Analysis of Structure and Function of Ladybird Leg and Subsequent Design and Fabrication of a Simplified Leg Structure for Robotic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procurement of Insects
2.2. Preparation of 3D X-ray Microscope Observation
2.3. Observation of Movement of the Tarsal Segments of the Ladybird Beetle
3. Relationship between the Tarsi Movement and the Tendons in the Ladybird Beetle, Coccinella septempunctata
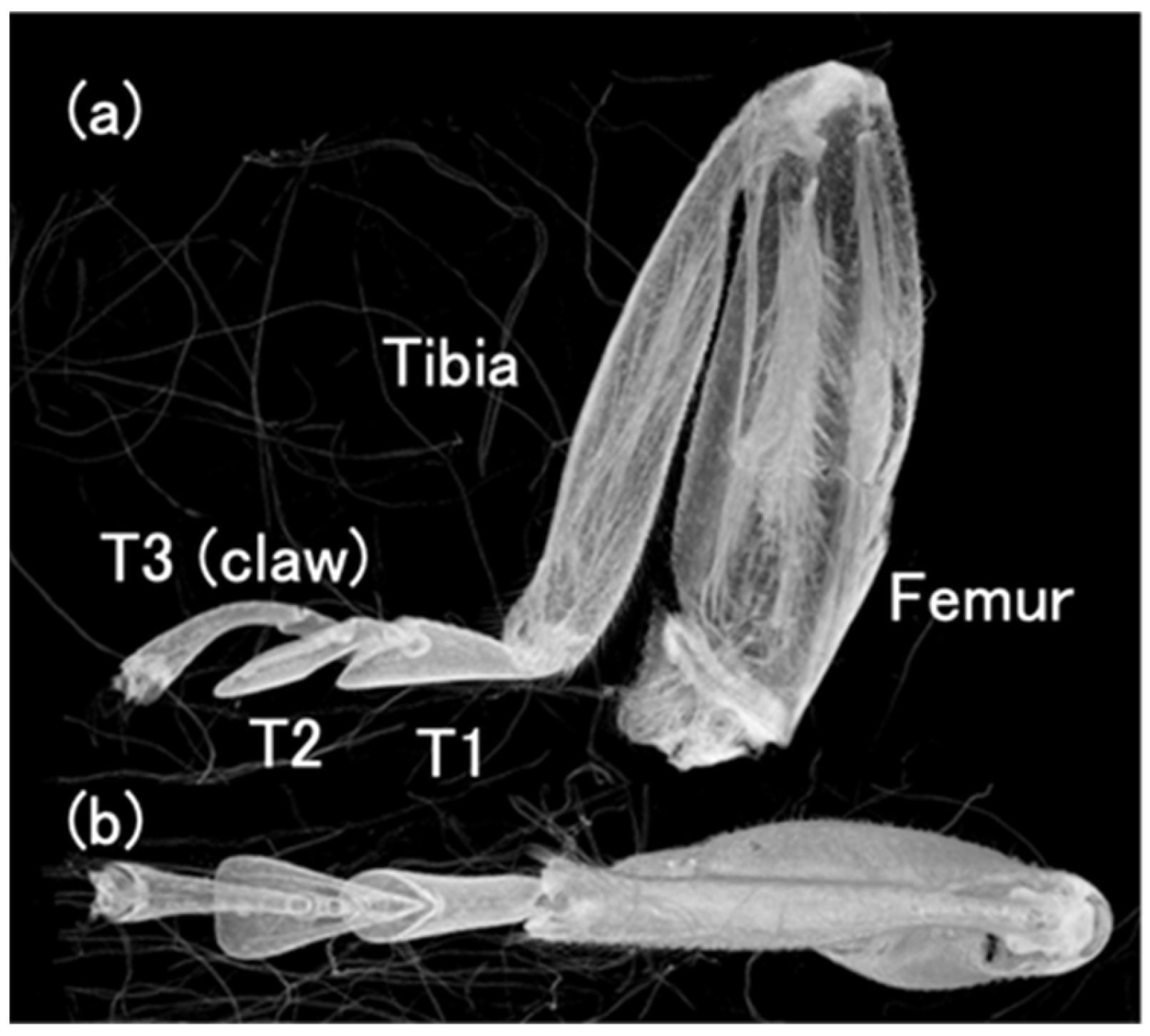
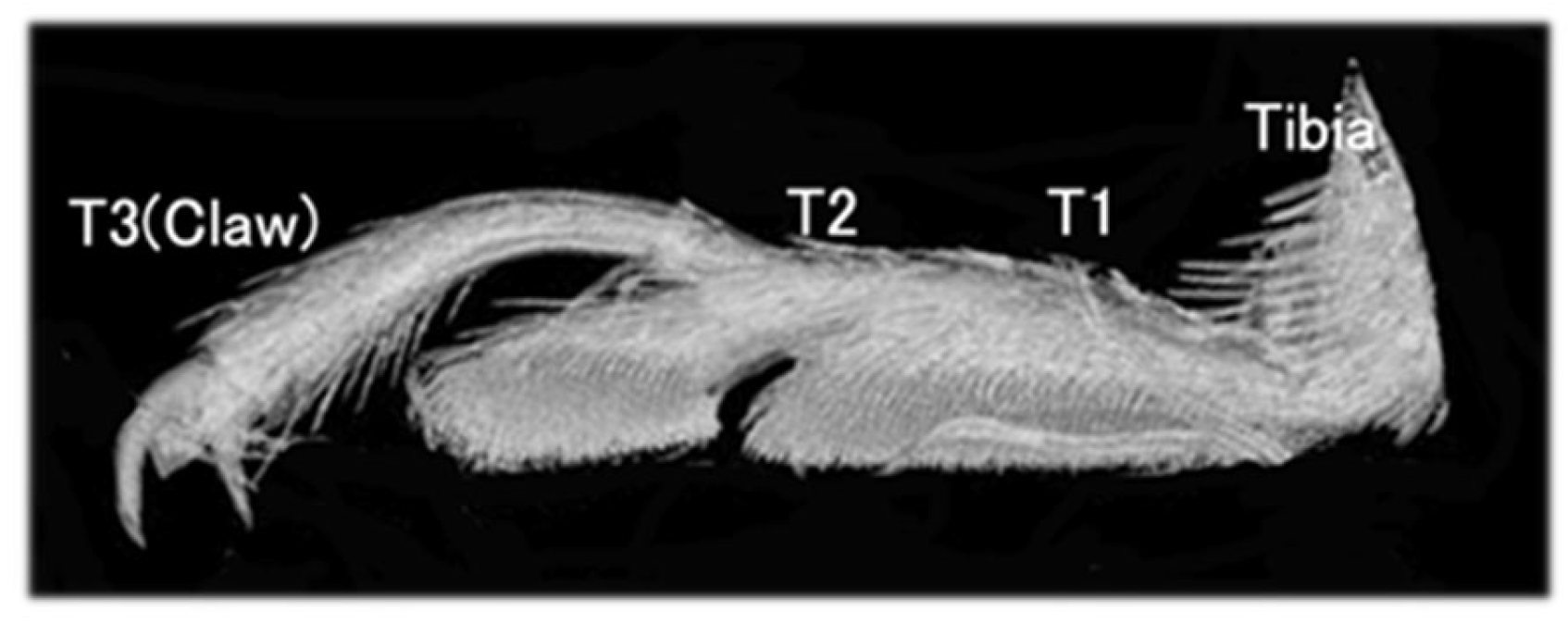
3.1. The Leg Morphology of the Ladybird Beetle C. septempunctata
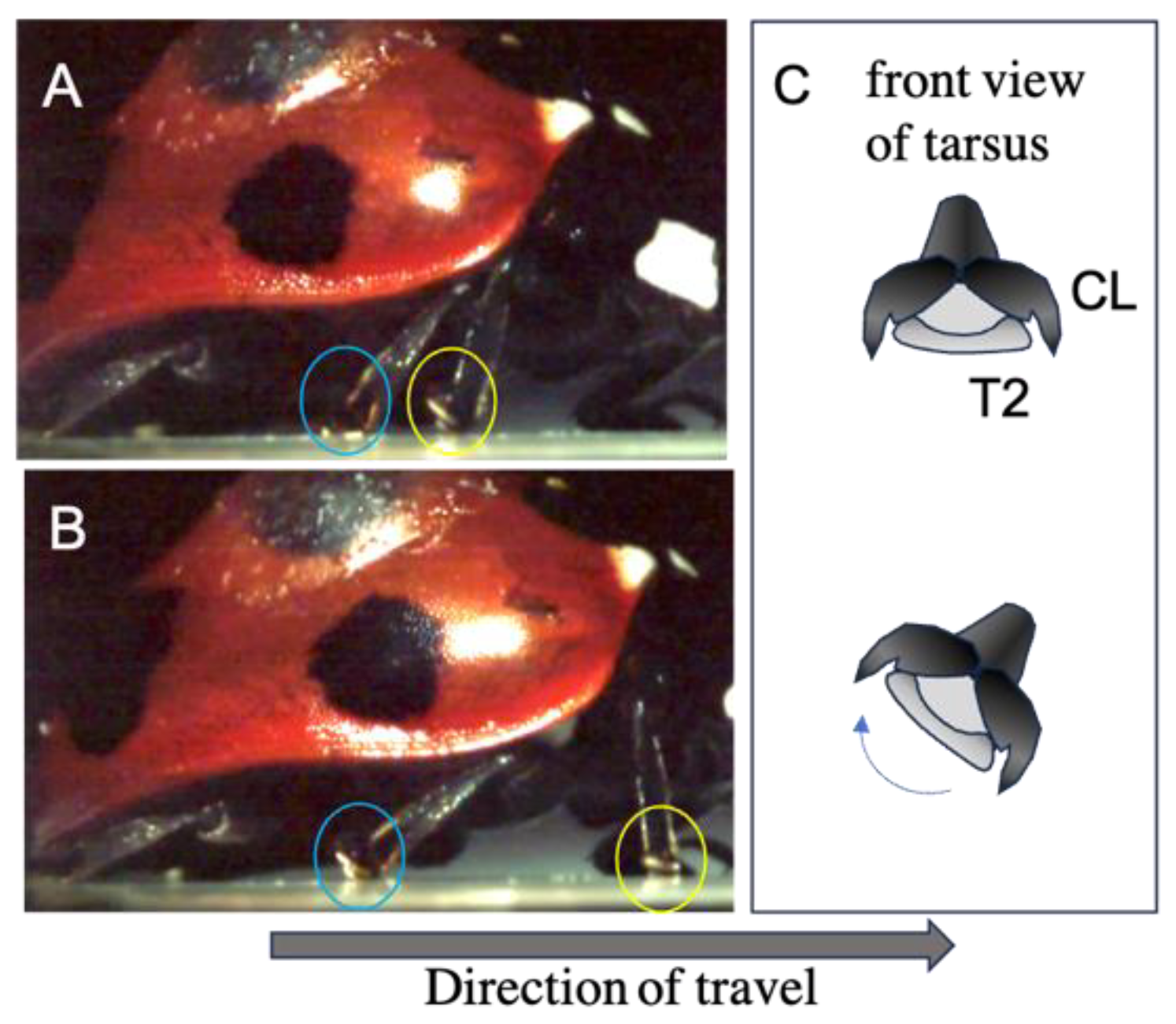
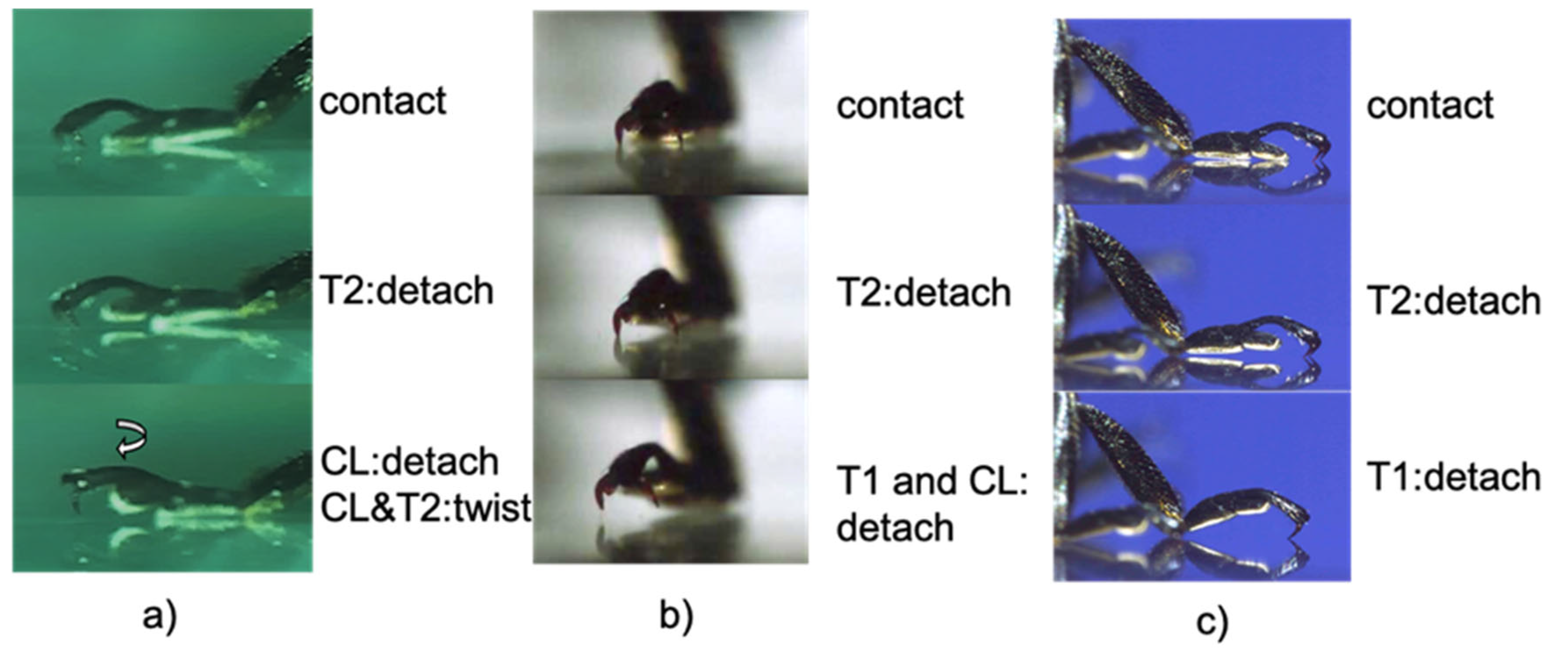
3.2. Movement of the Tarsal Segments of the Ladybird Beetle, Coccinella septempunctata, during Walking
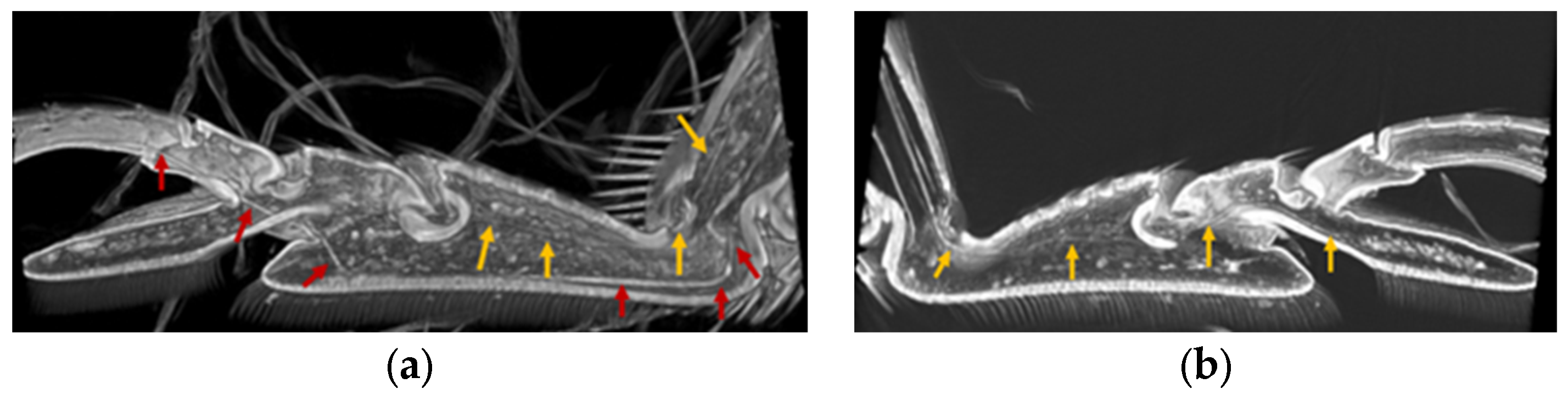
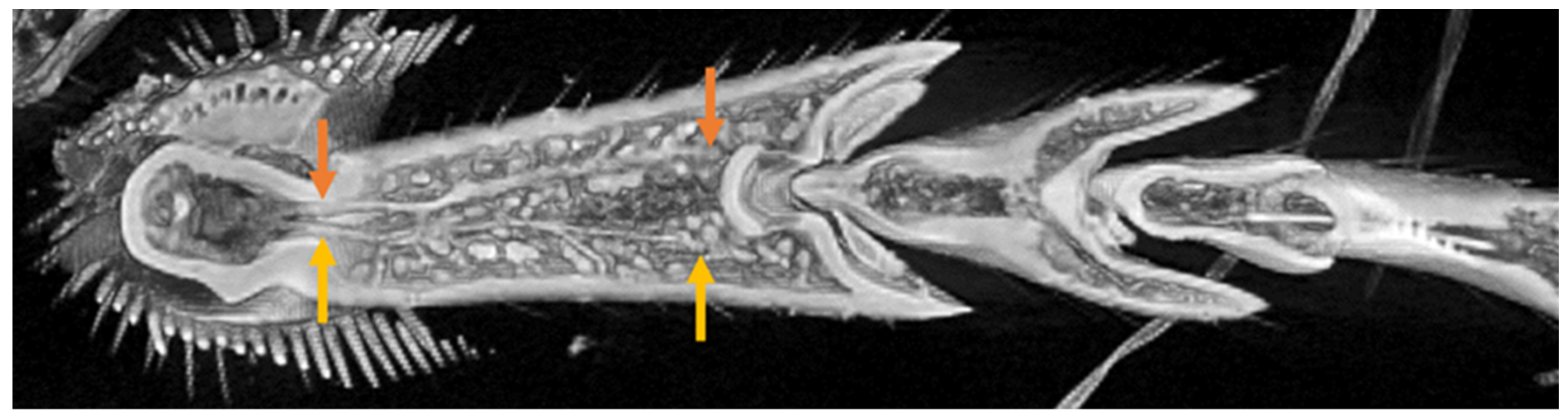
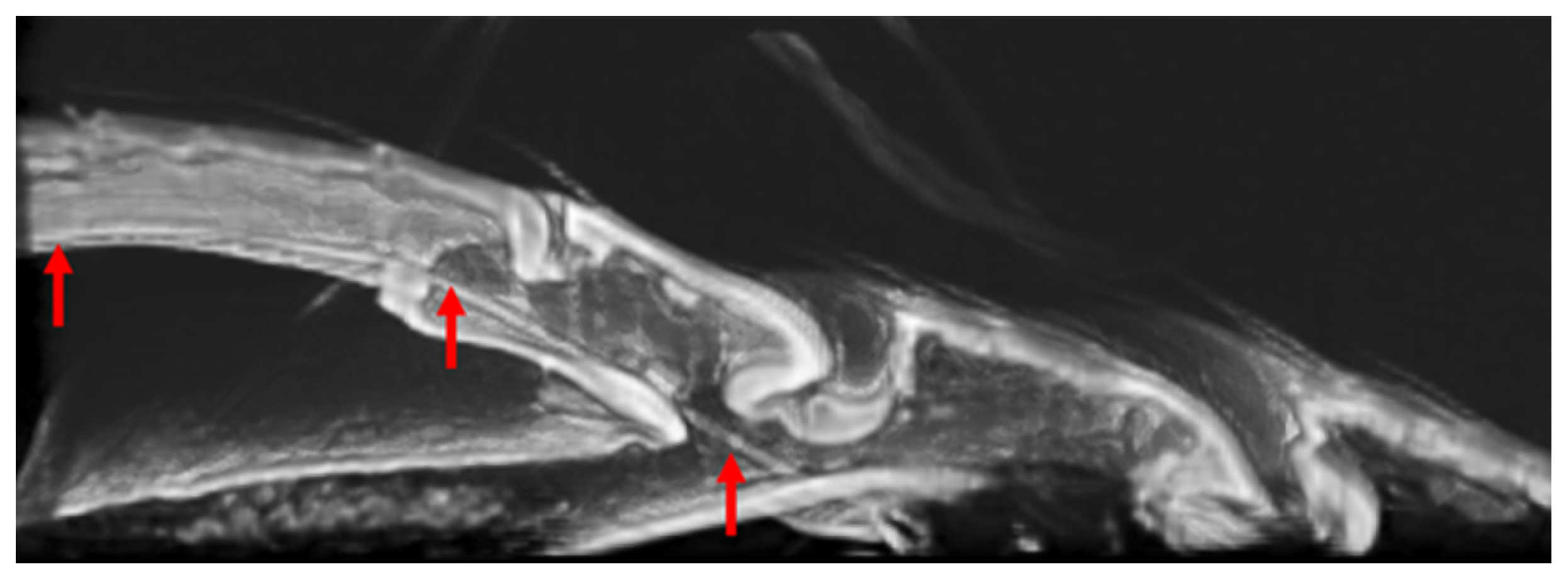
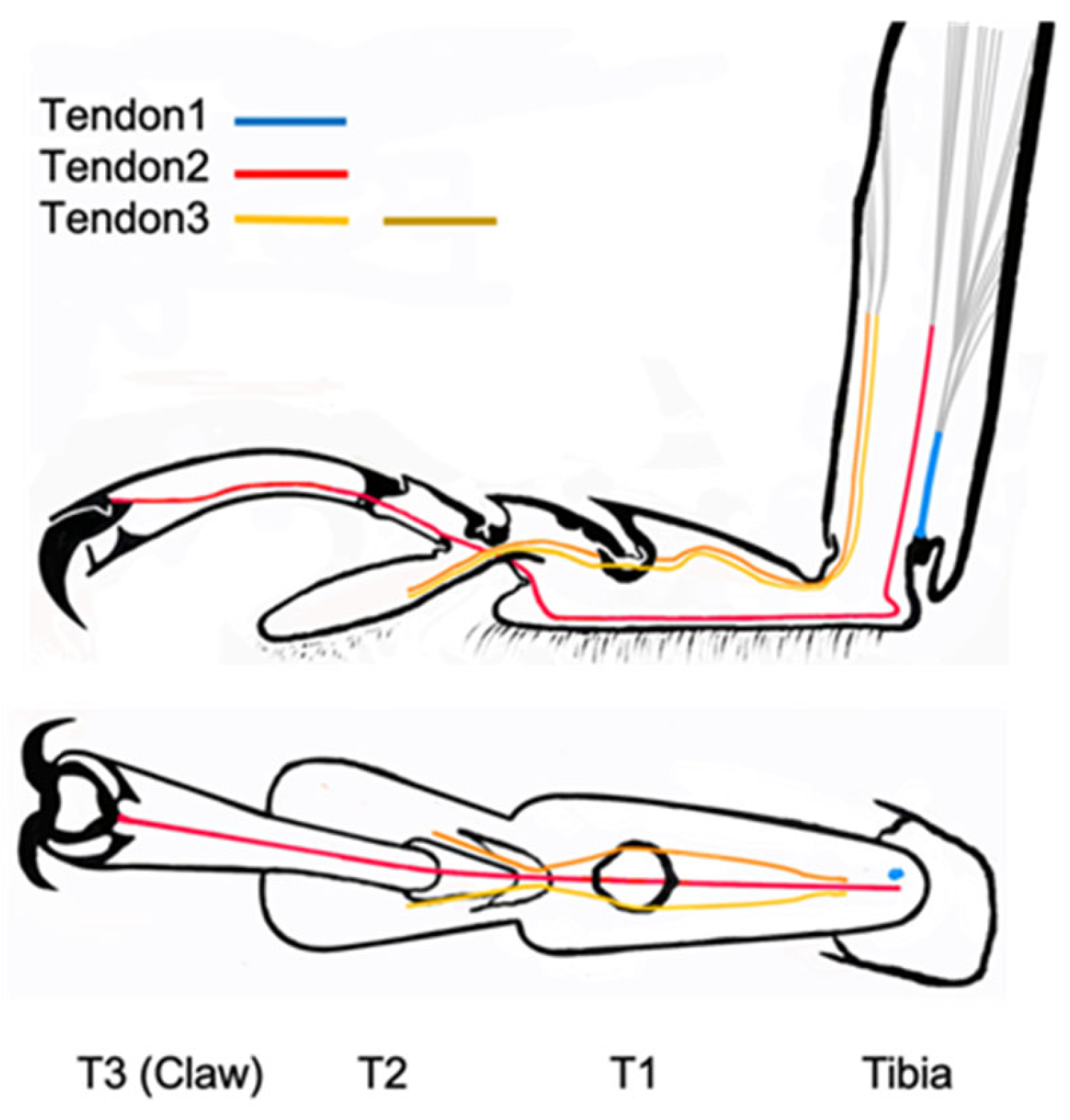
3.3. Location of Tendons in Tarsi of Ladybird Beetle
