Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases
Abstract
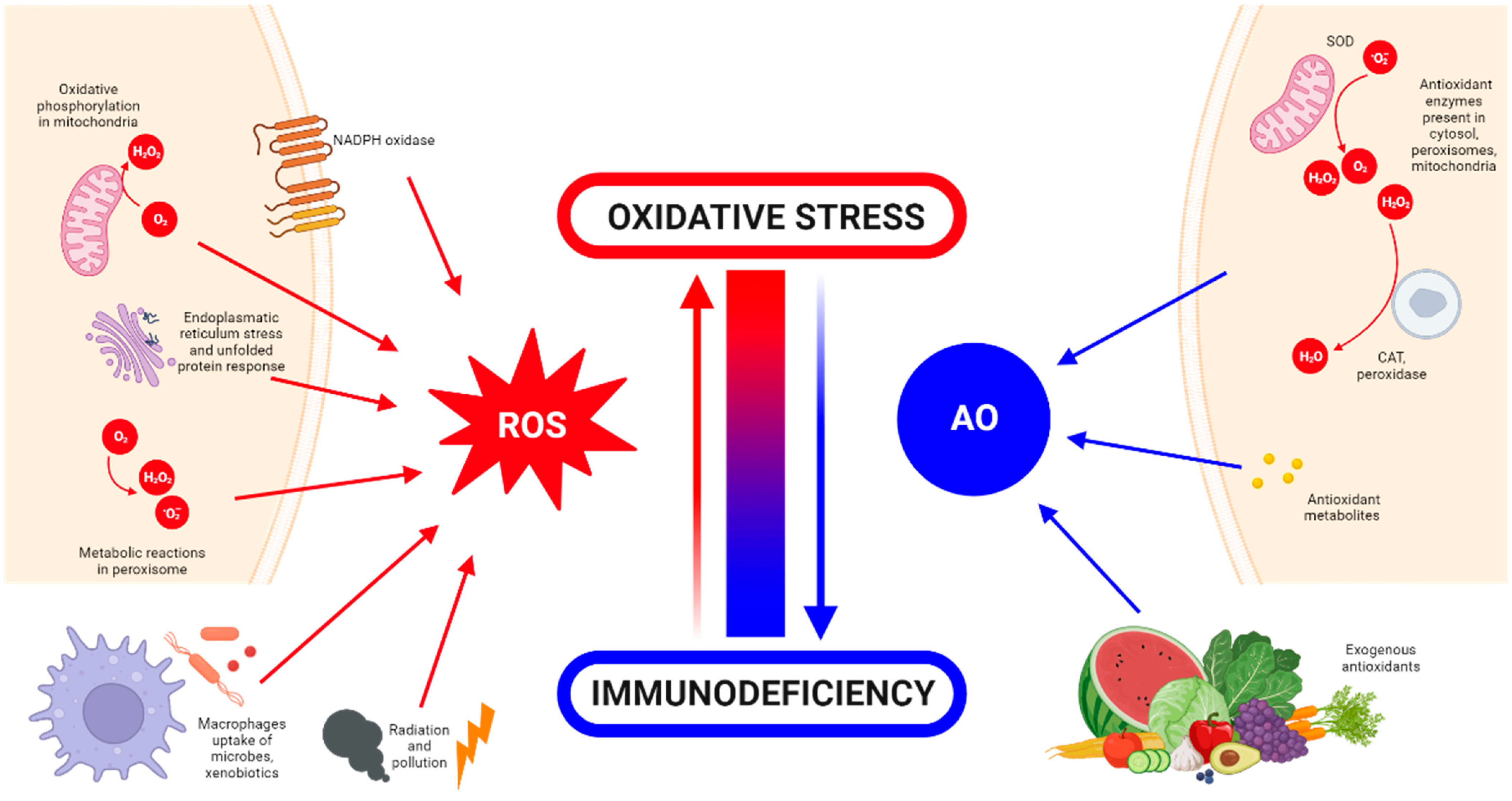
:1. Introduction
What Is Oxidative Stress?
2. Detrimental Effects of ROS
3. Oxidative Stress and Tissue Engineering
4. Biomaterials Employed for Oxidative Stress Diseases
4.1. Wound Healing
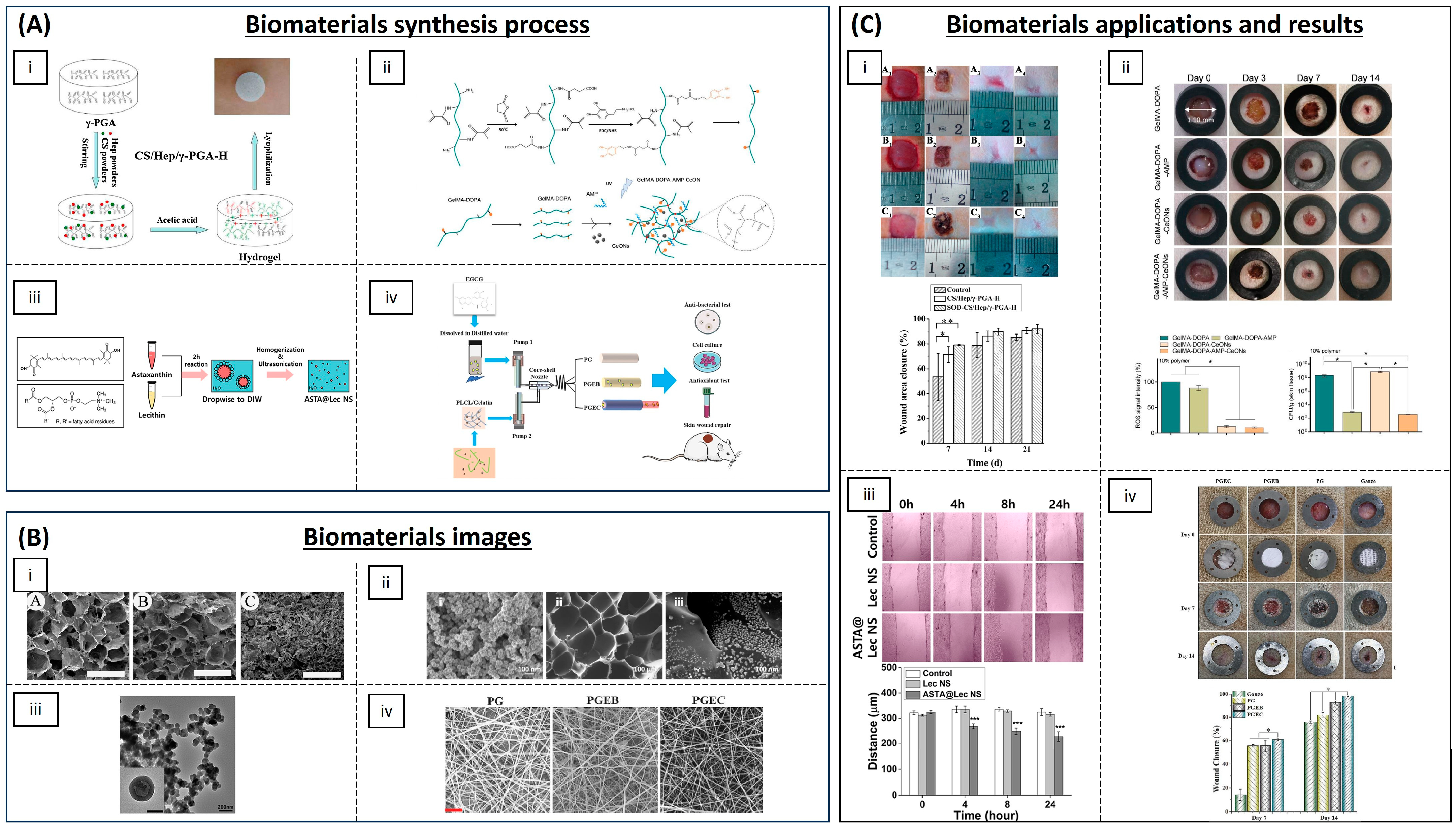
| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Hydrogels | Chitosan, heparin and poly(γ-glutamic acid) | SOD | Diabetic rat model | Accelerating re-epithelialization and collagen deposition | [61] |
| Poly(N-isopropyl-acrylamide)/poly(γ-glutamic acid) | SOD | Diabetic rat model | Antioxidant activity and high wound closure rate | [62] | |
| GelMA with dopamine motifs | Cerium oxide NPs and AMP | Rats | (ROS) scavenging and antibacterial properties | [65] | |
| SBMA, CBMA and HEMA | Cerium oxide and microRNA-146 | Mice | Accelerating wound healing | [66] | |
| Chitosan-PEG | Silver NPs | Diabetic rabbits | Antioxidant and antibacterial activity | [67] | |
| Chitosan | Eugenol | - | Antioxidant activity | [71] | |
| Chitosan-g-polyaniline and benzaldehyde | PEG-co-poly(glycerol sebacate) | Mice | Good self-healing, electro-activity and free radical scavenging capacity | [72] | |
| Carboxybetaine dextran and sulphobetaine dextran | - | Mice | Self-healing, antioxidative and antifouling properties | [73] | |
| Alginate | Edudragit NPs and Edavarone | Mice | Wound healing promoting and efficient free radical scavenging | [74] | |
| Polyvinyl alcohol | Mupirocin and GM-CSF | Diabetic mice | Antibacterial activity and wound closure promoting | [75] | |
| Silk fibroin | Melanin and berberine | Diabetic rat | Re-epithelialization and wound repair promoting | [76] | |
| Inorganic NPs | Prussian Blue NPs | - | Mice | Antioxidant and collagen deposition | [64] |
| Liposomal particles | Lecithin nano-liposol | astaxanthin | NIH/3T3 cells | ROS scavenging and antioxidant capacity | [77] |
| Polymeric matrix | Cellulose | Nanochitosan dust | Human gingival cells | Antioxidant and antimicrobial activity | [70] |
| PLA | Asiatic acid | Diabetic mouse model | Accelerating re-epithelization, angiogenesis and ECM formation | [79] | |
| Poly(L-Lactic-co-caprolactone) (PLCL) | EGCG | Rat liver trauma model | Promoting wound healing and tissue organization | [81] |
4.2. Neurodegenerative Diseases
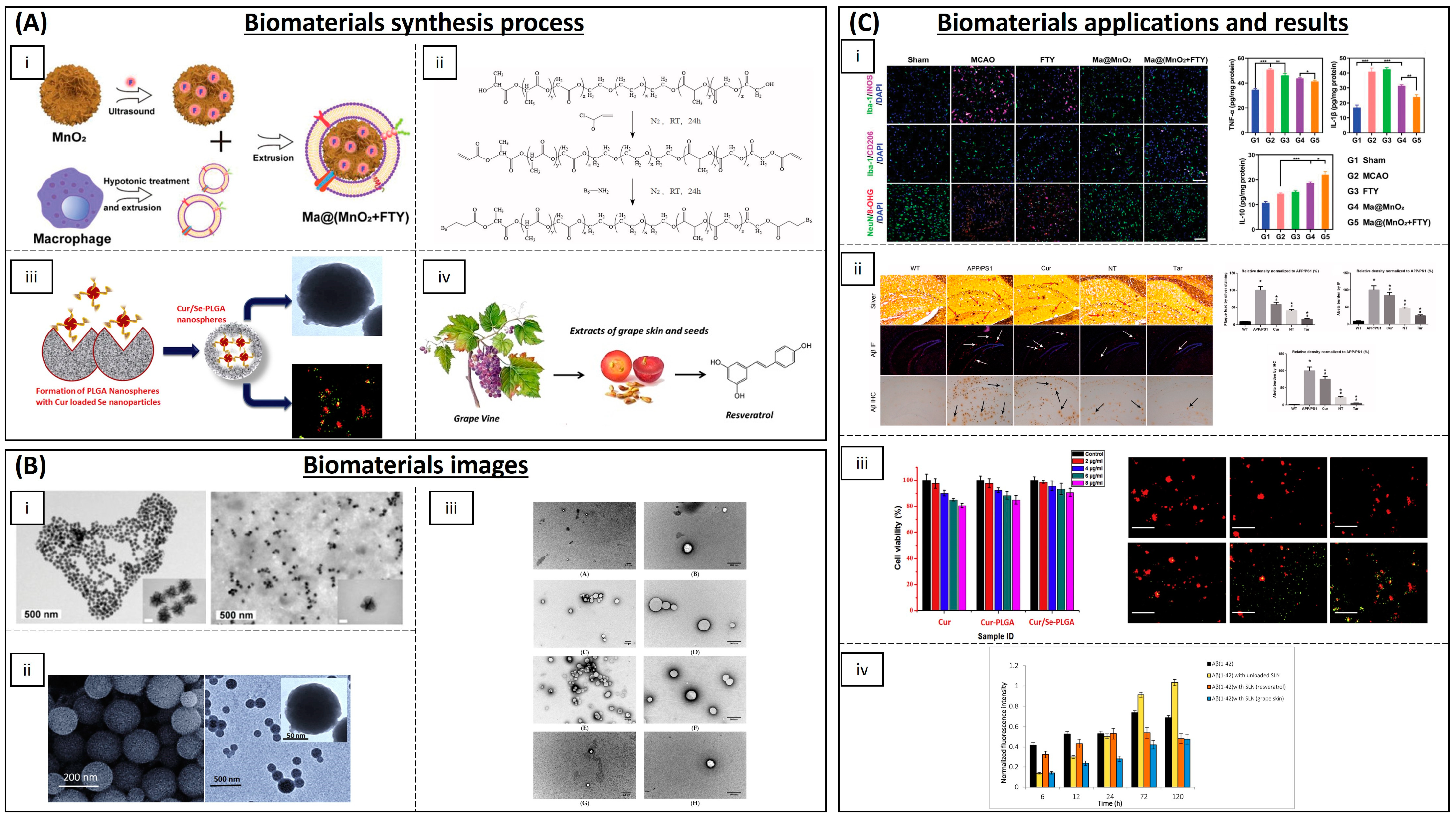
| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Inorganic NPs | Cerium oxide (CeONPs) | - | P12 neuronal cells | Anti-amyloid aggregation, antioxidant activity | [93,94,95] |
| Ceria/Polyoxometalates | - | P12 neuronal cells | Inhibition of Aβ-induced microglial cell activation | [96] | |
| Iron oxide (IONPs) | - | Drosophila Alzheimer’s disease model | Anti-ROS activity | [97] | |
| Yttrium oxide | - | P12 neuronal cells | Reduction in oxidative stress and apoptosis | [98] | |
| Yttrium NPs and CeONPs | - | Wistar rats | Reduction in oxidative stress | [99] | |
| MnO2 | Fingolimod | Mice | ROS and microglia pro-inflammatory state reduction | [100] | |
| Selenium NPs | Resveratrol | AD rat model | Anti-inflammatory activity | [116] | |
| Carbon materials | Partially reduced graphene oxide | - | Mouse-substantia-nigra-derived dopaminergic cell line | Prevention of dopaminergic neuron loss and α-syn depletion | [101] |
| PEG-HCCs | - | Brain endothelial cell line and primary cortical neuron cells | Protection against hydrogen peroxide | [102] | |
| Polymeric NPs | (PLGA-PEG) and B6 peptide | Curcumin | APP/PS1 Al transgenic mice | Improvement in spatial learning and memory | [104] |
| PLGA | Curcumin | Rats | Neuronal differentiation | [105,106,107,108,109] | |
| PEGylated PLGA NPs | Ascorbic acid and EGCG | Mice | Neuroinflammation and neuronal loss | [119] | |
| Solid lipid NPs | Glycerol behenate | Curcumin | AD mouse model | Cellular damage reduction in brain | [111] |
| Cetylpalmitate and OX26 mAb | Resveratrol | Human brain-like endothelial cells | Inhibition of protein aggregation | [117] | |
| Vitamin E and sefsol | Resveratrol | In vitro | Increasing the levels of GSH and SOD | [118] | |
| Unspecified | EGCG | Rat | Increasing bioavailability of EGCG | [120] |
4.3. Cardiovascular Diseases
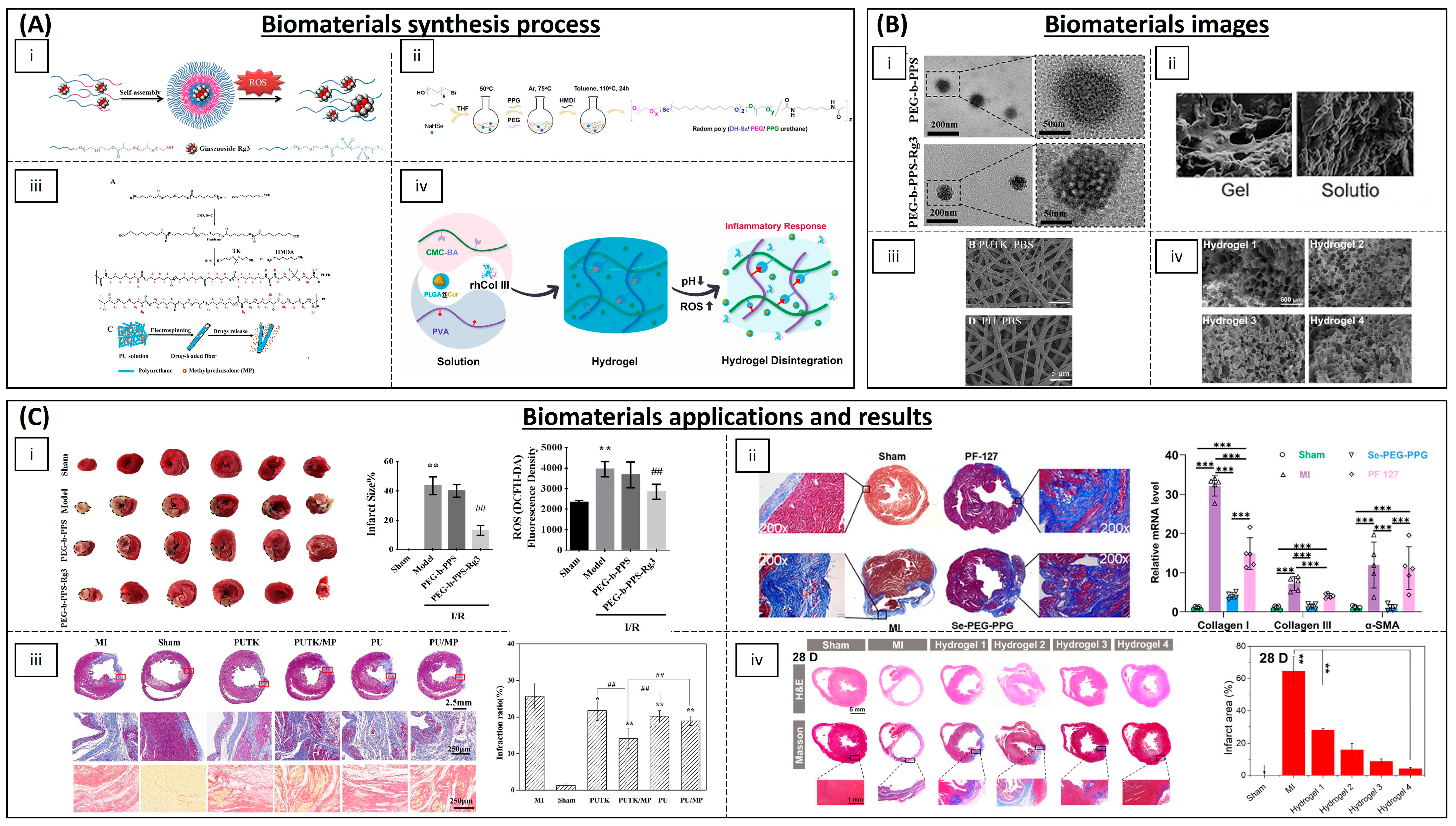
| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Polymeric NPs | Copolyoxalate | Vanillyl alcohol | I/R mouse model | Reduction in ROS | [130] |
| PEG and poly-(propylene sulphide) | Ginsenoside Rg3 | I/R rat model | Inhibition of oxidative stress, inflammation and fibrosis | [131] | |
| (PLL-PEG-PLL) | - | I/R rat model | Decreased oxidative stress and promoted myocardial function | [134] | |
| PLGA | Quercetin | H9C2 cells | Increased quercetin bioavailability | [135] | |
| PLGA | Resveratrol | H9C2 cells | ROS scavenging | [136] | |
| PLGA | Resveratrol | Rat | Preventing myocardial necrosis | [137] | |
| PLGA | Pioglitazone | Mouse and porcine model | Cardioprotection | [138] | |
| PLGA | Irbesartan | I/R mouse model | Anti-inflammatory activity and reduced infarct size | [139] | |
| PLGA | Mdivi-1 | I/R mouse model | Cardioprotection against I/R | [140] | |
| PLGA | CoQ-10 | Mice | Increased bioavailability | [142] | |
| PGMA | AID and cur/res | Rat | Decreased oxidative stress | [145] | |
| Solid lipid NPs | PEG-modified solid lipid NPs | Baicalin, schisandrin B | Rat | Reduction in the infarction size | [132,133] |
| Egg phosphatidylcholine, cholesterol, PEG2000-DSPE | CoQ-10 | I/R rabbit model | Limiting the fraction of damaged myocardium | [143] | |
| Inorganic NPs | Ceria NPs | - | Murine cardiac progenitor cells | Protecting cardiac progenitor cells | [147] |
| AuNP-MIBI | - | I/R rat model | Reduction in inflammation | [150] | |
| Inorganic fibres | Polyurethane | Methylprednisolone | Rat | Reconstruction of cardiac function | [152] |
| Hydrogels | Modified chitosan (CS-B-NO) | NO | I/R mouse model | Attenuation of cardiac damage | [154] |
| PMNT-PEG-PMNT | - | Mouse | ROS scavenging | [155] | |
| PVA/Dex | Astaxanthin | Rat | Reduction in oxidative stress | [156] | |
| Chitosan | α-tocopherol | Neonatal rat cardiomyocytes | Suppression of oxidative stress | [157] | |
| Chitosan chloride–glutathione | - | Neonatal rat cardiomyocytes | Scavenging superoxide anion and hydroxyl radical | [158] | |
| Chitosan–vitamin E | - | Neonatal rat cardiomyocytes | Reducing ROS | [159] | |
| Chitosan | Ferulic acid | Rabbit | Protection from oxidative stress | [160] | |
| CMC-BA | Curcumin, collagen III | Rat | Anti-inflammatory | [161] | |
| Alginate | Fullerenol nps | Brown adipose-derived stem cells | Scavenging the superoxide anion and hydroxyl radicals | [162] | |
| N-isopropyl acrylamide and methoxy-PEG methacrylate | - | Sheep | Increased contractile function and decreased ROS | [163] | |
| Poly(DH-SE/PEG/PPG urethane | - | Mouse | Inhibition of inflammation and fibrosis | [164] | |
| GO-IG | MSCs | WJ-MSCs and rat cardiomyocytes | Decreasing the oxidative damage | [166] | |
| Hyaluronic acid and 2-hydroxy-β-cyclodextrin | Resveratrol and MSCs | Rat | Proangiogenic, anti-inflammatory and anti-apoptotic activity | [167] | |
| Polymeric scaffolds | Cellulose | Statin and heparin | - | Anti-thrombogenic and anti-inflammatory functions | [168] |
