Complex Modal Characteristic Analysis of a Tensegrity Robotic Fish’s Body Waves
Abstract
:1. Introduction
2. Complex Modal Characteristic Analysis of Fish Body Wave
2.1. Fish Body Wave Curve
2.2. The Complex Orthogonal Decomposition Method of Fish Body Wave
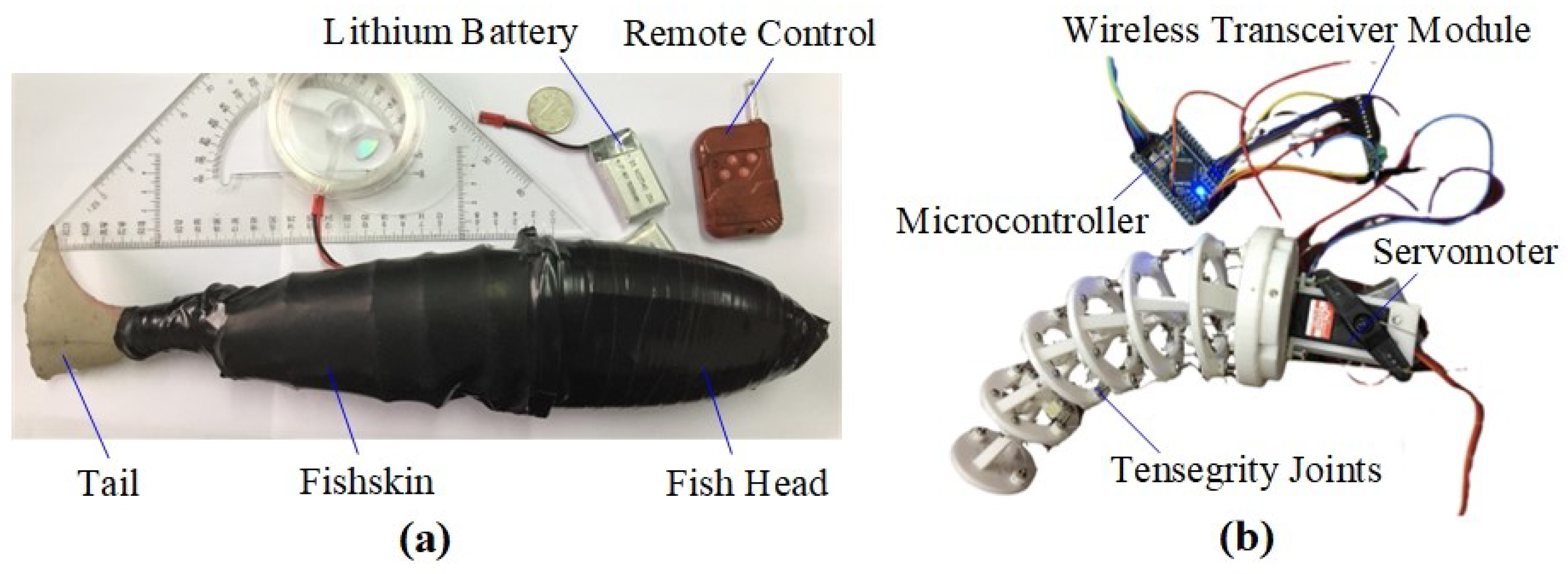
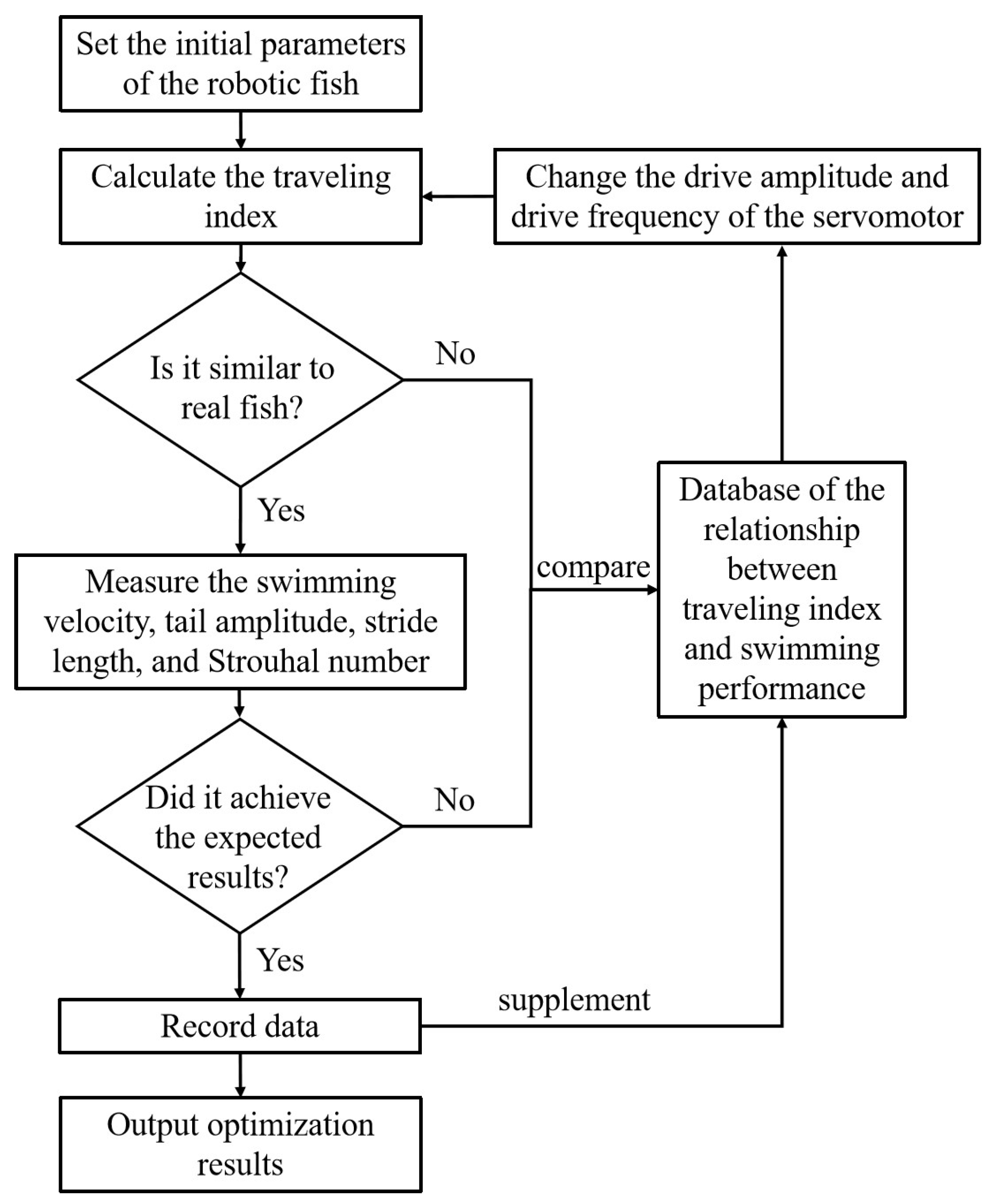
3. Experimental Program of the Tensegrity Robotic Fish
4. Analysis of Experimental Results
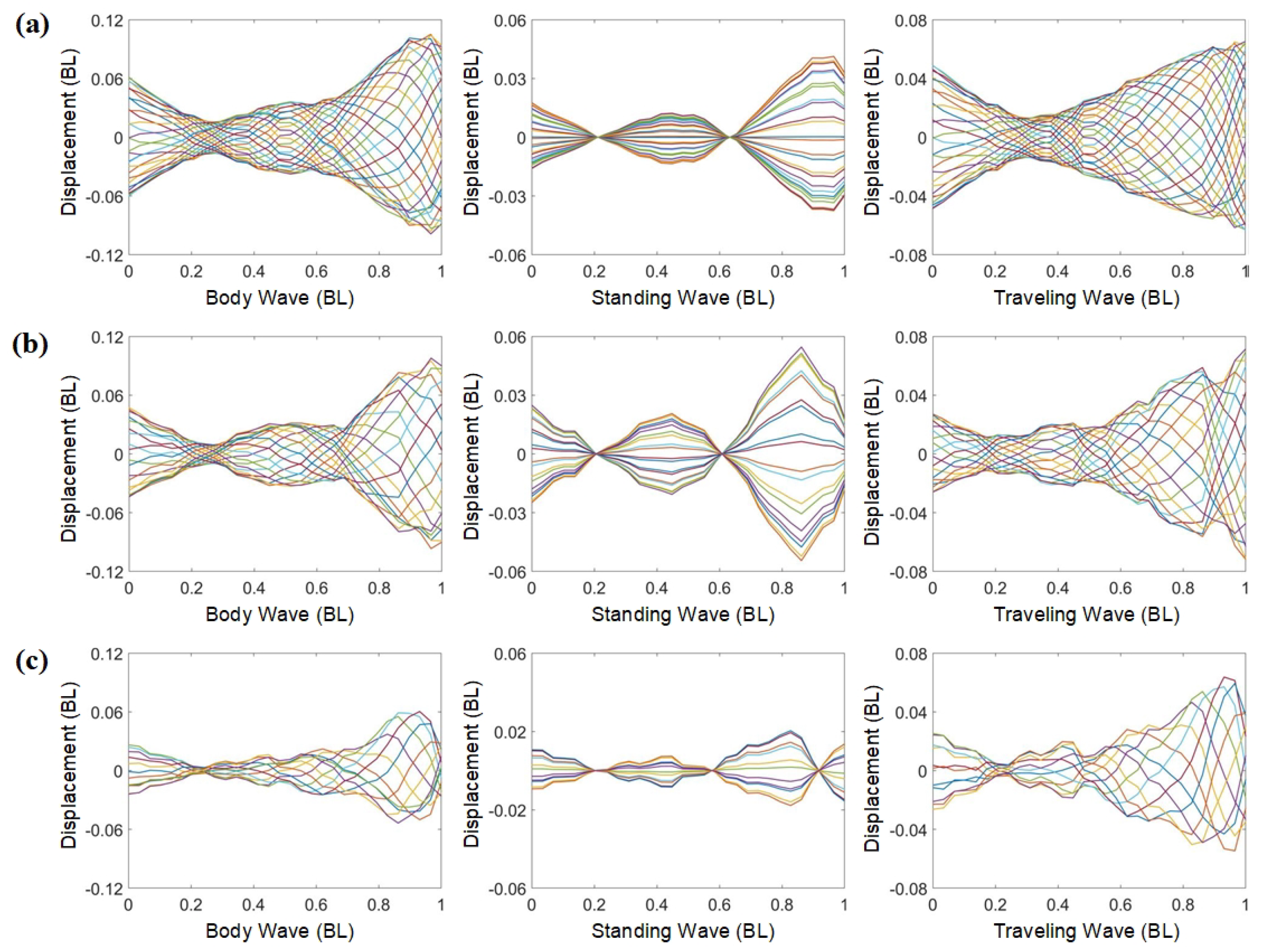
4.1. Comparison of the Fish Body Wave’s Complex Modal Characteristics between the Tensegrity Robotic Fish and Real Fish
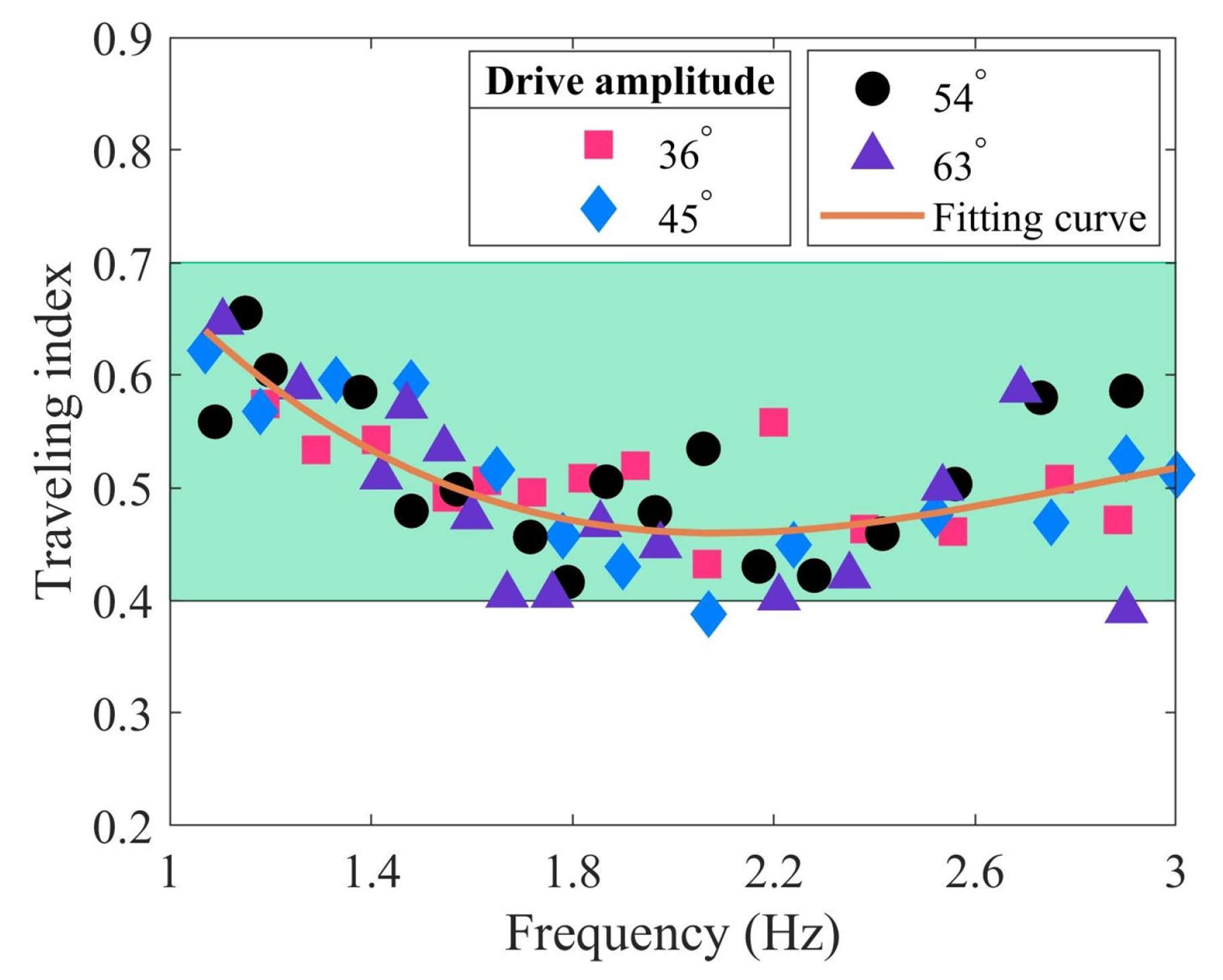
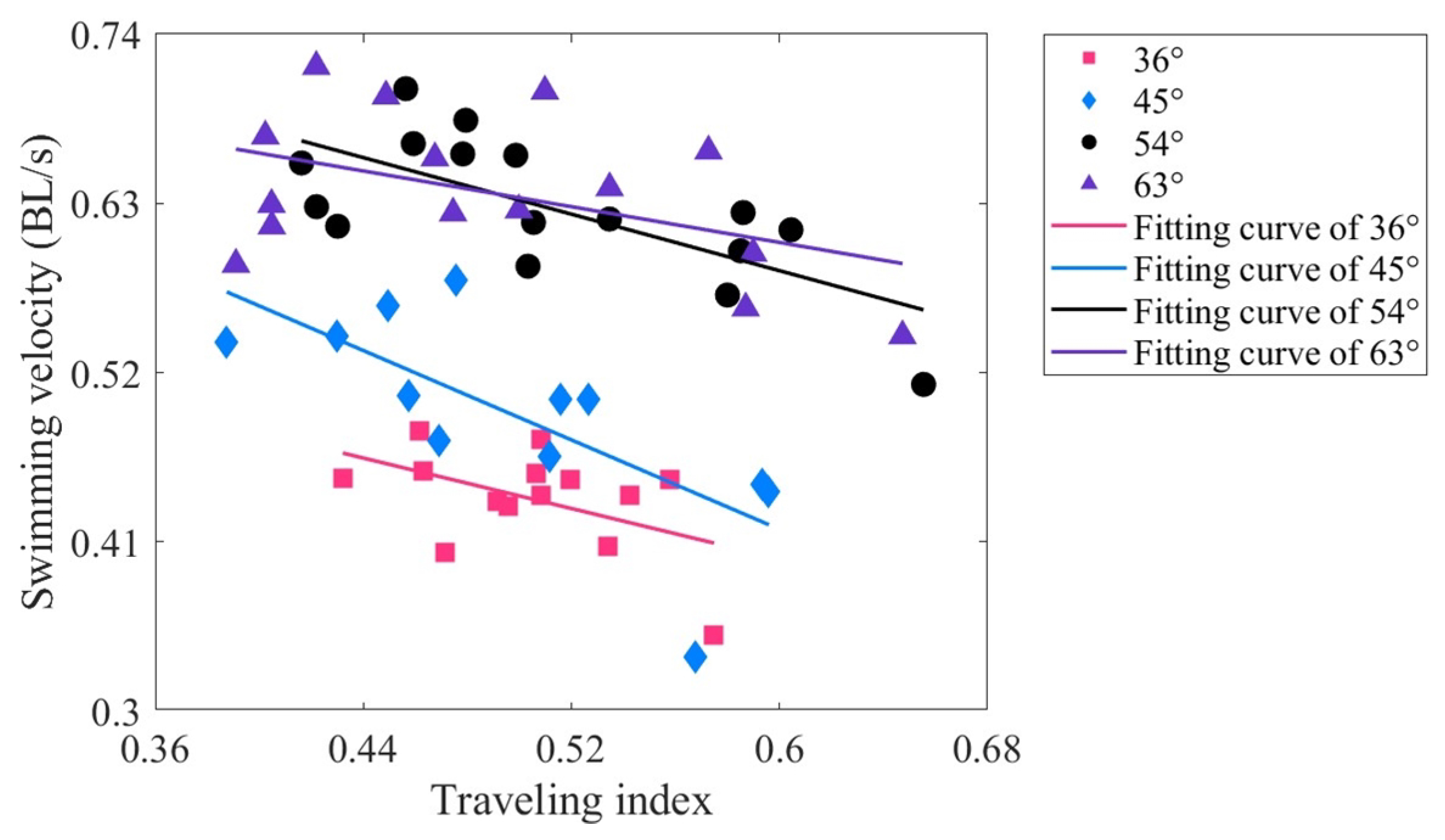
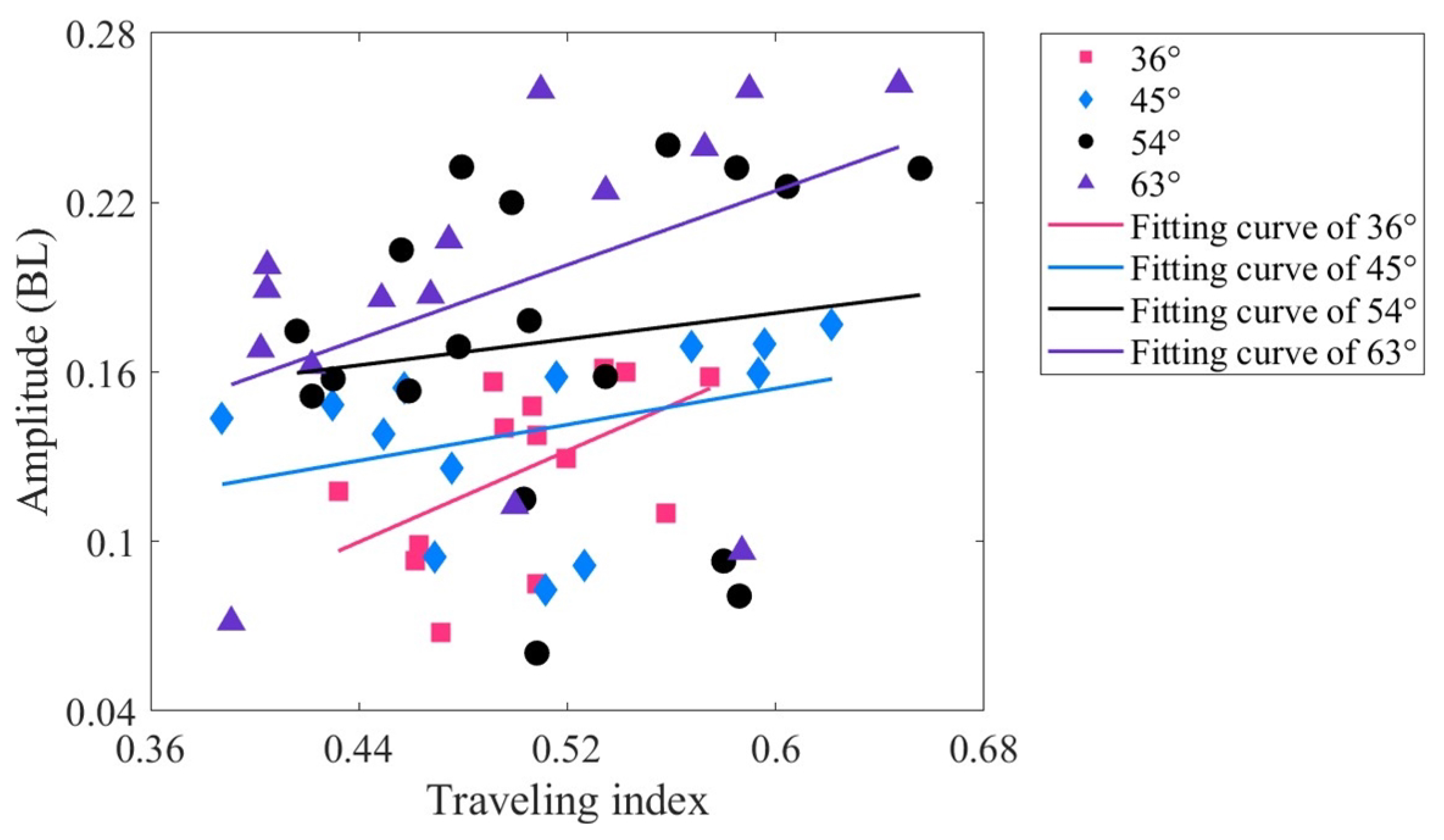
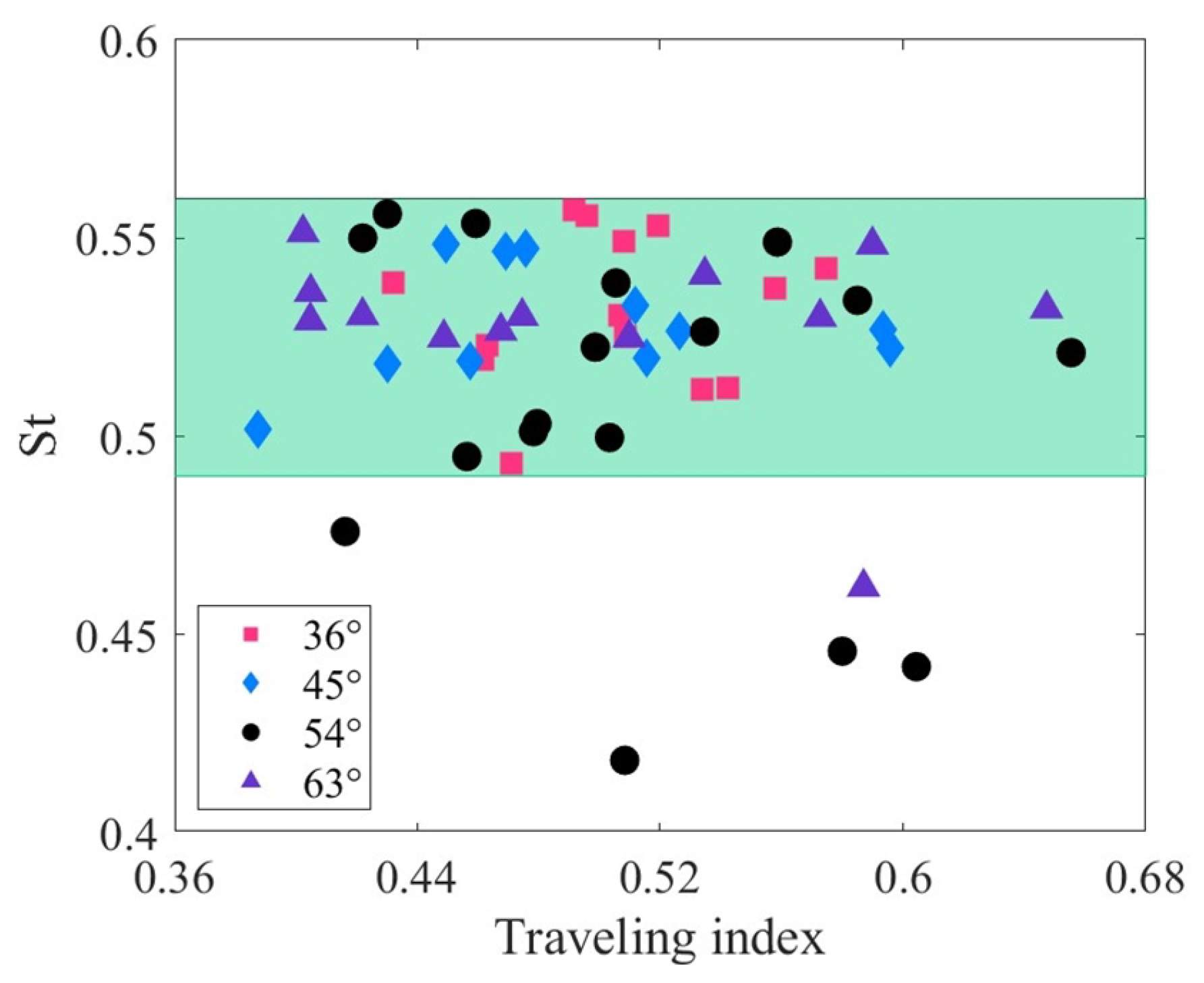
4.2. Relationship between the Traveling Index and Swimming Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Park, Y.J.; Jeong, U.; Lee, J.; Kwon, S.R.; Kim, H.Y.; Cho, K.J. Kinematic condition for maximizing the thrust of a robotic fish using a compliant caudal fin. IEEE Trans. Robot. 2012, 28, 1216–1227. [Google Scholar] [CrossRef]
- Quinn, D.B.; Lauder, G.V.; Smits, A.J. Scaling the propulsive performance of heaving flexible panels. J. Fluid Mech. 2014, 738, 250–267. [Google Scholar] [CrossRef]
- Tytell, E.D.; Leftwich, M.C.; Hsu, C.Y.; Griffith, B.E.; Cohen, A.H.; Smits, A.J.; Hamlet, C.; Fauci, L.J. Role of body stiffness in undulatory swimming: Insights from robotic and computational models. Phys. Rev. Fluids 2016, 1, 073202. [Google Scholar] [CrossRef]
- Xue, P.; Valenzuela, C.; Ma, S.; Zhang, X.; Ma, J.; Chen, Y.; Xu, X.; Wang, L. Highly Conductive MXene/PEDOT: PSS-Integrated Poly (N-Isopropylacrylamide) Hydrogels for Bioinspired Somatosensory Soft Actuators. Adv. Funct. Mater. 2023, 33, 2214867. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.; Zhang, Y.; Tan, W.; Chen, W.; Liu, L. Soft Underwater Swimming Robots Based on Artificial Muscle. Adv. Mater. Technol. 2023, 8, 2200962. [Google Scholar] [CrossRef]
- Li, T.; Li, G.; Liang, Y.; Cheng, T.; Dai, J.; Yang, X.; Liu, B.; Zeng, Z.; Huang, Z.; Luo, Y.; et al. Fast-moving soft electronic fish. Sci. Adv. 2017, 3, e1602045. [Google Scholar] [CrossRef]