3.4. Summary of the Role of Tendons on Tarsi Movements
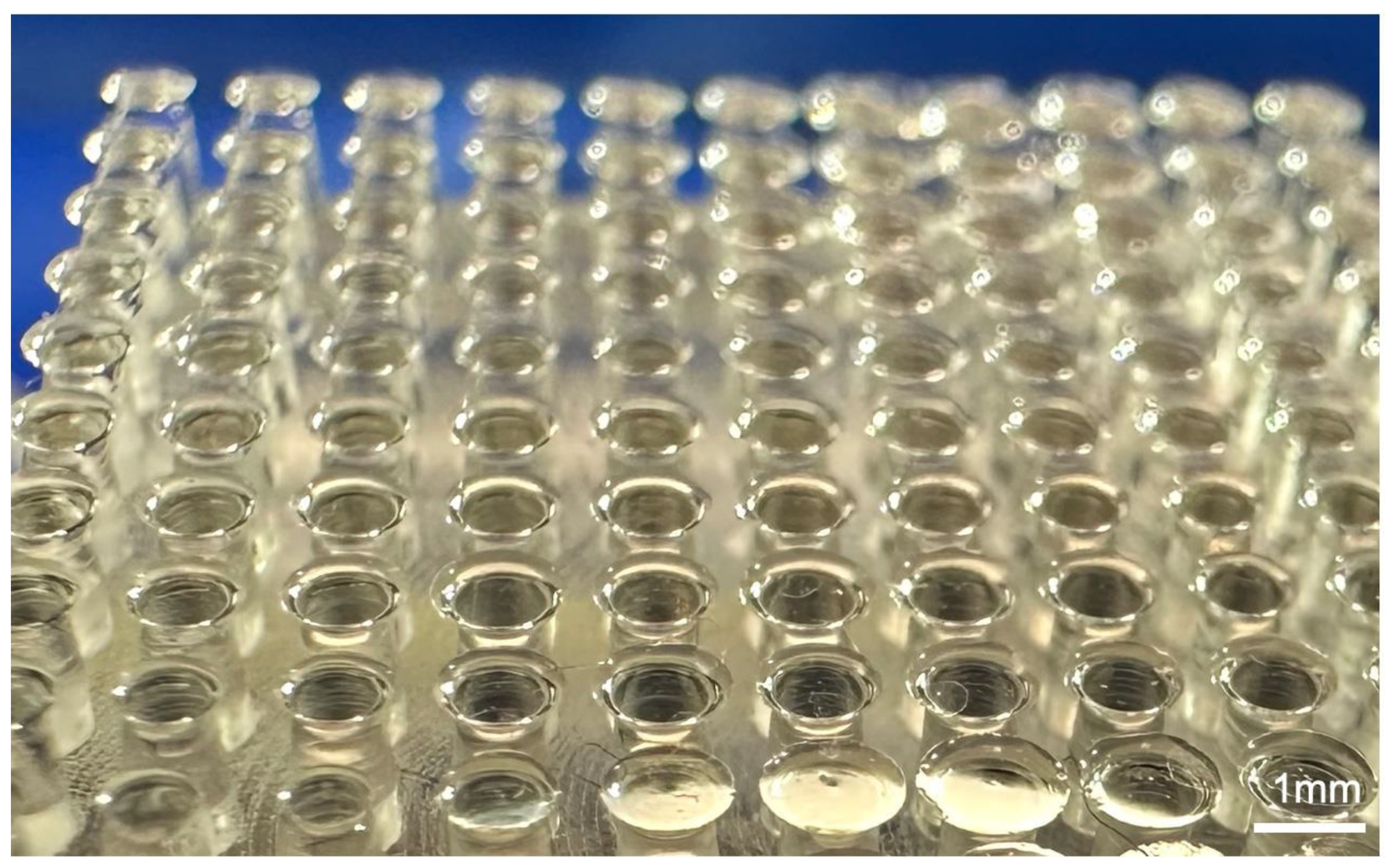
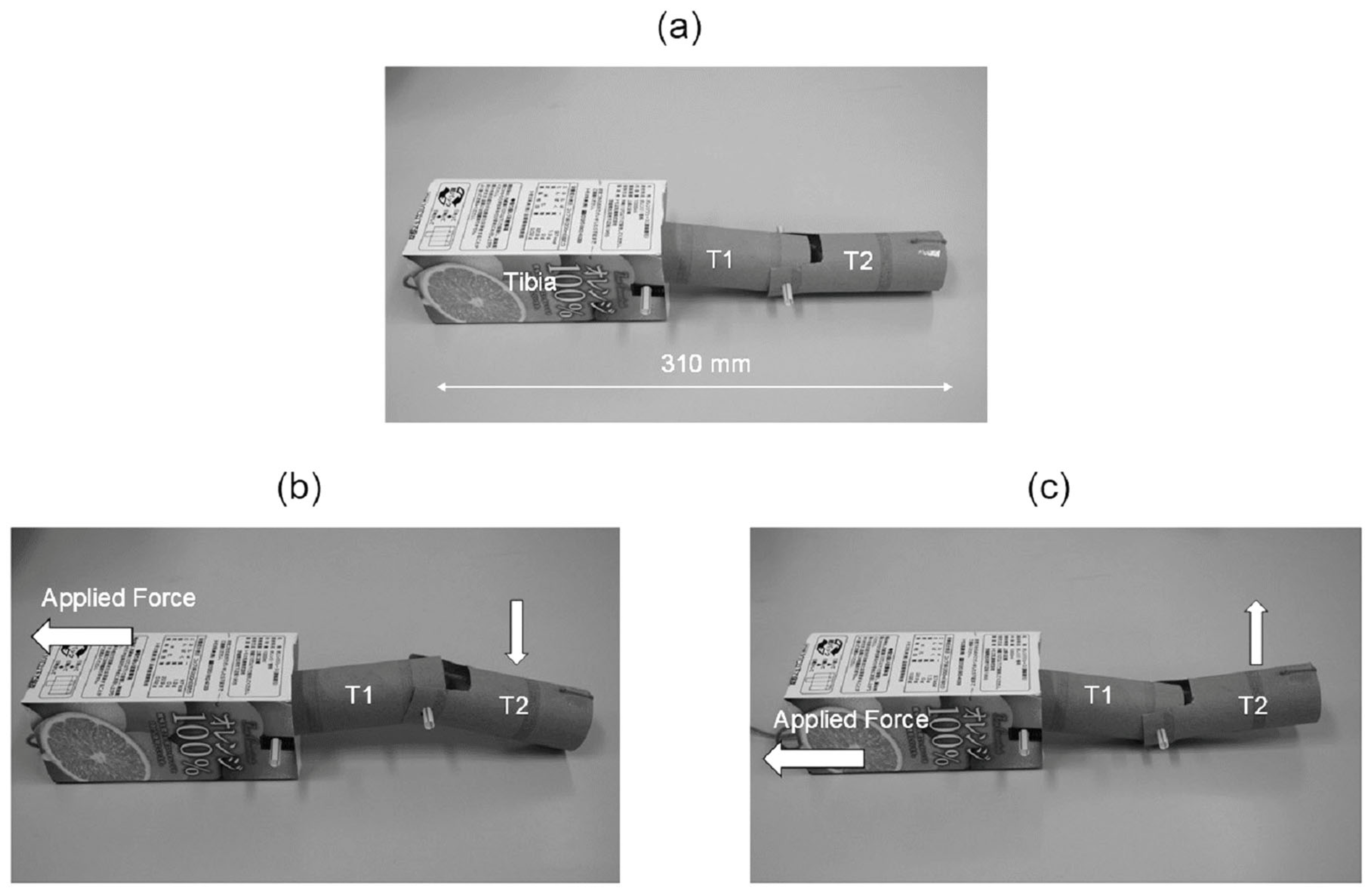
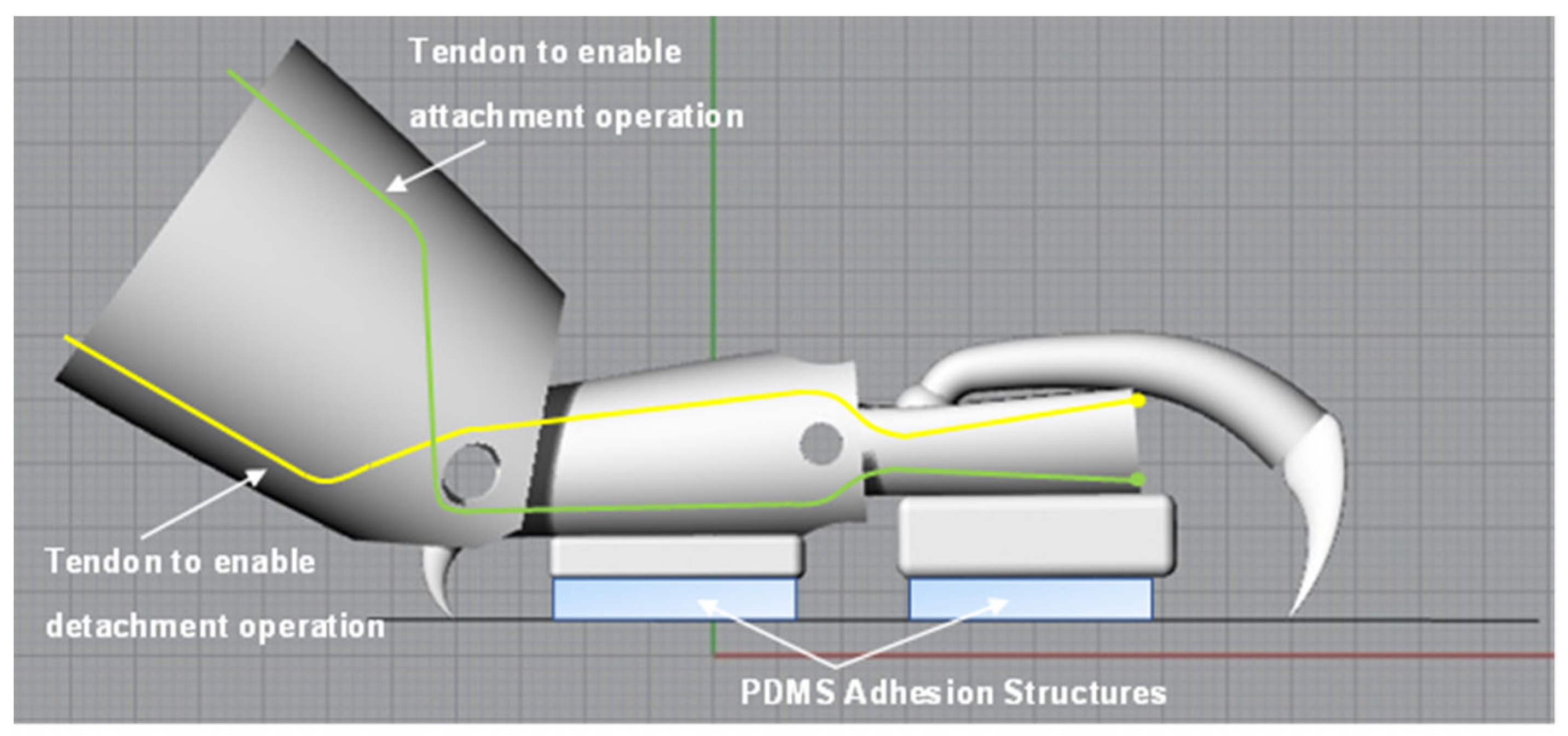
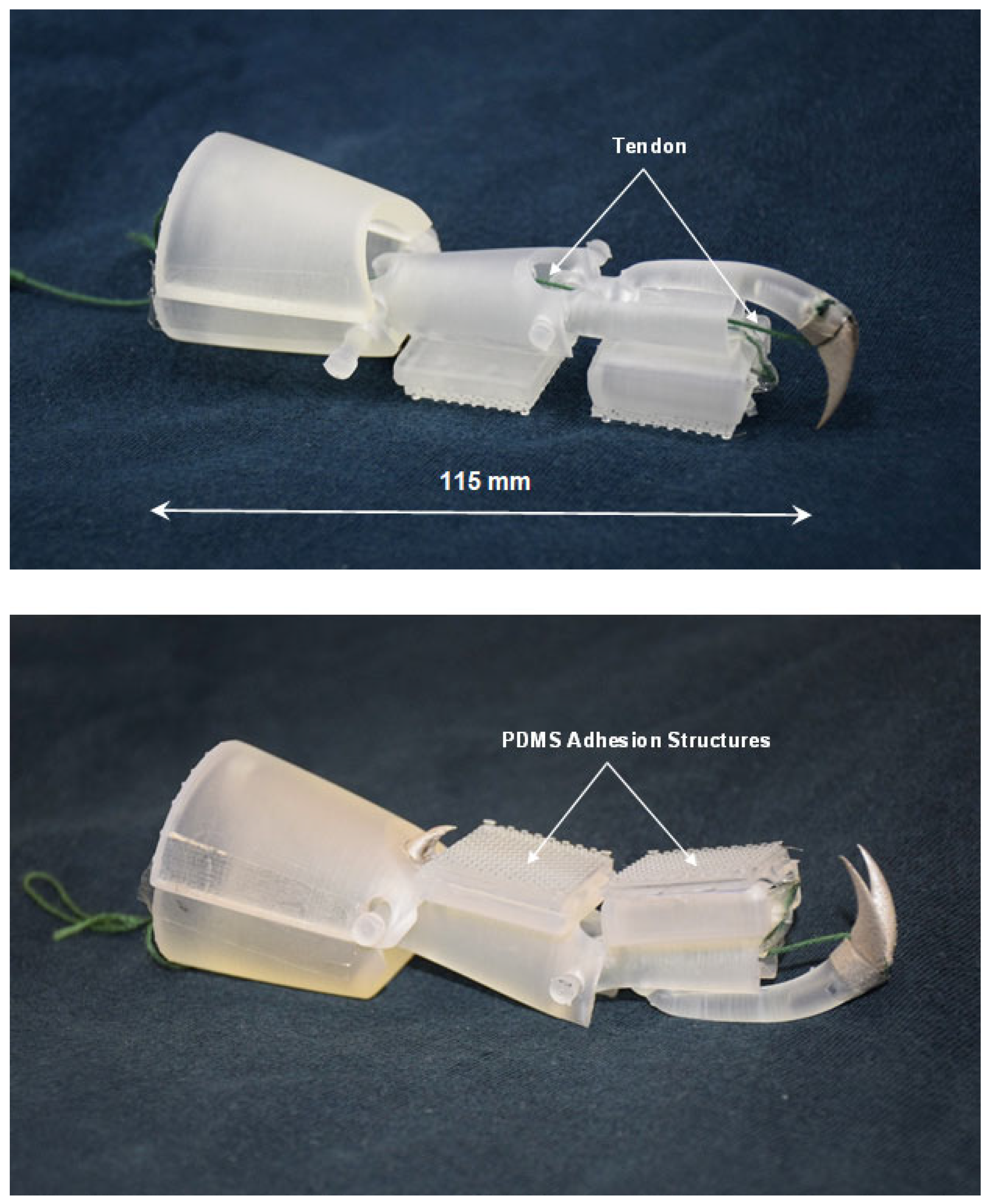
4. Design and Fabrication of Simplified Leg Structure
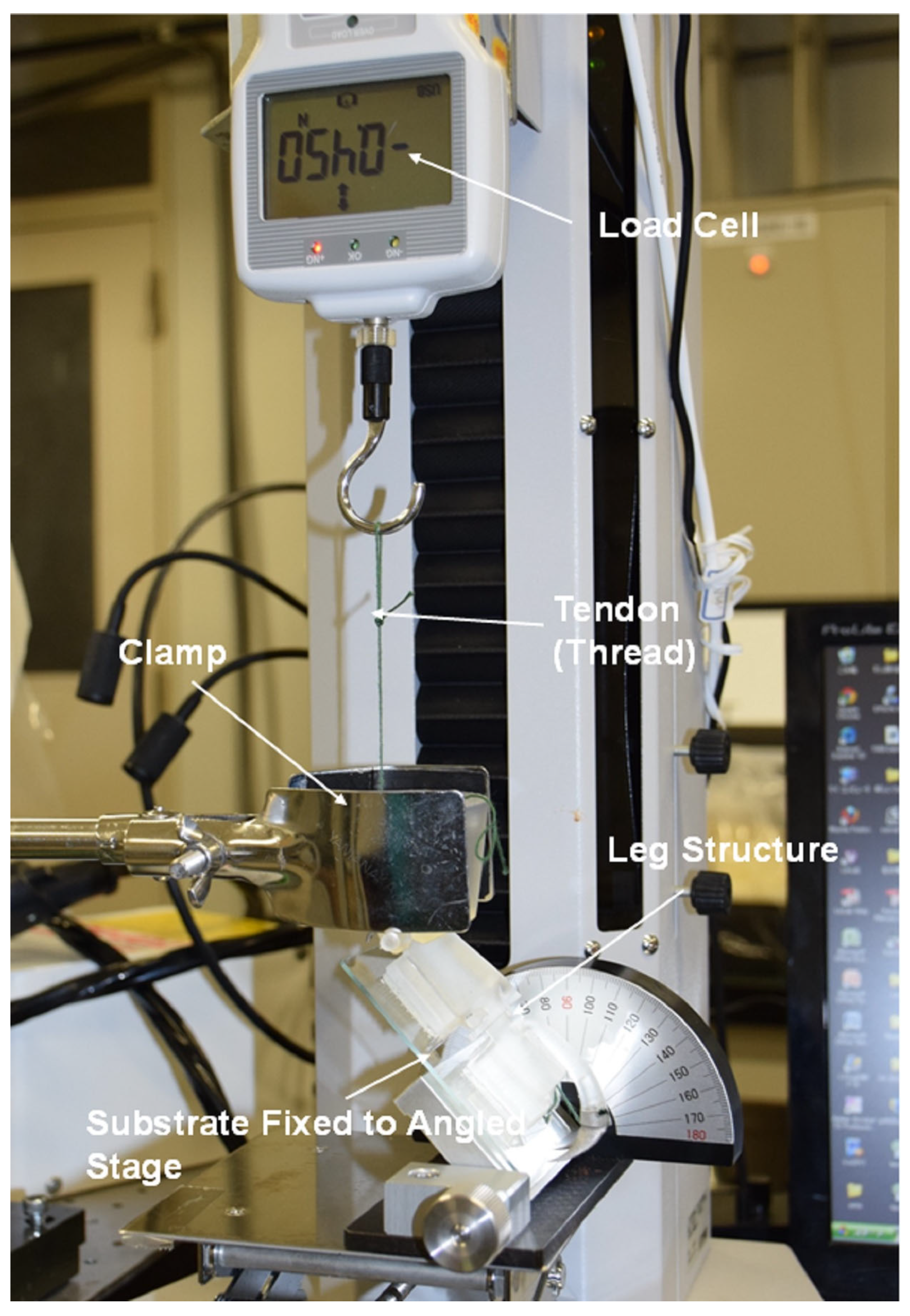
5. Experimental Procedures for Analysis of Ladybird Leg
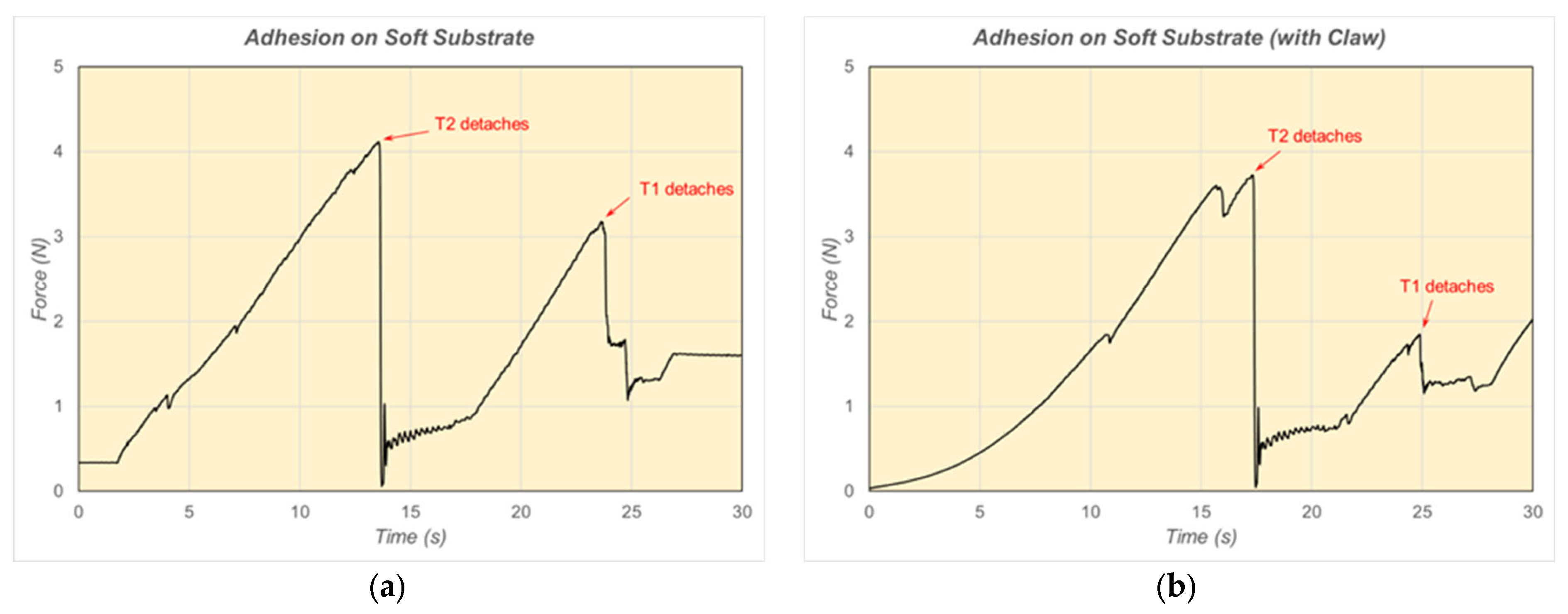
6. Results and Discussion of Adhesion Force Experiments
7. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorb, S.N. Attachment Devices of Insect Cuticle; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Stork, N.E. Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae: Coleoptera) on a variety of surfaces. J. Exp. Biol. 1980, 88, 91–108. [Google Scholar] [CrossRef]
- Gernay, S.M.; Labousse, S.; Lambert, P.; Compère, P.; Gilet, T. Multi-scale tarsal adhesion kinematics of freely-walking dock beetles. J. R. Soc. Interface 2017, 14, 20170493. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, N.; Nakamoto, M.; Suga, T.; Gorb, S.N. Evidence for intermolecular forces involved in ladybird beetle tarsal setae adhesion. Sci. Rep. 2021, 11, 7729. [Google Scholar] [CrossRef] [PubMed]
- Glassmaker, N.J.; Jagota, A.; Hui, C.-Y.; Kim, J. Design of biomimetic fibrillar interfaces: 1. Making contact. J. R. Soc. Interface 2004, 1, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Dai, L. Gecko-foot-mimetic aligned single-walled carbon nanotube dry adhesives with Unique electrical and thermal properties. Adv. Mater. 2007, 19, 3844–3849. [Google Scholar] [CrossRef]
- Santos, D.; Spenko, M.; Parness, A.; Kim, S.; Cutkosky, M. Directional adhesion for climbing: Theoretical and practical considerations. J. Adhes. Sci. Technol. 2007, 21, 1317–1341. [Google Scholar] [CrossRef]
- Gorb, S.S.; Varenberg, M.; Peressadko, A.; Tuma, J. Biomimetic mushroom-shaped fibrillar adhesive microstructure. J. R. Soc. Interface 2007, 4, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.H.; Lee, S.H.; Kim, P.; Suh, Y.K. Stretched Polymer Nanohairs by Nanodrawing. Nano Lett. 2006, 6, 1508–1513. [Google Scholar] [CrossRef]