| PLA/PVA | TEMPOL, rapamycin | Porcine model | Favours endothelialization and mitigates local inflammation | [169] |
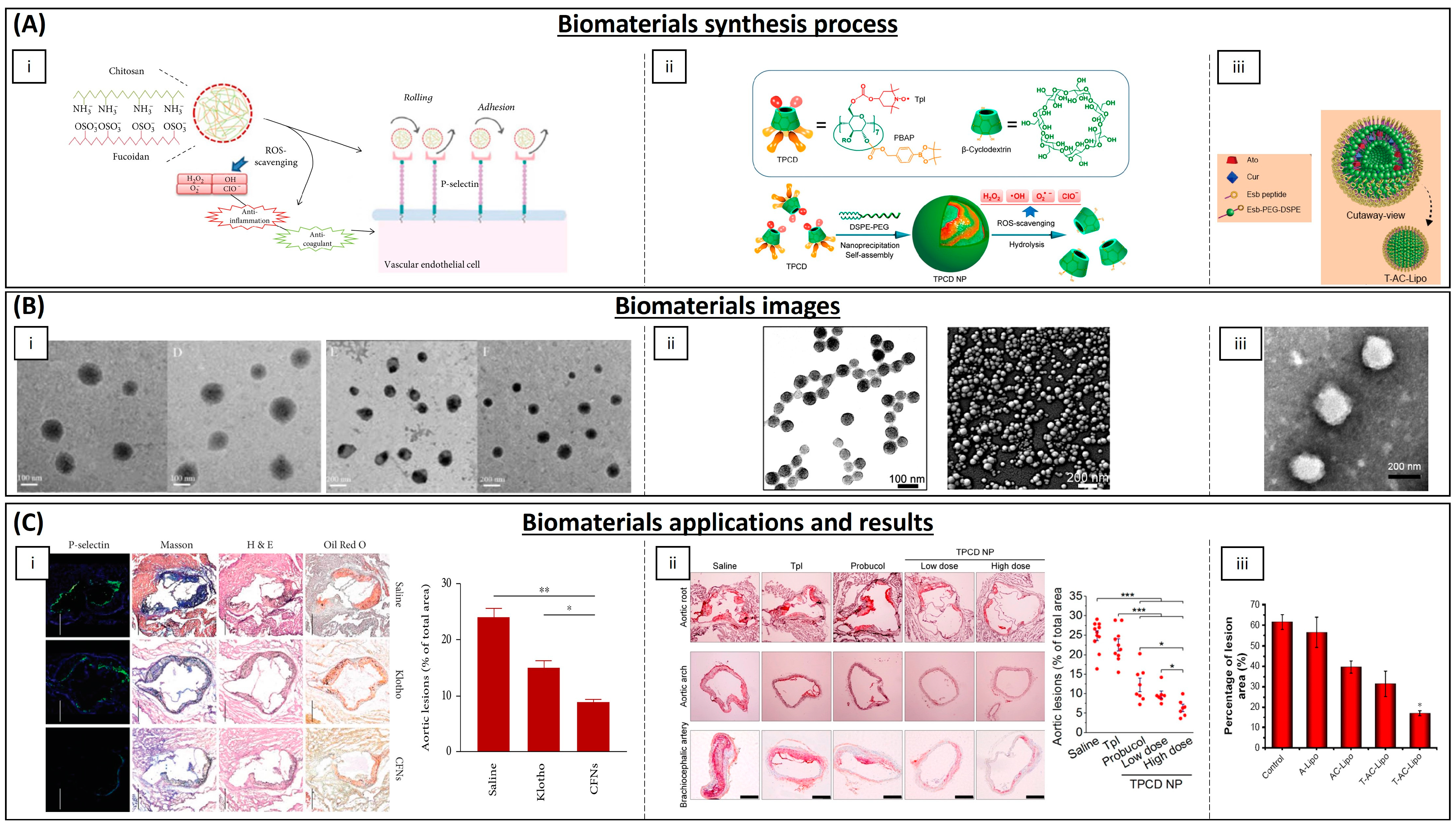
| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Polymeric NPs | Chitosan | EGCG | THP-1 cells | Decreasing cholesterol content and chemoattractant protein expression in macrophages | [179] |
| PLGA | Curcumin–bioperine | THP-1 cells | Anti-inflammatory activity | [180] | |
| Poly lactide–glycolidechitin | Diosmin | Rat | Downregulation of inflammatory molecules levels | [182] | |
| Chitosan | Selenium | Mice | Alleviation of early atherosclerotic lesions | [183] | |
| Chitosan–fucoidan | - | Mice | Suppression of local oxidative stress and inflammation | [185] | |
| β-cyclodextrin | Tempol, phenylboronic acid pinacol ester | Mice | Antioxidant and anti-inflammatory properties | [186] | |
| Lipid NPs | Phosphatidylcholines | EGCG and α-tocopherol | Mice | Smaller lesion surface areas on aortic arches | [178] |
| Cholesterol, DPPC and Mal-PEG2000-DSPE | Atorvastatin calcium and curcumin | Mice | Reduction in plasma lipid levels | [181] | |
| Inorganic NPs | Iron oxide | Spinacia oleracea | Mice | Increased activity of SOD and catalase enzymes | [184] |
4.4. Bone Diseases

| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Hydrogel | Poloxamer 407 and selenium | Silibinin | Rat | Bone regeneration and mineralization | [201] |
| EGCG, 3-acrylamido phenylboronic acid and acrylamide | MSCs | Rabbit | Antioxidant and anti-inflammatory activity, and improved osteogenesis | [220] | |
| gelatine methacryloyl–dopamine | Melatonin | Rat | Promotion of osteogenesis and improved bone quality | [221] | |
| Polymers | Silica NPs | Cerium oxide | RAW264.7 and MC3T3-E1 cells | Antioxidant capability and stimulated cell proliferation and osteogenic responses | [202] |
| Chitosan NPs | Shilajit water extract | Rat | Antioxidant and anti-inflammatory activity | [205] | |
| Fe2O3@PSC NPs | - | Mice | ROS scavenging, pro-osteogenic and inhibition of osteoclast differentiation | [206] | |
| Lycopene NPs | - | BMSCs | Pro-osteoblast differentiation | [211] | |
| PLGA NPs | Tocotrienol | Rat | Improvement in bone strength and mineralization | [207] | |
| polycaprolactone/gelatine NPs | Polaprezinc | Rat | Promotion of bone formation | [208] | |
| Titanium dioxide nanotubes | - | Rat calvarial osteoblasts | Improvement in osteoblast adhesion and osteogenic differentiation | [218] | |
| Inorganic NPs | Selenium | - | hESC-derived hMSCs | Increased antioxidant levels | [209] |
| Cerium oxide | - | MC3T3-E1 cells | Antioxidant activity | [203] | |
| Iron oxide | - | Mice | Antioxidant, osteogenic differentiation and inhibition of osteoclast differentiation | [212] | |
| Platinum | - | Mice | Decreased osteoclast activity levels and antioxidant capacity | [213] | |
| Manganese | β-tricalcium | Rat | Promotion of the differentiation of osteoblasts and accelerate bone regeneration | [214] | |
| Manganese oxide | Zn2+ | MC3T3-E1 cells | Catalase-like activity | [216] |
5. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Juan, C.; Pérez de la Lastra, J.; Plou, F.J.; Pérez-Lebeña, E.; Reinbothe, S. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Jaimes, E.A. Mitochondria and Reactive Oxygen Species: Physiology and Pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306–6344. [Google Scholar] [CrossRef] [PubMed]
- Magnani, F.; Mattevi, A. Structure and Mechanisms of ROS Generation by NADPH Oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D.; Pinchuk, I. Oxidative Stress, the Term and the Concept. Biochem. Biophys. Res. Commun. 2015, 461, 441–444. [Google Scholar] [CrossRef]
- Patten, D.A.; Lafleur, V.N.; Robitaille, G.A.; Chan, D.A.; Giaccia, A.J.; Richard, D.E. Hypoxia-Inducible Factor-1 Activation in Nonhypoxic Conditions: The Essential Role of Mitochondrial-Derived Reactive Oxygen Species. Mol. Biol. Cell 2010, 21, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.M.; Persechini, P.M.; Ojcius, D.M. ATP Activates a Reactive Oxygen Species-Dependent Oxidative Stress Response and Secretion of Proinflammatory Cytokines in Macrophages. J. Biol. Chem. 2007, 282, 2871–2879. [Google Scholar] [CrossRef]
- Gao, Q. Oxidative Stress and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 179–198. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Tal, M.C.; Sasai, M.; Lee, H.K.; Yordy, B.; Shadel, G.S.; Iwasaki, A. Absence of Autophagy Results in Reactive Oxygen Species-Dependent Amplification of RLR Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 2770–2775. [Google Scholar] [CrossRef]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR Signalling Augments Macrophage Bactericidal Activity through Mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef]
- Guo, C.; Dong, G.; Liang, X.; Dong, Z. NOX2-Dependent Regulation of Inflammation. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis versus Free Radical Scavenging In Vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Allen, R.G. Oxidative Stress as a Causal Factor in Differentiation and Aging: A Unifying Hypothesis. Exp. Gerontol. 1990, 25, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA Damage and Reactive Oxygen Species in Neurodegenerative Disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Abuja, P.M.; Albertini, R. Methods for Monitoring Oxidative Stress, Lipid Peroxidation and Oxidation Resistance of Lipoproteins. Clin. Chim. Acta 2001, 306, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho-junior, H.J.; Marzetti, E. Cell Death and Inflammation: The Role of Mitochondria in Health and Disease. Cells 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Env. Sci. Health C Env. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, M.; Mori, M.P.; Souza-Pinto, N.C. de Formation and Repair of Oxidative Damage in the Mitochondrial DNA. Mitochondrion 2014, 17, 164–181. [Google Scholar] [CrossRef]
- Poetsch, A.R. The Genomics of Oxidative DNA Damage, Repair, and Resulting Mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Jelic, M.; Mandic, A.; Maricic, S.; Srdjenovic, B. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.; Georgakilas, A.G.; Panayiotidis, M.I. Oxidative Stress, DNA Methylation and Carcinogenesis. Cancer Lett. 2008, 266, 6–11. [Google Scholar] [CrossRef]
- Ramana, K.; Srivastava, S.; Singhal, S.S. Lipid Peroxidation Products in Human Health and Disease. Oxid. Med. Cell. Longev. 2013, 2013, 583438. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.H.; Chaudhary, S.; Kim, M.H. Molecular Dynamic Simulations of Oxidized Skin Lipid Bilayer and Permeability of Reactive Oxygen Species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Feric, N.T.; Pallotta, I.; Singh, R.; Bogdanowicz, D.R.; Gustilo, M.M.; Chaudhary, K.W.; Willette, R.N.; Chendrimada, T.P.; Xu, X.; Graziano, M.P.; et al. Engineered Cardiac Tissues Generated in the Biowire II: A Platform for Human-Based Drug Discovery. Toxicol. Sci. 2019, 172, 89–97. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Mastikhina, O.; Moon, B.U.; Williams, K.; Hatkar, R.; Gustafson, D.; Mourad, O.; Sun, X.; Koo, M.; Lam, A.Y.L.; Sun, Y.; et al. Human Cardiac Fibrosis-on-a-Chip Model Recapitulates Disease Hallmarks and Can Serve as a Platform for Drug Testing. Biomaterials 2020, 233, 119741. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Desai, R.M.; Joly, P.; Li, J.; Bagrodia, R.K.; Lewin, S.A.; Joshi, N.S.; Mooney, D.J. Click-Crosslinked Injectable Gelatin Hydrogels. Adv. Healthc. Mater. 2016, 5, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Ott, H.C. Organ Engineering Based on Decellularized Matrix Scaffolds. Trends Mol. Med. 2011, 17, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Belviso, I.; Romano, V.; Sacco, A.M.; Ricci, G.; Massai, D.; Cammarota, M.; Catizone, A.; Schiraldi, C.; Nurzynska, D.; Terzini, M.; et al. Decellularized Human Dermal Matrix as a Biological Scaffold for Cardiac Repair and Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 229. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef]