- Breder, C.M. The locomotion of fishes. Zoologica 1926, 4, 159–297. [Google Scholar] [CrossRef]
- Tytell, E.D.; Hsu, C.Y.; Williams, T.L.; Cohen, A.H.; Fauci, L.J. Interactions between internal forces, body stiffness, and fluid environment in a neuromechanical model of lamprey swimming. Proc. Natl. Acad. Sci. USA 2010, 107, 19832–19837. [Google Scholar] [CrossRef]
- Valdivia y Alvarado, P.P.A. Design of Biomimetic Compliant Devices for Locomotion in Liquid Environments. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2007. [Google Scholar]
- Maertens, A.; Triantafyllou, M.S.; Yue, D.K. Efficiency of fish propulsion. Bioinspir. Biomimetics 2015, 10, 046013. [Google Scholar] [CrossRef]
- Ramananarivo, S.; Godoy-Diana, R.; Thiria, B. Propagating waves in bounded elastic media: Transition from standing waves to anguilliform kinematics. Europhys. Lett. 2014, 105, 54003. [Google Scholar] [CrossRef]
- Putrevu, H.; Subramanian, H.; Mulay, S.S. On the viscoelastic dynamic beam modelling. Int. J. Adv. Eng. Sci. Appl. Math. 2021, 13, 18–32. [Google Scholar] [CrossRef]
- Tytell, E.D.; Cooper, L.O.; Lin, Y.L.; Reis, P.M. Regulation of the swimming kinematics of lampreys Petromyzon marinus across changes in viscosity. J. Exp. Biol. 2023, 226, jeb245457. [Google Scholar] [CrossRef] [PubMed]
- Tytell, E.D.; Hsu, C.Y.; Fauci, L.J. The role of mechanical resonance in the neural control of swimming in fishes. Zoology 2014, 117, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Tytell, E.D.; Carr, J.A.; Danos, N.; Wagenbach, C.; Sullivan, C.M.; Kiemel, T.; Cowan, N.J.; Ankarali, M.M. Body stiffness and damping depend sensitively on the timing of muscle activation in lampreys. Integr. Comp. Biol. 2018, 58, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Tytell, E.D.; Lauder, G.V. The hydrodynamics of eel swimming: I. Wake structure. J. Exp. Biol. 2004, 207, 1825–1841. [Google Scholar] [CrossRef] [PubMed]
- Feeny, B. A complex orthogonal decomposition for wave motion analysis. J. Sound Vib. 2008, 310, 77–90. [Google Scholar] [CrossRef]