- Hosoda, N. The Mechanisms of Organisms as Eco-Materials Design Tools. In Handbook of Sustainable Engineering; Kauffman, J., Lee, K.-M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 1249–1261. [Google Scholar]
- Eisenhaure, J.; Kim, S. A Review of the State of Dry Adhesives: Biomimetic Structures and the Alternative Designs They Inspire. Micromachines 2017, 8, 125. [Google Scholar] [CrossRef]
- Niederegger, S.; Gorb, S. Tarsal movements in flies during leg attachment and detachment on a smooth substrate. J. Insect Physiol. 2003, 49, 611–620. [Google Scholar] [CrossRef]
- Soler, C.; Daczewska, M.; Da Ponte, J.P.; Dastugue, B.; Jagla, K. Coordinated development of muscles and tendons of the Drosophila leg. Development 2004, 131, 6041–6051. [Google Scholar] [CrossRef] [PubMed]
- Radnikow, G.; Bässler, U. Function of a Muscle Whose Apodeme Travels through a Joint Moved by Other Muscles: Why the Retractor Unguis Muscle in Stick Insects is Tripartite and Has No Antagonist. J. Exp. Biol. 1991, 157, 87–99. [Google Scholar] [CrossRef]
- Bußhardt, P.; Gorb, S.N.; Wolf, H. Activity of the claw retractor muscle in stick insects in wall and ceiling situations. J. Exp. Biol. 2011, 214, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Frantsevicha, L.; Gorb, S. Structure and mechanics of the tarsal chain in the hornet, Vespa crabro (Hymenoptera: Vespidae): Implications on the attachment mechanism. Arthropod Struct. Dev. 2004, 33, 77–89. [Google Scholar] [CrossRef]
- Bußhardt, P.; Gorb, S.N. Walking on smooth and rough ground: Activity and timing of the claw retractor muscle in the beetle Pachnoda marginata peregrina (Coleoptera, Scarabaeidae). J. Exp. Biol. 2013, 216, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Parietti, F.; Harry Asada, H. Supernumerary Robotic Limbs for Human Body Support. IEEE Trans. Robot. 2016, 32, 301–311. [Google Scholar] [CrossRef]