- Montero, P.; Flandes, M.; Musquiz, S.; Araluce, M.P.; Plano, D.; Sanmartín, C.; Gavira, J.J.; Mazo, M.M.; Prosper, F. Cells, Materials and Fabrication Processes for Cardiac Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 955. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer-Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Montero-Calle, P.; Flandes-Iparraguirre, M.; Mountris, K.; de la Nava, A.S.; Laita, N.; Rosales, R.M.; Iglesias-García, O.; de-Juan-Pardo, E.M.; Atienza, F.; Fernández-Santos, M.E.; et al. Fabrication of Human Myocardium Using Multidimensional Modelling of Engineered Tissues. Biofabrication 2022, 14, 045017. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Nikkhah, M.; Shin, S.R.; Annabi, N.; Masoumi, N.; Gaharwar, A.K.; Camci-Unal, G.; Khademhosseini, A. PGS:Gelatin Nanofibrous Scaffolds with Tunable Mechanical and Structural Properties for Engineering Cardiac Tissues. Biomaterials 2013, 34, 6355–6366. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S.; Scalia, A.C.; Cochis, A.; Rimondini, L.; Cruz-Maya, I.; Guarino, V.; Varesano, A.; Vineis, C. Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers 2020, 12, 2896. [Google Scholar] [CrossRef]
- Bas, O.; De-Juan-Pardo, E.M.; Chhaya, M.P.; Wunner, F.M.; Jeon, J.E.; Klein, T.J.; Hutmacher, D.W. Enhancing Structural Integrity of Hydrogels by Using Highly Organised Melt Electrospun Fibre Constructs. Eur. Polym. J. 2015, 72, 451–463. [Google Scholar] [CrossRef]
- Shakiba, N.; Zandstra, P.W. Engineering Cell Fitness: Lessons for Regenerative Medicine. Curr. Opin. Biotechnol. 2017, 47, 7–15. [Google Scholar] [CrossRef]
- Marrazzo, P.; O’leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef]
- Gamna, F.; Yamaguchi, S.; Cochis, A.; Ferraris, S.; Kumar, A.; Rimondini, L.; Spriano, S. Conferring Antioxidant Activity to an Antibacterial and Bioactive Titanium Surface through the Grafting of a Natural Extract. Nanomaterials 2023, 13, 479. [Google Scholar] [CrossRef]
- Bommakanti, V.; Banerjee, M.; Shah, D.; Manisha, K.; Sri, K.; Banerjee, S. An Overview of Synthesis, Characterization, Applications and Associated Adverse Effects of Bioactive Nanoparticles. Environ. Res. 2022, 214, 113919. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Xu, G.M.; Zhang, G.J.; Liu, J.R.; Wu, Y.M.; Gao, L.G.; Yang, Y.; Chang, Z.S.; Yao, C.W. Low-Temperature Plasma Promotes Fibroblast Proliferation in Wound Healing by ROS-Activated NF-ΚB Signaling Pathway. Curr. Med. Sci. 2018, 38, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.A.; Hee Hong, S.; Hee Lee, M.; Kwon, B.J.; Mi Seon, G.; Sung Kim, M.; Kim, D.; Chang Nam, K.; Park, J.C. Effective Stacking and Transplantation of Stem Cell Sheets Using Exogenous ROS-Producing Film for Accelerated Wound Healing. Acta Biomater. 2019, 95, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, 1801210. [Google Scholar] [CrossRef]
- Zhu, Y.; Hoshi, R.; Chen, S.; Yi, J.; Duan, C.; Galiano, R.D.; Zhang, H.F.; Ameer, G.A. Sustained Release of Stromal Cell Derived Factor-1 from an Antioxidant Thermoresponsive Hydrogel Enhances Dermal Wound Healing in Diabetes. J. Control. Release 2016, 238, 114–122. [Google Scholar] [CrossRef]
- Helary, C.; Abed, A.; Mosser, G.; Louedec, L.; Letourneur, D.; Coradin, T.; Giraud-Guille, M.M.; Meddahi-Pellé, A. Evaluation of Dense Collagen Matrices as Medicated Wound Dressing for the Treatment of Cutaneous Chronic Wounds. Biomater. Sci. 2015, 3, 373–382. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Gong, C.Y.; Wu, Q.J.; Wang, Y.J.; Zhang, D.D.; Luo, F.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. A Biodegradable Hydrogel System Containing Curcumin Encapsulated in Micelles for Cutaneous Wound Healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A Composite Hydrogel of Chitosan/Heparin/Poly (γ-Glutamic Acid) Loaded with Superoxide Dismutase for Wound Healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhuang, H.; Hao, Y.; Zhang, L.; Yang, Q.; Liu, Y.; Qi, C.; Wang, S. Poly(N-Isopropyl-Acrylamide)/Poly(γ-Glutamic Acid) Thermo-Sensitive Hydrogels Loaded with Superoxide Dismutase for Wound Dressing Application. Int. J. Nanomed. 2020, 15, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, S.; Yin, J.J.; He, W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Jeon, J.; Lee, M.S.; Yang, H.S.; Tae, G. Antioxidant and Anti-Inflammatory Activities of Prussian Blue Nanozyme Promotes Full-Thickness Skin Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111596. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Shi, Z.; Yue, K.; Huang, X.; Xu, Y.; Gao, C.; Yao, Z.; Zhang, Y.S.; Wang, J. Sprayable Hydrogel Dressing Accelerates Wound Healing with Combined Reactive Oxygen Species-Scavenging and Antibacterial Abilities. Acta Biomater. 2021, 124, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Hilton, S.A.; Osmond, M.J.; Zgheib, C.; Newsom, J.P.; Dewberry, L.; Singh, S.; Sakthivel, T.S.; Seal, S.; Liechty, K.W.; et al. Injectable, Self-Healable Zwitterionic Cryogels with Sustained MicroRNA-Cerium Oxide Nanoparticle Release Promote Accelerated Wound Healing. Acta Biomater. 2020, 101, 262–272. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver Nanoparticle Impregnated Chitosan-PEG Hydrogel Enhances Wound Healing in Diabetes Induced Rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Marković, Z.M.; Budimir, M.D.; Zdravković, N.M.; Trišić, D.D.; Bugárová, N.; Danko, M.; Kozyrovska, N.O.; Špitalský, Z.; Kleinová, A.; et al. Photoactive and Antioxidant Nanochitosan Dots/Biocellulose Hydrogels for Wound Healing Treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111925. [Google Scholar] [CrossRef]
- Jung, B.O.; Chung, S.J.; Lee, S.B. Preparation and Characterization of Eugenol-Grafted Chitosan Hydrogels and Their Antioxidant Activities. J. Appl. Polym. Sci. 2006, 99, 3500–3506. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, J.; Cao, L.; Jiao, Q.; Zhou, J.; Yang, L.; Zhang, H.; Wei, Y. Antifouling Antioxidant Zwitterionic Dextran Hydrogels as Wound Dressing Materials with Excellent Healing Activities. ACS Appl. Mater. Interfaces 2021, 13, 7060–7069. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wu, W.; Lei, Y.; Gaucher, C.; Pei, S.; Zhang, J.; Xia, X. Edaravone-Loaded Alginate-Based Nanocomposite Hydrogel Accelerated Chronic Wound Healing in Diabetic Mice. Mar. Drugs 2019, 17, 285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef] [PubMed]