- Feeny, B.; Feeny, A. Complex modal analysis of the swimming motion of a whiting. J. Vib. Acoust. 2013, 135, 021004. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, Z.; Shen, L.; Jiang, H. Complex modal analysis of the movements of swimming fish propelled by body and/or caudal fin. Wave Motion 2018, 78, 83–97. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, X. Computational Study of Stiffness-Tuning Strategies in Anguilliform Fish. Biomimetics 2023, 8, 263. [Google Scholar] [CrossRef]
- Leroy-Calatayud, P.; Pezzulla, M.; Keiser, A.; Mulleners, K.; Reis, P.M. Tapered foils favor traveling-wave kinematics to enhance the performance of flapping propulsion. Phys. Rev. Fluids 2022, 7, 074403. [Google Scholar] [CrossRef]
- Muller, U.K.; Smit, J.; Stamhuis, E.J.; Videler, J.J. How the body contributes to the wake in undulatory fish swimming: Flow fields of a swimming eel (Anguilla anguilla). J. Exp. Biol. 2001, 204, 2751–2762. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, A.; Paez, L.; Melo, K.; Tytell, E.; Ijspeert, A.J.; Mulleners, K. Identification of the trade-off between speed and efficiency in undulatory swimming using a bio-inspired robot. Res. Sq. 2023, preprint. [Google Scholar] [CrossRef] [PubMed]
- Lauder, G.V. Fish locomotion: Recent advances and new directions. Annu. Rev. Mar. Sci. 2015, 7, 521–545. [Google Scholar] [CrossRef] [PubMed]
- Roper, D.; Sharma, S.; Sutton, R.; Culverhouse, P. A review of developments towards biologically inspired propulsion systems for autonomous underwater vehicles. Proc. Inst. Mech. Eng. Part J. Eng. Marit. Environ. 2011, 225, 77–96. [Google Scholar] [CrossRef]
- Raj, A.; Thakur, A. Fish-inspired robots: Design, sensing, actuation, and autonomy—A review of research. Bioinspiration Biomimetics 2016, 11, 031001. [Google Scholar] [CrossRef] [PubMed]
- Lauder, G.V.; Anderson, E.J.; Tangorra, J.; Madden, P.G. Fish biorobotics: Kinematics and hydrodynamics of self-propulsion. J. Exp. Biol. 2007, 210, 2767–2780. [Google Scholar] [CrossRef] [PubMed]
- Shintake, J.; Cacucciolo, V.; Shea, H.; Floreano, D. Soft biomimetic fish robot made of dielectric elastomer actuators. Soft Robot. 2018, 5, 466–474. [Google Scholar] [CrossRef]