- Khazoom, C.; Caillouette, P.; Girard, A.; Plante, J.-B. A Supernumerary Robotic Leg Powered by Magnetorheological Actuators to Assist Human Locomotion. IEEE Robot. Autom. Lett. 2020, 5, 5143–5150. [Google Scholar] [CrossRef]
- Rezazadeh, S.; Abate, A.; Hatton, R.L.; Hurst, J.W. Robot Leg Design: A Constructive Framework. IEEE Access 2018, 6, 54369–54387. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, Z.; Bai, X.; Xie, H.; Zhu, Y.; Chen, S.; Shang, J. Design and Experiments of a Human-Leg-Inspired Omnidirectional Robotic Leg. J. Bionic Eng. 2023, 20, 2570–2589. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.-S.; Kim, S. Design of a cat-inspired robotic leg for fast running. Adv. Robot. 2014, 28, 1587–1598. [Google Scholar] [CrossRef]
- Daltorio, K.A.; Horchler, A.D.; Gorb, S.; Ritzmann, R.E.; Quinn, R.D. A Small Wall-Walking Robot with Compliant Adhesive Feet. In Proceedings of the 2005 IEEE/RSJ International Conference on Intelligent Robots and Systems, Edmonton, AB, Canada, 2–6 August 2005; pp. 4018–4023. [Google Scholar]