- Maity, B.; Alam, S.; Samanta, S.; Prakash, R.G.; Govindaraju, T. Antioxidant Silk Fibroin Composite Hydrogel for Rapid Healing of Diabetic Wound. Macromol. Biosci. 2022, 22, e2200097. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.S.; Sung, D.; Lim, J.M.; Choi, W. Il Potential Antioxidant and Wound Healing Effect of Nano-Liposol with High Loading Amount of Astaxanthin. Int. J. Nanomed. 2020, 15, 9231–9240. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Sharma, A.; Zhang, T.; Wu, Y.; Ding, X. Pharmacological Review on Asiatic Acid and Its Derivatives: A Potential Compound. SLAS Technol. 2018, 23, 111–127. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Li, Y.; Wang, M.; Fan, T.; Liu, M.; Ke, Q.; Xu, H.; Yi, Z. An Aligned Porous Electrospun Fibrous Scaffold with Embedded Asiatic Acid for Accelerating Diabetic Wound Healing. J. Mater. Chem. B 2019, 7, 6125–6138. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Li, A.; Li, L.; Zhao, B.; Li, X.; Liang, W.; Lang, M.; Cheng, B.; Li, J. Antibacterial, Antioxidant and Anti-Inflammatory PLCL/Gelatin Nanofiber Membranes to Promote Wound Healing. Int. J. Biol. Macromol. 2022, 194, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Marasco, R.A. Economic Burden of Alzheimer Disease and Managed Care Considerations. Am. J. Manag. Care 2020, 26, S171–S183. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and Away from Alzheimer’s Disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Samii, A.; Nutt, J.G.; Ransom, B.R. Parkinson’s Disease. Lancet 2004, 363, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Forman, M.S.; Higuchi, M.; Golbe, L.I.; Graves, C.L.; Kotzbauer, P.T.; Trojanowski, J.Q.; Lee, V.M.Y. Initiation and Synergistic Fibrillization of Tau and Alpha-Synuctein. Science 2003, 300, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Mizuno, Y. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol. 2015, 24, 406–411. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative Stress and Parkinson’s Disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Aguilera, G.; Colín-González, A.L.; Rangel-López, E.; Chavarría, A.; Santamaría, A. Redox Signaling, Neuroinflammation, and Neurodegeneration. Antioxid. Redox Signal. 2018, 28, 1626–1651. [Google Scholar] [CrossRef]
- Bello-Medina, P.C.; González-Franco, D.A.; Vargas-Rodríguez, I.; Díaz-Cintra, S. Oxidative Stress, the Immune Response, Synaptic Plasticity, and Cognition in Transgenic Models of Alzheimer Disease. Neurologia 2022, 37, 682–690. [Google Scholar] [CrossRef]
- Zuo, L.; Motherwell, M.S. The Impact of Reactive Oxygen Species and Genetic Mitochondrial Mutations in Parkinson’s Disease. Gene 2013, 532, 18–23. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium Oxide Nanoparticles in Neuroprotection and Considerations for Efficacy and Safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef] [PubMed]
- Zand, Z.; Khaki, P.A.; Salihi, A.; Sharifi, M.; Nanakali, N.M.Q.; Alasady, A.A.B.; Aziz, F.M.; Shahpasand, K.; Hasan, A.; Falahati, M. Cerium Oxide NPs Mitigate the Amyloid Formation of α-Synuclein and Associated Cytotoxicity. Int. J. Nanomed. 2019, 14, 6989–7000. [Google Scholar] [CrossRef] [PubMed]
- Siposova, K.; Huntosova, V.; Garcarova, I.; Shlapa, Y.; Timashkov, I.; Belous, A.; Musatov, A. Dual-Functional Antioxidant and Antiamyloid Cerium Oxide Nanoparticles Fabricated by Controlled Synthesis in Water-Alcohol Solutions. Biomedicines 2022, 10, 942. [Google Scholar] [CrossRef]
- Ciofani, G.; Genchi, G.G.; Liakos, I.; Cappello, V.; Gemmi, M.; Athanassiou, A.; Mazzolai, B.; Mattoli, V. Effects of Cerium Oxide Nanoparticles on PC12 Neuronal-like Cells: Proliferation, Differentiation, and Dopamine Secretion. Pharm. Res. 2013, 30, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Li, M.; Dong, K.; Gao, N.; Ren, J.; Zheng, Y.; Qu, X. Ceria/POMs Hybrid Nanoparticles as a Mimicking Metallopeptidase for Treatment of Neurotoxicity of Amyloid-β Peptide. Biomaterials 2016, 98, 92–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Li, X.; Wang, L.; Yin, M.; Wang, L.; Chen, N.; Fan, C.; Song, H. Dietary Iron Oxide Nanoparticles Delay Aging and Ameliorate Neurodegeneration in Drosophila. Adv. Mater. 2016, 28, 1387–1393. [Google Scholar] [CrossRef]
- Ghaznavi, H.; Najafi, R.; Mehrzadi, S.; Hosseini, A.; Tekyemaroof, N.; Shakeri-Zadeh, A.; Rezayat, M.; Sharifi, A.M. Neuro-Protective Effects of Cerium and Yttrium Oxide Nanoparticles on High Glucose-Induced Oxidative Stress and Apoptosis in Undifferentiated PC12 Cells. Neurol. Res. 2015, 37, 624–632. [Google Scholar] [CrossRef]
- Hosseini, A.; Mohammad Sharifi, A.; Abdollahi, M.; Najafi, R.; Baeeri, M.; Rayegan, S.; Cheshmehnour, J.; Hassani, S.; Bayrami, Z.; Safa, M. Cerium and Yttrium Oxide Nanoparticles Against Lead-Induced Oxidative Stress and Apoptosis in Rat Hippocampus. Biol. Trace Elem. Res. 2015, 164, 80–89. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Luo, Y.; Ning, T.; Liu, P.; Chen, Q.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke. Adv. Sci. 2021, 8, 2101526. [Google Scholar] [CrossRef]
- Rodriguez-Losada, N.; Wendelbob, R.; Ocaña, M.C.; Casares, A.D.; Guzman de Villoría, R.; Aguirre Gomez, J.A.; Arraez, M.A.; Gonzalez-Alegre, P.; Medina, M.A.; Arenas, E.; et al. Graphene Oxide and Reduced Derivatives, as Powder or Film Scaffolds, Differentially Promote Dopaminergic Neuron Differentiation and Survival. Front. Neurosci. 2020, 14, 570409. [Google Scholar] [CrossRef] [PubMed]
- Fabian, R.H.; Derry, P.J.; Rea, H.C.; Dalmeida, W.V.; Nilewski, L.G.; Sikkema, W.K.A.; Mandava, P.; Tsai, A.L.; Mendoza, K.; Berka, V.; et al. Efficacy of Novel Carbon Nanoparticle Antioxidant Therapy in a Severe Model of Reversible Middle Cerebral Artery Stroke in Acutely Hyperglycemic Rats. Front. Neurol. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chena, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-Loaded PLGA-PEG Nanoparticles Conjugated with B6 Peptide for Potential Use in Alzheimer’s Disease. Drug Deliv. 2018, 25, 1044–1055. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model via Canonical Wnt/β-Catenin Pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A Novel Synthesis of Selenium Nanoparticles Encapsulated PLGA Nanospheres with Curcumin Molecules for the Inhibition of Amyloid β Aggregation in Alzheimer’s Disease. J. Photochem. Photobiol. B 2019, 190, 98–102. [Google Scholar] [CrossRef]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel Curcumin Loaded Nanoparticles Engineered for Blood-Brain Barrier Crossing and Able to Disrupt Abeta Aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef]
- Iemolo, A.; Moein Moghimi, S.; Decuzzi, P.; Ameruoso, A.; Palomba, R.; Lisa Palange, A.; Cervadoro, A.; Lee, A.; Di Mascolo, D. Ameliorating Amyloid-β Fibrils Triggered Inflammation via Curcumin-Loaded Polymeric Nanoconstructs. Front. Immunol. 2017, 8, 31. [Google Scholar] [CrossRef]
- Huang, N.; Lu, S.; Liu, X.G.; Zhu, J.; Wang, Y.J.; Liu, R.T. PLGA Nanoparticles Modified with a BBB-Penetrating Peptide Co-Delivering Aβ Generation Inhibitor and Curcumin Attenuate Memory Deficits and Neuropathology in Alzheimer’s Disease Mice. Oncotarget 2017, 8, 81001–81013. [Google Scholar] [CrossRef]
- Maiti, P.; Paladugu, L.; Dunbar, G.L. Solid Lipid Curcumin Particles Provide Greater Anti-Amyloid, Anti-Inflammatory and Neuroprotective Effects than Curcumin in the 5xFAD Mouse Model of Alzheimer’s Disease BMC Neuroscience. BMC Neurosci. 2018, 19, 7. [Google Scholar] [CrossRef]