- Xia, Q.; Li, H.; Song, N.; Wu, Z.; Wang, X.; Sun, X.; Zhang, S.; Yang, C. Research on flexible collapsible fluid-driven bionic robotic fish. Ocean. Eng. 2023, 276, 114203. [Google Scholar] [CrossRef]
- Di Santo, V.; Goerig, E.; Wainwright, D.K.; Akanyeti, O.; Liao, J.C.; Castro-Santos, T.; Lauder, G.V. Convergence of undulatory swimming kinematics across a diversity of fishes. Proc. Natl. Acad. Sci. USA 2021, 118, e2113206118. [Google Scholar] [CrossRef]
- Barrett, D.; Triantafyllou, M.; Yue, D.; Grosenbaugh, M.; Wolfgang, M. Drag reduction in fish-like locomotion. J. Fluid Mech. 1999, 392, 183–212. [Google Scholar] [CrossRef]
- Stefanini, C.; Orofino, S.; Manfredi, L.; Mintchev, S.; Marrazza, S.; Assaf, T.; Capantini, L.; Sinibaldi, E.; Grillner, S.; Wallén, P.; et al. A novel autonomous, bioinspired swimming robot developed by neuroscientists and bioengineers. Bioinspiration Biomimetics 2012, 7, 025001. [Google Scholar] [CrossRef] [PubMed]
- Porez, M.; Boyer, F.; Ijspeert, A.J. Improved Lighthill fish swimming model for bio-inspired robots: Modeling, computational aspects and experimental comparisons. Int. J. Robot. Res. 2014, 33, 1322–1341. [Google Scholar] [CrossRef]
- Liu, J.D.; Hu, H. Biologically inspired behaviour design for autonomous robotic fish. Int. J. Autom. Comput. 2006, 3, 336–347. [Google Scholar] [CrossRef]
- Ziegler, M.; Hoffmann, M.; Carbajal, J.P.; Pfeifer, R. Varying body stiffness for aquatic locomotion. In Proceedings of the 2011 IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; pp. 2705–2712. [Google Scholar]
- Yu, J.; Su, Z.; Wu, Z.; Tan, M. Development of a fast-swimming dolphin robot capable of leaping. IEEE/ASME Trans. Mechatronics 2016, 21, 2307–2316. [Google Scholar] [CrossRef]
- Su, Z.; Yu, J.; Tan, M.; Zhang, J. Implementing flexible and fast turning maneuvers of a multijoint robotic fish. IEEE/ASME Trans. Mechatronics 2013, 19, 329–338. [Google Scholar] [CrossRef]
- Xie, F.; Li, Z.; Ding, Y.; Zhong, Y.; Du, R. An experimental study on the fish body flapping patterns by using a biomimetic robot fish. IEEE Robot. Autom. Lett. 2019, 5, 64–71. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Z.; Du, R. A novel robot fish with wire-driven active body and compliant tail. IEEE/ASME Trans. Mechatronics 2017, 22, 1633–1643. [Google Scholar] [CrossRef]
