [Google Scholar] [CrossRef]
- Babu, A.S.; Manju, V.; Gopal, V.K. Effect of Chemical Disinfectants and Accelerated Aging on Maxillofacial Silicone Elastomers: An In Vitro Study. Indian J. Dent. Res. 2018, 29, 67–73. [Google Scholar] [CrossRef]
- Çevik, P. Evaluation of Shore A Hardness of Maxillofacial Silicones: The Effect of Dark Storage and Nanoparticles. Eur. Oral Res. 2018, 52, 99–104. [Google Scholar] [CrossRef]


| Date (2022) | Temperature (°C) | Average Humidity (%) | Pressure (m bar) | ||
|---|---|---|---|---|---|
| Max | Min | Average | |||
| June | 37.4 | 24.6 | 31.0 | 28.3 | 910.1 |
| July | 40.7 | 26.8 | 33.7 | 24.2 | 906.4 |
| August | 42.1 | 28.0 | 35.0 | 23.9 | 908.5 |
| September | 36.9 | 23.3 | 30.1 | 29.8 | 912.3 |
| October | 30.5 | 18.0 | 24.2 | 42.8 | 917.6 |
| November | 20.0 | 9.8 | 14.9 | 62.1 | 919.2 |
| December | 14.7 | 6.6 | 10.7 | 65.5 | 920.2 |
| Condition | 1 wt.% Chitosan–TiO2 | 1 wt.% TC | 2 wt.% TiO2 | 3wt.% Chitosan | Control (Zero Nano) | p-Value |
|---|---|---|---|---|---|---|
| Baseline (No Weathering) | 9.88 ± 0.94 | 9.53 ± 0.97 | 9.65 ± 0.92 | 8.35 ± 0.57 | 9.35 ± 0.60 | 0.07 |
| Sweat (6 Months) | 10.23 ± 0.70 a | 8.76 ± 0.92 | 9.88 ± 0.74 | 9.18 ± 0.57 | 9.88 ± 0.97 | 0.05 |
| Sebum (6 Months) | 10.53 ± 1.81 | 10.76 ± 0.68 * | 10.53 ± 1.05 | 9.82 ± 0.90 | 10.53 ± 1.46 | 0.80 |
| UV Weathering (720 h) | 11.29 ± 1.01 abcd | 8.94 ± 0.53 | 9.71 ± 1.16 | 9.00 ± 0.26 | 8.88 ± 0.70 | 0.00 |
| Outdoor Weathering (6 Months) | 11.29 ± 0.85 abcd | 8.71 ± 0.79 | 8.94 ± 0.64 | 8.76 ± 0.92 | 8.53 ± 1.98 | 0.005 |
| Antibacterial (30 h) | 10.35 ± 1.13 | 9.47 ± 1.20 | 9.71 ± 1.06 | 8.82 ± 0.91 | 9.76 ± 1.52 | 0.386 |
| p-Value | 0.29 | 0.01 | 0.24 | 0.08 | 0.21 |
| Condition | 1 wt.% Chitosan–TiO2 | 1 wt.% TC | 2 wt.% TiO2 | 3 wt.% Chitosan | Control (Zero Nano) | p-Value |
|---|---|---|---|---|---|---|
| Baseline (No Weathering) | 422.63 ± 55.88 ab | 587.6 ± 39.49 c | 602.35 ± 72.90 c | 415.56 ± 37.40 | 628.65 ± 110.26 | 0.00 |
| Sweat (6 Months) | 373.21 ± 35.97 abd | 575.63 ± 31.16 cd | 526.09 ± 64.11 c | 377.32 ± 42.47 d | 461.14 ± 43.59 * | 0.00 |
| Sebum (6 Months) | 389.03 ± 79.51 ab | 519.38 ± 27.36 c | 541.24 ± 34.70 c | 379.12 ± 41.26 d | 485.23 ± 65.54 * | 0.00 |
| UV Weathering (720 h) | 418.32 ± 42.85 a | 582.05 ± 60.14 | 489.51 ± 60.40 | 445.79 ± 84.47 | 516.44 ± 49.11 | 0.004 |
| Outdoor Weathering (6 Months) | 410.14 ± 38.10 a | 577.95 ± 55.13 d | 504.90 ± 86.85 | 448.00 ± 87.70 | 426.92 ± 108.11 * | 0.02 |
| Antibacterial (30 h) | 409.87 ± 44.42 abd | 551.15 ± 72.00 c | 574.82 ± 90.06 c | 410.61 ± 84.47 d | 573.75 ± 112.04 | 0.002 |
| p-Value | 0.65 | 0.29 | 0.15 | 0.27 | 0.01 |
| Condition | 1 wt.% Chitosan–TiO2 | 1 wt.% TC | 2 wt.% TiO2 | 3 wt.% Chitosan | Control (Zero Nano) | p-Value |
|---|---|---|---|---|---|---|
| Baseline (No Weathering) | 29.33 ± 3.90 | 27.33 ± 4.62 | 33.33 ± 1.57 c | 26.22 ± 1.69 | 30.44 ± 2.30 | 0.01 |
| Sweat (6 Months) | 29.56 ± 4.49 | 31.78 ± 4.35 | 34.22 ± 5.18 c | 26.67 ± 2.08 d | 34.22 ± 4.54 | 0.05 |
| Sebum (6 Months) | 33.33 ± 7.20 | 33.56 ± 5.35 | 30.44 ± 4.94 | 33.33 ± 3.42 * | 33.78 ± 7.44 | 0.89 |
| UV Weathering (720 h) | 26.44 ± 3.96 | 32.00 ± 6.87 | 29.33 ± 3.39 | 26.00 ± 2.56 | 29.11 ± 4.61 | 0.253 |