- Campisi, A.; Sposito, G.; Pellitteri, R.; Santonocito, D.; Bisicchia, J.; Raciti, G.; Russo, C.; Nardiello, P.; Pignatello, R.; Casamenti, F.; et al. Effect of Unloaded and Curcumin-Loaded Solid Lipid Nanoparticles on Tissue Transglutaminase Isoforms Expression Levels in an Experimental Model of Alzheimer’s Disease. Antioxidants 2022, 11, 1863. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Esmaeili, A.; Zarrabi, A.; Zarepour, A. Superparamagnetic Iron Oxide Nanoparticles and Curcumin Equally Promote Neuronal Branching Morphogenesis in the Absence of Nerve Growth Factor in PC12 Cells. Pharmaceutics 2022, 14, 2692. [Google Scholar] [CrossRef] [PubMed]
- Palmal, S.; Maity, A.R.; Singh, B.K.; Basu, S.; Jana, N.R.; Jana, N.R. Inhibition of Amyloid Fibril Growth and Dissolution of Amyloid Fibrils by Curcumin-Gold Nanoparticles. Chemistry 2014, 20, 6184–6191. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Redox Nanoparticles: Synthesis, Properties and Perspectives of Use for Treatment of Neurodegenerative Diseases. J. Nanobiotechnol. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Aβ Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef]
- Abozaid, O.A.R.; Sallam, M.W.; El-Sonbaty, S.; Aziza, S.; Emad, B.; Ahmed, E.S.A. Resveratrol-Selenium Nanoparticles Alleviate Neuroinflammation and Neurotoxicity in a Rat Model of Alzheimer’s Disease by Regulating Sirt1/MiRNA-134/GSK3β Expression. Biol. Trace Elem. Res. 2022, 200, 5104–5114. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.N.; et al. Resveratrol and Grape Extract-Loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Pangeni, R.; Sharma, S.; Mustafa, G.; Ali, J.; Baboota, S. Vitamin E Loaded Resveratrol Nanoemulsion for Brain Targeting for the Treatment of Parkinson’s Disease by Reducing Oxidative Stress. Nanotechnology 2014, 25, 485102. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Folch, J.; Kühne, B.A.; Barenys, M.; Sánchez-López, E.; Souto, E.B.; García, M.L.; et al. Epigallocatechin-3-Gallate PEGylated Poly(Lactic-Co-Glycolic) Acid Nanoparticles Mitigate Striatal Pathology and Motor Deficits in 3-Nitropropionic Acid Intoxicated Mice. Nanomedicine 2021, 16, 19–35. [Google Scholar] [CrossRef]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic Particles Improve the Bioavailability and Alpha-Secretase Inducing Ability of Epigallocatechin-3-Gallate (EGCG) for the Treatment of Alzheimer’s Disease. Int. J. Pharm. 2010, 389, 207–212. [Google Scholar] [CrossRef]
- Vishwas, S.; Kumar, R.; Khursheed, R.; Ramanunny, A.K.; Kumar, R.; Awasthi, A.; Corrie, L.; Porwal, O.; Arshad, M.F.; Alshammari, M.K.; et al. Expanding Arsenal against Neurodegenerative Diseases Using Quercetin Based Nanoformulations: Breakthroughs and Bottlenecks. Curr. Neuropharmacol. 2022, 20, 1558–1574. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Bondi, M.L.; Montana, G.; Bruno, A.; Pitarresi, G.; Giammona, G.; Di Carlo, M. Ferulic Acid Inhibits Oxidative Stress and Cell Death Induced by Ab Oligomers: Improved Delivery by Solid Lipid Nanoparticles. Free Radic. Res. 2009, 43, 1133–1145. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Kutscher, H.L.; Singh, A.; Cwiklinski, K.; Khechen, N.; Schwartz, S.A.; Prasad, P.N.; Mahajan, S.D. Neuroprotective Effects of a Biodegradable Poly(Lactic-Co-Glycolic Acid)-Ginsenoside Rg3 Nanoformulation: A Potential Nanotherapy for Alzheimer’s Disease? J. Drug Target. 2018, 26, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Soudi, S.A.; Nounou, M.I.; Sheweita, S.A.; Ghareeb, D.A.; Younis, L.K.; El-Khordagui, L.K. Protective Effect of Surface-Modified Berberine Nanoparticles against LPS-Induced Neurodegenerative Changes: A Preclinical Study. Drug Deliv. Transl. Res. 2019, 9, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, M.; Lanthier, P.; Miller, H.; Beyers, M.; Sodja, C.; Zurakowski, B.; Gangaraju, S.; Pandey, S.; Sandhu, J.K. Nanomicellar Formulation of Coenzyme Q10 (Ubisol-Q10) Effectively Blocks Ongoing Neurodegeneration in the Mouse 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Model: Potential Use as an Adjuvant Treatment in Parkinson’s Disease. Neurobiol. Aging 2014, 35, 2329–2346. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Jimenez, M.T.B.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid Med Cell Longev 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative Stress and Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, 2181–2190. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative Stress and Inflammation in the Evolution of Heart Failure: From Pathophysiology to Therapeutic Strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef]
- Bae, S.; Park, M.; Kang, C.; Dilmen, S.; Kang, T.H.; Kang, D.G.; Ke, Q.; Lee, S.U.; Lee, D.; Kang, P.M. Hydrogen Peroxide-Responsive Nanoparticle Reduces Myocardial Ischemia/Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003697. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Guo, R.; Li, S.; Ni, J.; Gao, S.; Gao, X.; Mao, J.; Zhu, Y.; Wu, P.; et al. Ginsenoside Rg3-Loaded, Reactive Oxygen Species-Responsive Polymeric Nanoparticles for Alleviating Myocardial Ischemia-Reperfusion Injury. J. Control. Release 2020, 317, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Pan, J. Drug Delivery Baicalin-Loaded PEGylated Lipid Nanoparticles: Characterization, Pharmacokinetics, and Protective Effects on Acute Myocardial Ischemia in Rats Baicalin-Loaded PEGylated Lipid Nanoparticles: Characterization, Pharmacokinetics, and Protective Effects on Acute Myocardial Ischemia in Rats. Drug Deliv. 2016, 23, 3696–3703. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Yang, W.; Han, G. Protective Effects on Myocardial Infarction Model: Delivery of Schisandrin B Using Matrix Metalloproteinase-Sensitive Peptide-Modified, Pegylated Lipid Nanoparticles. Int. J. Nanomed. 2017, 12, 7121. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, P.; Zhou, H.; Shen, R.; Hu, B.; Shen, Y.; Zhang, X.; Shen, X.; Xu, G.; Jin, L. Pharmacological Signatures of the Exenatide Nanoparticles Complex Against Myocardial Ischemia Reperfusion Injury. Kidney Blood Press. Res. 2018, 43, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Lozano, O.; Lázaro-Alfaro, A.; Silva-Platas, C.; Oropeza-Almazán, Y.; Torres-Quintanilla, A.; Bernal-Ramírez, J.; Alves-Figueiredo, H.; García-Rivas, G. Nanoencapsulated Quercetin Improves Cardioprotection during Hypoxia-Reoxygenation Injury through Preservation of Mitochondrial Function. Oxid. Med. Cell. Longev. 2019, 2019, 7683051. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, D.Z.; Zhang, C.X.; Cui, H.; Liu, M.; Zhang, B.L.; Mei, Q.B.; Lu, Z.F.; Zhou, S.Y. Mitochondria-Targeted Antioxidant Delivery for Precise Treatment of Myocardial Ischemia–Reperfusion Injury through a Multistage Continuous Targeted Strategy. Nanomedicine 2019, 16, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hu, Y.; Mishra, A.; Sreeharsha, N.; Moktan, J.B.; Kumar, P.; Wang, L. Protective Role of Poly(Lactic-Co-Glycolic) Acid Nanoparticle Loaded with Resveratrol against Isoproterenol-Induced Myocardial Infarction. Biofactors 2020, 46, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Tokutome, M.; Matoba, T.; Nakano, Y.; Okahara, A.; Fujiwara, M.; Koga, J.I.; Nakano, K.; Tsutsui, H.; Egashira, K. Peroxisome Proliferator-Activated Receptor-Gamma Targeting Nanomedicine Promotes Cardiac Healing after Acute Myocardial Infarction by Skewing Monocyte/Macrophage Polarization in Preclinical Animal Models. Cardiovasc. Res. 2019, 115, 419–431. [Google Scholar] [CrossRef]