- Zhong, Y.; Song, J.; Yu, H.; Du, R. Toward a transform method from lighthill fish swimming model to biomimetic robot fish. IEEE Robot. Autom. Lett. 2018, 3, 2632–2639. [Google Scholar] [CrossRef]
- McHenry, M.J.; Pell, C.A.; Long, J.H., Jr. Mechanical control of swimming speed: Stiffness and axial wave form in undulating fish models. J. Exp. Biol. 1995, 198, 2293–2305. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, H. Swimming performance of a tensegrity robotic fish. Soft Robot. 2019, 6, 520–531. [Google Scholar] [CrossRef]
- Micheletti, A.; Podio-Guidugli, P. Seventy years of tensegrities (and counting). Arch. Appl. Mech. 2022, 92, 2525–2548. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, Q.; Yue, X.; Wu, J.; Yang, B.; Li, Y. A review on tensegrity structures-based robots. Mech. Mach. Theory 2022, 168, 104571. [Google Scholar] [CrossRef]
- Shah, D.S.; Booth, J.W.; Baines, R.L.; Wang, K.; Vespignani, M.; Bekris, K.; Kramer-Bottiglio, R. Tensegrity robotics. Soft Robot. 2022, 9, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; White, C.; Wainwright, D.K.; Di Santo, V.; Lauder, G.V.; Bart-Smith, H. Tuna robotics: A high-frequency experimental platform exploring the performance space of swimming fishes. Sci. Robot. 2019, 4, eaax4615. [Google Scholar] [CrossRef] [PubMed]
- Jusufi, A.; Vogt, D.M.; Wood, R.J.; Lauder, G.V. Undulatory swimming performance and body stiffness modulation in a soft robotic fish-inspired physical model. Soft Robot. 2017, 4, 202–210. [Google Scholar] [CrossRef]
- White, C.H.; Lauder, G.V.; Bart-Smith, H. Tunabot Flex: A tuna-inspired robot with body flexibility improves high-performance swimming. Bioinspir. Biomimetics 2021, 16, 026019. [Google Scholar] [CrossRef]
- Wang, W.; Dai, X.; Li, L.; Gheneti, B.H.; Ding, Y.; Yu, J.; Xie, G. Three-dimensional modeling of a fin-actuated robotic fish with multimodal swimming. IEEE/ASME Trans. Mechatronics 2018, 23, 1641–1652. [Google Scholar] [CrossRef]
- Blake, R.; Li, J.; Chan, K. Swimming in four goldfish Carassius auratus morphotypes: Understanding functional design and performance employing artificially selected forms. J. Fish Biol. 2009, 75, 591–617. [Google Scholar] [CrossRef]