| Outdoor Weathering (6 Months) | 34.44 ± 2.36 *c | 35.56 ± 7.49 | 33.33 ± 3.85 | 27.11 ± 2.56 | 34.22 ± 4.80 | 0.064 |
| Antibacterial (30 h) | 36.44 ± 4.47 * | 31.11 ± 5.72 | 30.00 ± 5.39 | 28.22 ± 2.79 | 28.89 ± 8.28 | 0.191 |
| p-Value | 0.02 | 0.39 | 0.34 | 0.00 | 0.43 |
| Condition | 1 wt.% Chitosan–TiO2 | 1 wt.% TC | 2 wt.% TiO2 | 3 wt.% Chitosan | Control (Zero Nano) | p-Value |
|---|---|---|---|---|---|---|
| Baseline (No Weathering) | 36.62 ± 2.53 | 36.19 ± 0.90 | 35.75 ± 0.97 | 37.05 ± 2.30 | 34.75 ± 1.49 | 0.325 |
| Sweat (6 Months) | 40.42 ± 2.62 a | 36.42 ± 1.68 c | 37.57 ± 1.52 | 40.13 ± 1.81 * | 37.1 ± 1.34 * | 0.006 |
| Sebum (6 Months) | 40.43 ± 1.79 a | 35.96 ± 1.18 bcd | 38.41 ± 0.52 * | 39.25 ± 0.79 | 39.556 ± 0.89 * | 0.000 |
| UV Weathering (720 h) | 38.50 ± 2.70 | 37.65 ± 1.96 | 38.88 ± 1.21 * | 40.84 ± 1.62 *d | 36.82 ± 0.65 * | 0.021 |
| Outdoor Weathering (6 Months) | 37.36 ± 2.64 c | 37.58 ± 0.50 | 38.6 ± 0.59 * | 40.76 ± 2.61 * | 38.23 ± 1.12 * | 0.046 |
| Antibacterial (30 h) | 39.43 ± 1.91 ad | 36.53 ± 1.12 c | 38.4 ± 0.80 * | 39.4 ± 1.04 d | 36.73 ± 0.95 * | 0.002 |
| p-Value | 0.088 | 0.227 | 0.000 | 0.03 | 0.000 |
| Condition | 1 wt.% Chitosan–TiO2 | 1 wt.% TC | 2 wt.% TiO2 | 3 wt.% Chitosan | Control (Zero Nano) | p-Value |
|---|---|---|---|---|---|---|
| Baseline (No Weathering) | 0.27 ± 0.001 abcd | 0.27 ± 0.0008 cd | 0.27 ± 0.0008 cd | 0.68 ± 0.001 d | 0.64 ± 0.0008 | 0.00 |
| Sweat (6 Months) | 0.34 ± 0.0008 *abcd | 0.21 ± 0.0008 *bcd | 0.28 ± 0.0008 *cd | 0.64 ± 0.0005 *d | 0.27 ± 0.0008 * | 0.00 |
| Sebum (6 Months) | 0.80 ± 0.0008 *abcd | 0.24 ± 0.0008 *bcd | 0.22 ± 0.0004 *cd | 0.60 ± 0.0008 *d | 0.59 ± 0.0005 * | 0.00 |
| UV Weathering (720 h) | 0.48 ± 0.0005 *abcd | 0.20 ± 0.0007 *bcd | 0.26 ± 0.0005 *cd | 0.42 ± 0.0008 *d | 0.60 ± 0.0008 * | 0.00 |
| Outdoor Weathering (6 Months) | 0.24 ± 0.0008 *abcd | 0.29 ± 0.005 *bcd | 0.22 ± 0.0005 *cd | 0.61 ± 0.0008 *d | 0.68 ± 0.0005 * | 0.00 |
| Antibacterial (30 h) | 0.47 ± 0.0005 *abcd | 0.24 ± 0.0008 *bcd | 0.28 ± 0.0005 *cd | 0.49 ± 0.0008 *d | 0.44 ± 0.0008 * | 0.00 |
| p-Value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Kadi, F.K.; Adbulkareem, J.F.; Azhdar, B.A. Evaluation of the Mechanical and Physical Properties of Maxillofacial Silicone Type A-2186 Impregnated with a Hybrid Chitosan–TiO2 Nanocomposite Subjected to Different Accelerated Aging Conditions. Biomimetics 2023, 8, 539. https://doi.org/10.3390/biomimetics8070539
Al-Kadi FK, Adbulkareem JF, Azhdar BA. Evaluation of the Mechanical and Physical Properties of Maxillofacial Silicone Type A-2186 Impregnated with a Hybrid Chitosan–TiO2 Nanocomposite Subjected to Different Accelerated Aging Conditions. Biomimetics. 2023; 8(7):539. https://doi.org/10.3390/biomimetics8070539
Chicago/Turabian StyleAl-Kadi, Faten K., Jwan Fateh Adbulkareem, and Bruska A. Azhdar. 2023. "Evaluation of the Mechanical and Physical Properties of Maxillofacial Silicone Type A-2186 Impregnated with a Hybrid Chitosan–TiO2 Nanocomposite Subjected to Different Accelerated Aging Conditions" Biomimetics 8, no. 7: 539. https://doi.org/10.3390/biomimetics8070539