- Nakano, Y.; Matoba, T.; Tokutome, M.; Funamoto, D.; Katsuki, S.; Ikeda, G.; Nagaoka, K.; Ishikita, A.; Nakano, K.; Koga, J.-I.; et al. Nanoparticle-Mediated Delivery of Irbesartan Induces Cardioprotection from Myocardial Ischemia-Reperfusion Injury by Antagonizing Monocyte-Mediated Inflammation. Sci. Rep. 2016, 6, 29601. [Google Scholar] [CrossRef]
- Ishikita, A.; Matoba, T.; Ikeda, G.; Koga, J.I.; Mao, Y.; Nakano, K.; Takeuchi, O.; Sadoshima, J.; Egashira, K. Nanoparticle-Mediated Delivery of Mitochondrial Division Inhibitor 1 to the Myocardium Protects the Heart From Ischemia-Reperfusion Injury Through Inhibition of Mitochondria Outer Membrane Permeabilization: A New Therapeutic Modality for Acute Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003872. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Coenzyme Q10 Supplementation: Efficacy, Safety, and Formulation Challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef]
- Simoń-Yarza, T.; Tamayo, E.; Benavides, C.; Lana, H.; Formiga, F.R.; Grama, C.N.; Ortiz-de-Solorzano, C.; Ravi Kumar, M.N.V.; Prosper, F.; Blanco-Prieto, M.J. Functional Benefits of PLGA Particulates Carrying VEGF and CoQ10 in an Animal of Myocardial Ischemia. Int. J. Pharm. 2013, 454, 784–790. [Google Scholar] [CrossRef]
- Verma, D.D.; Hartner, W.C.; Thakkar, V.; Levchenko, T.S.; Torchilin, V.P. Protective Effect of Coenzyme Q10-Loaded Liposomes on the Myocardium in Rabbits with an Acute Experimental Myocardial Infarction. Pharm. Res. 2007, 24, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.M.; Arthur, P.G.; Hool, L.C. Transient Exposure to Hydrogen Peroxide Causes an Increase in Mitochondria-Derived Superoxide as a Result of Sustained Alteration in L-Type Ca2+ Channel Function in the Absence of Apoptosis in Ventricular Myocytes. Circ. Res. 2007, 100, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Hardy, N.; Viola, H.M.; Johnstone, V.P.A.; Clemons, T.D.; Cserne Szappanos, H.; Singh, R.; Smith, N.M.; Iyer, K.S.; Hool, L.C. Nanoparticle-Mediated Dual Delivery of an Antioxidant and a Peptide against the L-Type Ca2+ Channel Enables Simultaneous Reduction of Cardiac Ischemia-Reperfusion Injury. ACS Nano 2015, 9, 279–289. [Google Scholar] [CrossRef]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K.; et al. Ceria Nanoparticles That Can Protect against Ischemic Stroke. Angew. Chem. Int. Ed. Engl. 2012, 51, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G.; Licoccia, S.; Minieri, M.; Di Nardo, P.; Traversa, E. Cerium Oxide Nanoparticles Protect Cardiac Progenitor Cells from Oxidative Stress. ACS Nano 2012, 6, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Pala, N.; DessÌ, G.; Manconi, P.; Mariani, A.; Dedola, S.; Rassu, M.; Crosio, C.; Iaccarino, C.; Sechi, M. Single-Step Green Synthesis and Characterization of Gold-Conjugated Polyphenol Nanoparticles with Antioxidant and Biological Activities. Int. J. Nanomed. 2014, 9, 4935–4951. [Google Scholar] [CrossRef]
- Babu, B.; Palanisamy, S.; Vinosha, M.; Anjali, R.; Kumar, P.; Pandi, B.; Tabarsa, M.; You, S.G.; Prabhu, N.M. Bioengineered Gold Nanoparticles from Marine Seaweed Acanthophora Spicifera for Pharmaceutical Uses: Antioxidant, Antibacterial, and Anticancer Activities. Bioprocess Biosyst. Eng. 2020, 43, 2231–2242. [Google Scholar] [CrossRef]
- Tartuce, L.P.; Pacheco Brandt, F.; dos Santos Pedroso, G.; Rezende Farias, H.; Barros Fernandes, B.; da Costa Pereira, B.; Gonçalves Machado, A.; Feuser, P.E.; Lock Silveira, P.C.; Tiscoski Nesi, R.; et al. 2-Methoxy-Isobutyl-Isonitrile-Conjugated Gold Nanoparticles Improves Redox and Inflammatory Profile in Infarcted Rats. Colloids Surf. B Biointerfaces 2020, 192, 111012. [Google Scholar] [CrossRef]
- Jarrar, Q.; Al-Doaiss, A.; Jarrar, B.M.; Alshehri, M. On the Toxicity of Gold Nanoparticles: Histological, Histochemical and Ultrastructural Alterations. Toxicol. Ind. Health 2022, 38, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ding, J.; Wang, Z.; Zhang, H.; Xie, J.; Wang, Y.; Hong, L.; Mao, Z.; Gao, J.; Gao, C. ROS-Responsive Polyurethane Fibrous Patches Loaded with Methylprednisolone (MP) for Restoring Structures and Functions of Infarcted Myocardium in Vivo. Biomaterials 2020, 232, 119726. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate-Nitrite-Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Qian, M.; Zhang, Y.; Liu, Q.; Midgley, A.C.; Liu, Y.; Che, Y.; Hou, J.; Zhao, Q. An Injectable Dual-Function Hydrogel Protects Against Myocardial Ischemia/Reperfusion Injury by Modulating ROS/NO Disequilibrium. Adv. Sci. 2022, 9, e2105408. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel Angiogenesis Therapeutics by Redox Injectable Hydrogel-Regulation of Local Nitric Oxide Generation for Effective Cardiovascular Therapy. Biomaterials 2018, 167, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.; Gregnanin, G.; Cencetti, C.; Di Meo, C.; Gueguen, V.; Letourneur, D.; Meddahi-Pellé, A.; Pavon-Djavid, G.; Matricardi, P. PVA/Dextran Hydrogel Patches as Delivery System of Antioxidant Astaxanthin: A Cardiovascular Approach. Biomed. Mater. 2017, 13, 015020. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, L.; Song, L.L.; Chen, S.; Gao, Y. α-Tocopherol Liposome Loaded Chitosan Hydrogel to Suppress Oxidative Stress Injury in Cardiomyocytes. Int. J. Biol. Macromol. 2019, 125, 1192–1202. [Google Scholar] [CrossRef]
- Li, J.; Shu, Y.; Hao, T.; Wang, Y.; Qian, Y.; Duan, C.; Sun, H.; Lin, Q.; Wang, C. A Chitosan-Glutathione Based Injectable Hydrogel for Suppression of Oxidative Stress Damage in Cardiomyocytes. Biomaterials 2013, 34, 9071–9081. [Google Scholar] [CrossRef]
- Guo, Y.; Qu, Y.; Yu, J.; Song, L.; Chen, S.; Qin, Z.; Gong, J.; Zhan, H.; Gao, Y.; Zhang, J. A Chitosan-Vitamin C Based Injectable Hydrogel Improves Cell Survival under Oxidative Stress. Int. J. Biol. Macromol. 2022, 202, 102–111. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Lin, F.H.; Wang, C.Y.; Hsiao, C.Y.; Chen, H.C.; Kuo, H.Y.; Tsai, T.F.; Chiou, S.H. Recovery of Oxidative Stress-Induced Damage in Cisd2-Deficient Cardiomyocytes by Sustained Release of Ferulic Acid from Injectable Hydrogel. Biomaterials 2016, 103, 207–218. [Google Scholar] [CrossRef]