- Borazjani, I. Numerical Simulations of Fluid-Structure Interaction Problems in Biological Flows. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2008. [Google Scholar]
- Borazjani, I.; Sotiropoulos, F. Numerical investigation of the hydrodynamics of carangiform swimming in the transitional and inertial flow regimes. J. Exp. Biol. 2008, 211, 1541–1558. [Google Scholar] [CrossRef]
- Videler, J.; Wardle, C. Fish swimming stride by stride: Speed limits and endurance. Rev. Fish Biol. Fish. 1991, 1, 23–40. [Google Scholar] [CrossRef]
- Videler, J.J. Fish Swimming; Chapman & Hall: London, UK, 1993; Volume 10. [Google Scholar]
- Bhayadia, A.; Olivett, A.; Singh, T.; Karami, M.A. Input shaping for travelling wave generation. Smart Mater. Struct. 2022, 31, 055006. [Google Scholar] [CrossRef]
- Wilkening, J. Traveling-Standing Water Waves. Fluids 2021, 6, 187. [Google Scholar] [CrossRef]
- Akers, B.F.; Reeger, J.A. Three-dimensional overturned traveling water waves. Wave Motion 2017, 68, 210–217. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; He, B.; Yan, T. Hydrodynamic numerical simulation and prediction of bionic fish based on computational fluid dynamics and multilayer perceptron. Eng. Appl. Comput. Fluid Mech. 2022, 16, 858–878. [Google Scholar] [CrossRef]
- Shen, Q.; Olsen, Z.; Stalbaum, T.; Trabia, S.; Lee, J.; Hunt, R.; Kim, K.; Kim, J.; Oh, I.K. Basic design of a biomimetic underwater soft robot with switchable swimming modes and programmable artificial muscles. Smart Mater. Struct. 2020, 29, 035038. [Google Scholar] [CrossRef]
- Feeny, B. Complex modal decomposition for estimating wave properties in one-dimensional media. J. Vib. Acoust. 2013, 135, 031010. [Google Scholar] [CrossRef]
- Khalid, M.S.U.; Wang, J.; Akhtar, I.; Dong, H.; Liu, M. Modal decompositions of the kinematics of Crevalle Jack and the fluid–caudal fin interaction. Bioinspir. Biomimetics 2020, 16, 016018. [Google Scholar]
- Cui, Z.; Jiang, H. Numerical study of complex modal characteristics in anguilliform mode of fish swimming. J. Mech. Sci. Technol. 2021, 35, 4511–4521. [Google Scholar] [CrossRef]