- Hu, C.; Liu, W.; Long, L.; Wang, Z.; Zhang, W.; He, S.; Lu, L.; Fan, H.; Yang, L.; Wang, Y. Regeneration of Infarcted Hearts by Myocardial Infarction-Responsive Injectable Hydrogels with Combined Anti-Apoptosis, Anti-Inflammatory and pro-Angiogenesis Properties. Biomaterials 2022, 290, 121849. [Google Scholar] [CrossRef]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, K.A.; Zhu, Y.; Takaba, K.; Ramasubramanian, A.; Badathala, A.; Haraldsson, H.; Collins, A.; Aguayo, E.; Shah, C.; Wallace, A.W.; et al. Myocardial Injection of a Thermoresponsive Hydrogel with Reactive Oxygen Species Scavenger Properties Improves Border Zone Contractility. J. Biomed. Mater. Res. A 2020, 108, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, C.; Li, Y.; Li, Z.; Zhang, Z.; Xu, J.; Chen, M.; Li, R.; Liu, S.; Wu, Y.; et al. Injectable Selenium-Containing Polymeric Hydrogel Formulation for Effective Treatment of Myocardial Infarction. Front. Bioeng. Biotechnol. 2022, 10, 912562. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Cheng, S.J.; Chen, H.H.; Hsu, W.C. Development of Injectable Graphene Oxide/Laponite/Gelatin Hydrogel Containing Wharton’s Jelly Mesenchymal Stem Cells for Treatment of Oxidative Stress-Damaged Cardiomyocytes. Colloids Surf. B Biointerfaces 2022, 209, 112150. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, X.; Liu, Y.; Cui, C.; Sun, Y.; Liu, W. Wet Adhesive Hydrogel Cardiac Patch Loaded with Anti-Oxidative, Autophagy-Regulating Molecule Capsules and MSCs for Restoring Infarcted Myocardium. Bioact. Mater. 2022, 21, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Janjic, M.; Pappa, F.; Karagkiozaki, V.; Gitas, C.; Ktenidis, K.; Logothetidis, S. Surface Modification of Endovascular Stents with Rosuvastatin and Heparin-Loaded Biodegradable Nanofibers by Electrospinning. Int. J. Nanomed. 2017, 12, 6343–6355. [Google Scholar] [CrossRef]
- Wang, R.; Lu, J.; Yin, J.; Chen, H.; Liu, H.; Xu, F.; Zang, T.; Xu, R.; Li, C.; Wu, Y.; et al. A TEMPOL and Rapamycin Loaded Nanofiber-Covered Stent Favors Endothelialization and Mitigates Neointimal Hyperplasia and Local Inflammation. Bioact. Mater. 2023, 19, 666–677. [Google Scholar] [CrossRef]
- Ghafoor, B.; Ali, N.; Riaz, Z. Synthesis and Appraisal of Natural Drug-Polymer-Based Matrices Relevant to the Application of Drug-Eluting Coronary Stent Coatings. Cardiol. Res. Pr. 2020, 2020, 4073091. [Google Scholar] [CrossRef]
- Wang, K.; Shang, T.; Zhang, L.; Zhou, L.; Liu, C.; Fu, Y.; Zhao, Y.; Li, X.; Wang, J. Application of a Reactive Oxygen Species-Responsive Drug-Eluting Coating for Surface Modification of Vascular Stents. ACS Appl. Mater. Interfaces 2021, 13, 35443. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Yang, H.; Wang, B.; Dai, Y.; Li, X.; Yan, K.; You, R.; Ma, L. Silk Fibroin/Chitosan Coating with Tunable Catalytic Nitric Oxide Generation for Surface Functionalization of Cardiovascular Stents. Int. J. Biol. Macromol. 2023, 228, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.K.; Goh, B.H.; Yap, W.H. Oxidative Stress in Cardiovascular Diseases: Involvement of Nrf2 Antioxidant Redox Signaling in Macrophage Foam Cells Formation. Int. J. Mol. Sci. 2017, 18, 2336. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Venkata, N.; Pothineni, K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Genet. Genom. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, S. Targeting Oxidative Stress for the Treatment of Ischemic Stroke: Upstream and Downstream Therapeutic Strategies. Brain Circ. 2016, 2, 153. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Urzúa, S.; Rojas, I.; Líbano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020, 26, 4246–4260. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, S.; Zu, Y.; Abbasi, M.; Cao, J.; Li, C.; Wu, D.; Labib, S.; Brackee, G.; Shen, C.L.; et al. Anti-Atherogenic Effects of CD36-Targeted Epigallocatechin Gallate-Loaded Nanoparticles. J. Control. Release 2019, 303, 263–273. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation Enhances Epigallocatechin-3-Gallate Stability and Its Antiatherogenic Bioactivities in Macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef]
- Pillai, S.C.; Borah, A.; Le, M.N.T.; Kawano, H.; Hasegawa, K.; Sakthi Kumar, D. Co-Delivery of Curcumin and Bioperine via Plga Nanoparticles to Prevent Atherosclerotic Foam Cell Formation. Pharmaceutics 2021, 13, 1420. [Google Scholar] [CrossRef]
- Li, X.; Xiao, H.; Lin, C.; Sun, W.; Wu, T.; Wang, J.; Chen, B.; Chen, X.; Cheng, D. Synergistic Effects of Liposomes Encapsulating Atorvastatin Calcium and Curcumin and Targeting Dysfunctional Endothelial Cells in Reducing Atherosclerosis. Int. J. Nanomed. 2019, 14, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Om, H.; El-Naggar, M.E.; El-Banna, M.; Fouda, M.M.G.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Combating Atherosclerosis with Targeted Diosmin Nanoparticles-Treated Experimental Diabetes. Investig. New Drugs 2020, 38, 1303–1315. [Google Scholar] [CrossRef]
- Xiao, J.; Li, N.; Xiao, S.; Wu, Y.; Liu, H. Comparison of Selenium Nanoparticles and Sodium Selenite on the Alleviation of Early Atherosclerosis by Inhibiting Endothelial Dysfunction and Inflammation in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2021, 22, 11612. [Google Scholar] [CrossRef]
- Obidah Abert, H.; Umaru Aduwamai, H.; Shehu Adamu, S. Effect of Green Synthesized Iron Oxide Nanoparticles Using Spinach Extract on Triton X-100-Induced Atherosclerosis in Rats. Biochem. Res. Int. 2022, 2022, 9311227. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Ma, X.; Zhang, B.; Huang, Y.; Zhao, J.; Wang, S.; Li, Y.; Zhu, Y.; Xiong, J.; et al. Synthesis and Characterization of Fucoidan-Chitosan Nanoparticles Targeting P-Selectin for Effective Atherosclerosis Therapy. Oxid. Med. Cell. Longev. 2022, 2022, 8006642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Zhao, W.; Dou, Y.; An, H.; Tao, H.; Xu, X.; Jia, Y.; Lu, S.; Zhang, J.; et al. Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-Inflammatory Activity. ACS Nano 2018, 12, 8943–8960. [Google Scholar] [CrossRef] [PubMed]
- Cymet, T.C.; Wood, B.; Orbach, N. Osteoporosis. J. Am. Osteopath Assoc. 2000, 100 Pt 1, S9–S15. [Google Scholar] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The Epidemiology of Osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Hadjidakis, D.J.; Androulakis, I.I. Bone Remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular Mechanisms of Bone Remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef]
- Prasad, G.; Dhillon, M.S.; Khullar, M.; Nagi, O.N. Evaluation of Oxidative Stress after Fractures. A Preliminary Study. Acta Orthop. Belg. 2003, 69, 546–551. [Google Scholar] [PubMed]
- Lee, D.H.; Lim, B.S.; Lee, Y.K.; Yang, H.C. Effects of Hydrogen Peroxide (H2O2) on Alkaline Phosphatase Activity and Matrix Mineralization of Odontoblast and Osteoblast Cell Lines. Cell Biol. Toxicol. 2006, 22, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal Involution by Age-Associated Oxidative Stress and Its Acceleration by Loss of Sex Steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative Stress Inhibits Osteoblastic Differentiation of Bone Cells by ERK and NF-ΚB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Noble, B.; Weinstein, R.S. Osteocyte Apoptosis. Bone 2013, 54, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.J.; Kim, J.M.; Kim, H.; Song, H.; So, H.; Lee, S.Y.; Kwon, S.B.; Kim, H.J.; Kim, H.H.; Lee, S.H.; et al. Regulation of Osteoclast Differentiation by the Redox-Dependent Modulation of Nuclear Import of Transcription Factors. Cell Death Differ. 2006, 13, 1138–1146. [Google Scholar] [CrossRef]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative Stress, Free Radicals and Bone Remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar] [CrossRef]
- Sumi, D.; Hayashi, T.; Matsui-Hirai, H.; Jacobs, A.T.; Ignarro, L.J.; Iguchi, A. 17β-Estradiol Inhibits NADPH Oxidase Activity through the Regulation of P47phox MRNA and Protein Expression in THP-1 Cells. Biochim. Biophys. Acta Mol. Cell. Res. 2003, 1640, 113–118. [Google Scholar] [CrossRef]