- Cui, Z.; Jiang, H. Complex Modal Analysis of Locomotive Motions of Soft Robotic Fish. Int. J. Robot. Autom. 2021, 36, 75–81. [Google Scholar] [CrossRef]
- Long, J.H., Jr.; Porter, M.E.; Root, R.G.; Liew, C.W. Go reconfigure: How fish change shape as they swim and evolve. Integr. Comp. Biol. 2010, 50, 1120–1139. [Google Scholar] [CrossRef]
- Webb, P.W. Kinematics of plaice, Pleuronectes platessa, and cod, Gadus morhua, swimming near the bottom. J. Exp. Biol. 2002, 205, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Bale, R.; Shirgaonkar, A.A.; Neveln, I.D.; Bhalla, A.P.S.; MacIver, M.A.; Patankar, N.A. Separability of drag and thrust in undulatory animals and machines. Sci. Rep. 2014, 4, 7329. [Google Scholar] [CrossRef] [PubMed]
- Long, J.H., Jr.; Nipper, K.S. The importance of body stiffness in undulatory propulsion. Am. Zool. 1996, 36, 678–694. [Google Scholar] [CrossRef]
- Zheng, C.; Ding, J.; Dong, B.; Lian, G.; He, K.; Xie, F. How non-uniform stiffness affects the propulsion performance of a biomimetic robotic fish. Biomimetics 2022, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Hannard, F.; Mirkhalaf, M.; Ameri, A.; Barthelat, F. Segmentations in fins enable large morphing amplitudes combined with high flexural stiffness for fish-inspired robotic materials. Sci. Robot. 2021, 6, eabf9710. [Google Scholar] [CrossRef]
- Asuero, A.G.; Sayago, A.; González, A. The correlation coefficient: An overview. Crit. Rev. Anal. Chem. 2006, 36, 41–59. [Google Scholar] [CrossRef]
- Schervish, M.J. P values: What they are and what they are not. Am. Stat. 1996, 50, 203–206. [Google Scholar] [CrossRef]
- Eloy, C. On the best design for undulatory swimming. J. Fluid Mech. 2013, 717, 48–89. [Google Scholar] [CrossRef]
- Sfakiotakis, M.; Lane, D.M.; Davies, J.B.C. Review of fish swimming modes for aquatic locomotion. IEEE J. Ocean. Eng. 1999, 24, 237–252. [Google Scholar] [CrossRef]
- Lauder, G.V.; Tytell, E.D. Hydrodynamics of undulatory propulsion. Fish Physiol. 2005, 23, 425–468. [Google Scholar]
- Eloy, C. Optimal Strouhal number for swimming animals. J. Fluids Struct. 2012, 30, 205–218. [Google Scholar] [CrossRef]
- Akanyeti, O.; Liao, J.C. A kinematic model of Kármán gaiting in rainbow trout. J. Exp. Biol. 2013, 216, 4666–4677. [Google Scholar] [CrossRef] [PubMed]
- Akanyeti, O.; Thornycroft, P.J.; Lauder, G.V.; Yanagitsuru, Y.R.; Peterson, A.N.; Liao, J.C. Fish optimize sensing and respiration during undulatory swimming. Nat. Commun. 2016, 7, 11044. [Google Scholar] [CrossRef] [PubMed]
- Clapham, R.J.; Hu, H. iSplash-I: High performance swimming motion of a carangiform robotic fish with full-body coordination. In Proceedings of the 2014 IEEE International Conference on Robotics and Automation (ICRA), Hong Kong, China, 31 May–7 June 2014; pp. 322–327. [Google Scholar]
- Clapham, R.J.; Hu, H. iSplash: Realizing fast carangiform swimming to outperform a real fish. In Robot Fish: Bio-Inspired Fishlike Underwater Robots; Springer: Berlin/Heidelberg, Germany, 2015; pp. 193–218. [Google Scholar]




| Swimming Performance | Driving Amplitude () | The Correlation Coefficient | p Value |
|---|---|---|---|
| Swimming velocity | 36 | −0.487 | p < 0.01 |
| 45 | −0.717 | p > 0.05 | |
| 54 | −0.700 | p < 0.01 | |
| 63 | −0.463 | p < 0.01 | |
| Tail amplitude | 36 | 0.519 | p < 0.01 |
| 45 | 0.360 | p < 0.01 | |
| 54 | 0.141 | p < 0.01 | |
| 63 | 0.452 | p < 0.01 | |
| Stride length | 36 | 0.522 | p < 0.01 |
| 45 | 0.262 | p < 0.01 | |
| 54 | 0.228 | p < 0.01 | |
| 63 | 0.484 | p < 0.01 | |
| Strouhal number | 36 | 0.121 | p < 0.05 |
| 45 | 0.127 | p > 0.05 | |
| 54 | −0.274 | p < 0.01 | |
| 63 | 0.059 | p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Zhang, J.; Meng, Q.; Dong, H.; Jiang, H. Complex Modal Characteristic Analysis of a Tensegrity Robotic Fish’s Body Waves. Biomimetics 2024, 9, 6. https://doi.org/10.3390/biomimetics9010006
Chen B, Zhang J, Meng Q, Dong H, Jiang H. Complex Modal Characteristic Analysis of a Tensegrity Robotic Fish’s Body Waves. Biomimetics. 2024; 9(1):6. https://doi.org/10.3390/biomimetics9010006
Chicago/Turabian StyleChen, Bingxing, Jie Zhang, Qiuxu Meng, Hui Dong, and Hongzhou Jiang. 2024. "Complex Modal Characteristic Analysis of a Tensegrity Robotic Fish’s Body Waves" Biomimetics 9, no. 1: 6. https://doi.org/10.3390/biomimetics9010006