- Sendur, O.F.; Turan, Y.; Tastaban, E.; Serter, M. Antioxidant Status in Patients with Osteoporosis: A Controlled Study. Joint Bone Spine 2009, 76, 514–518. [Google Scholar] [CrossRef]
- Tao, Z.; Li, T.L.; Yang, M.; Xu, H.G. Silibinin Can Promote Bone Regeneration of Selenium Hydrogel by Reducing the Oxidative Stress Pathway in Ovariectomized Rats. Calcif. Tissue Int. 2022, 110, 723–735. [Google Scholar] [CrossRef]
- Pinna, A.; Torki Baghbaderani, M.; Vigil Hernández, V.; Naruphontjirakul, P.; Li, S.; McFarlane, T.; Hachim, D.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Nanoceria Provides Antioxidant and Osteogenic Properties to Mesoporous Silica Nanoparticles for Osteoporosis Treatment. Acta Biomater. 2021, 122, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Plasma Sprayed Cerium Oxide Coating Inhibits H2O2-Induced Oxidative Stress and Supports Cell Viability. J. Mater. Sci. Mater. Med. 2016, 27, 100. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Sen, A.P.; Ghosal, S. Effects of Shilajit on Biogenic Free Radicals. Phytother. Res. 1995, 9, 56–59. [Google Scholar] [CrossRef]
- Alshubaily, F.A.; Jambi, E.J. Correlation between Antioxidant and Anti-Osteoporotic Activities of Shilajit Loaded into Chitosan Nanoparticles and Their Effects on Osteoporosis in Rats. Polymers 2022, 14, 3972. [Google Scholar] [CrossRef]
- Yu, P.; Zheng, L.; Wang, P.; Chai, S.; Zhang, Y.; Shi, T.; Zhang, L.; Peng, R.; Huang, C.; Guo, B.; et al. Development of a Novel Polysaccharide-Based Iron Oxide Nanoparticle to Prevent Iron Accumulation-Related Osteoporosis by Scavenging Reactive Oxygen Species. Int. J. Biol. Macromol. 2020, 165, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.I.; Mohd Noor, H.I.; Shuid, A.N.; Mohamad, S.; Abdul Malik, M.M.; Jayusman, P.A.; Shuid, A.N.; Naina Mohamed, I. Osteoprotective Effects in Postmenopausal Osteoporosis Rat Model: Oral Tocotrienol vs. Intraosseous Injection of Tocotrienol-Poly Lactic-Co-Glycolic Acid Combination. Front. Pharmacol. 2021, 12, 706747. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Al-Baadani, M.A.; Wu, M.; Tong, N.; Shen, X.; Ding, X.; Liu, J. Study on the Local Anti-Osteoporosis Effect of Polaprezinc-Loaded Antioxidant Electrospun Membrane. Int. J. Nanomed. 2022, 17, 17–29. [Google Scholar] [CrossRef]
- Fatima, S.; Alfrayh, R.; Alrashed, M.; Alsobaie, S.; Ahmad, R.; Mahmood, A. Selenium Nanoparticles by Moderating Oxidative Stress Promote Differentiation of Mesenchymal Stem Cells to Osteoblasts. Int. J. Nanomed. 2021, 16, 331–343. [Google Scholar] [CrossRef]
- Poleboina, S.; Sheth, V.G.; Sharma, N.; Sihota, P.; Kumar, N.; Tikoo, K. Selenium Nanoparticles Stimulate Osteoblast Differentiation via BMP-2/MAPKs/β-Catenin Pathway in Diabetic Osteoporosis. Nanomedicine 2022, 17, 607–625. [Google Scholar] [CrossRef]
- Ardawi, M.S.M.; Badawoud, M.H.; Hassan, S.M.; Ardawi, A.M.S.; Rouzi, A.A.; Qari, M.H.; Mousa, S.A. Lycopene Nanoparticles Promotes Osteoblastogenesis and Inhibits Adipogenesis of Rat Bone Marrow Mesenchymal Stem Cells. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6894–6907. [Google Scholar] [CrossRef]
- Zheng, L.; Zhuang, Z.; Li, Y.; Shi, T.; Fu, K.; Yan, W.; Zhang, L.; Wang, P.; Li, L.; Jiang, Q. Bone Targeting Antioxidative Nano-Iron Oxide for Treating Postmenopausal Osteoporosis. Bioact. Mater. 2021, 14, 250–261. [Google Scholar] [CrossRef]
- Kim, W.K.; Kim, J.C.; Park, H.J.; Sul, O.J.; Lee, M.H.; Kim, J.S.; Choi, H.S. Platinum Nanoparticles Reduce Ovariectomy-Induced Bone Loss by Decreasing Osteoclastogenesis. Exp. Mol. Med. 2012, 44, 432–439. [Google Scholar] [CrossRef]
- Li, J.; Deng, C.; Liang, W.; Kang, F.; Bai, Y.; Ma, B.; Wu, C.; Dong, S. Mn-Containing Bioceramics Inhibit Osteoclastogenesis and Promote Osteoporotic Bone Regeneration via Scavenging ROS. Bioact. Mater. 2021, 6, 3839–3850. [Google Scholar] [CrossRef]
- Brandi, M.L. Healing of the Bone with Anti-Fracture Drugs. Expert Opin. Pharmacother 2013, 14, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, K.; Hu, T.; Shao, D.; Huang, S.; Xie, Y.; Zheng, X. Zn-Doped MnO2 Nanocoating with Enhanced Catalase-Mimetic Activity and Cytocompatibility Protects Pre-Osteoblasts against H2O2-Induced Oxidative Stress. Colloids Surf. B Biointerfaces 2021, 202, 111666. [Google Scholar] [CrossRef] [PubMed]
- Riccucci, G.; Ferraris, S.; Reggio, C.; Bosso, A.; Rlygsson, G.O.; Ng, C.H.; Spriano, S. Polyphenols from Grape Pomace: Functionalization of Chitosan-Coated Hydroxyapatite for Modulated Swelling and Release of Polyphenols. Langmuir 2021, 37, 14793–14804. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, X.; Luo, Z.; Hu, Y.; Li, M.; Ma, P.; Ran, Q.; Dai, L.; He, Y.; Cai, K. Osteogenesis Potential of Different Titania Nanotubes in Oxidative Stress Microenvironment. Biomaterials 2018, 167, 44–57. [Google Scholar] [CrossRef]
- Ding, W.; Zhou, Q.; Lu, Y.; Wei, Q.; Tang, H.; Zhang, D.; Liu, Z.; Wang, G.; Wu, D. ROS-Scavenging Hydrogel as Protective Carrier to Regulate Stem Cells Activity and Promote Osteointegration of 3D Printed Porous Titanium Prosthesis in Osteoporosis. Front. Bioeng. Biotechnol. 2023, 11, 11103611. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; He, C. Melatonin Having Therapeutic Bone Regenerating Capacity in Biomaterials. Curr. Pharm. Biotechnol. 2022, 23, 707–718. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, J.; Chen, R.; Huang, Y.; Liu, Y.; Bai, J.; Ge, G.; Shi, X.; Chen, Y.; Shi, J.; et al. Sustained Release of Melatonin from GelMA Liposomes Reduced Osteoblast Apoptosis and Improved Implant Osseointegration in Osteoporosis. Oxid. Med. Cell. Longev. 2020, 2020, 6797154. [Google Scholar] [CrossRef] [PubMed]
- Ewart, L.; Apostolou, A.; Briggs, S.A.; Carman, C.V.; Chaff, J.T.; Heng, A.R.; Jadalannagari, S.; Janardhanan, J.; Jang, K.-J.; Joshipura, S.R.; et al. Performance Assessment and Economic Analysis of a Human Liver-Chip for Predictive Toxicology. Commun. Med. 2022, 2, 154. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Nicholson, M.W.; Wang, J.Y.; Ting, C.Y.; Tsai, M.H.; Cheng, Y.C.; Liu, C.L.; Chan, D.Z.H.; Lee, Y.C.; Hsu, C.C.; et al. Population-Based High-Throughput Toxicity Screen of Human IPSC-Derived Cardiomyocytes and Neurons. Cell Rep. 2022, 39, 110643. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Ahmed, S.M.; Shivnaraine, R.V.; Wu, J.C. FDA Modernization Act 2.0 Paves the Way to Computational Biology and Clinical Trials in a Dish. Circulation 2023, 148, 309–311. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Araluce, M.; Jüngst, T.; Sanmartin, C.; Prosper, F.; Plano, D.; Mazo, M.M. Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases. Biomimetics 2024, 9, 23. https://doi.org/10.3390/biomimetics9010023
Perez-Araluce M, Jüngst T, Sanmartin C, Prosper F, Plano D, Mazo MM. Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases. Biomimetics. 2024; 9(1):23. https://doi.org/10.3390/biomimetics9010023
Chicago/Turabian StylePerez-Araluce, Maria, Tomasz Jüngst, Carmen Sanmartin, Felipe Prosper, Daniel Plano, and Manuel M. Mazo. 2024. "Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases" Biomimetics 9, no. 1: 23. https://doi.org/10.3390/biomimetics9010023