- Kim, S.; Spenko, M.; Trujillo, S.; Heyneman, B.; Santos, D.; Cutkosky, M. Smooth Vertical Surface Climbing with Directional Adhesion. IEEE Trans. Robot. 2008, 24, 65–74. [Google Scholar]
- Bian, S.; Xu, F.; Wei, Y.; Kong, D. A Novel Type of Wall-Climbing Robot with a Gear Transmission System Arm and Adhere Mechanism Inspired by Cicada and Gecko. Appl. Sci. 2021, 11, 4137. [Google Scholar] [CrossRef]
- Autumn, K.; Hsieh, S.; Dudek, D.; Chen, J.; Chitaphan, C.; Full, R. Dynamics of geckos running vertically. J. Exp. Biol. 2006, 209, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K.; Dittmore, A.; Santos, D.; Spenko, M.; Cutkosky, M. Frictional adhesion: A new angle on gecko attachment. J. Exp. Biol. 2006, 209, 3569–3579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, X.; Li, M.; Wei, Y.; Liang, P. DP-Climb: A Hybrid Adhesion Climbing Robot Design and Analysis for Internal Transition. Machines 2022, 10, 678. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Yao, H.; Gorb, S.; Arzt, E. Mechanics of hierarchical adhesion structures of geckos. Mech. Mater. 2005, 37, 275–285. [Google Scholar] [CrossRef]
- Tran-Ngoc, P.T.; Lim, L.Z.; Gan, J.H.; Wang, H.; Vo-Doan, T.T.; Sato, H. A robotic leg inspired from an insect leg. Bioinspir. Biomim. 2022, 17, 056008. [Google Scholar] [CrossRef]






| Substrate | Maximum Force (N) | Average Force (N) | Standard Deviation |
|---|---|---|---|
| Dry Glass | 4.4 | 3.6 | 0.8 |
| Wet Glass | 6.8 | 4.7 | 1.6 |
| Soft Silicone Rubber | 4.1 | 2.2 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercer, C.; Hosoda, N. Analysis of Structure and Function of Ladybird Leg and Subsequent Design and Fabrication of a Simplified Leg Structure for Robotic Applications. Biomimetics 2024, 9, 184. https://doi.org/10.3390/biomimetics9030184
Mercer C, Hosoda N. Analysis of Structure and Function of Ladybird Leg and Subsequent Design and Fabrication of a Simplified Leg Structure for Robotic Applications. Biomimetics. 2024; 9(3):184. https://doi.org/10.3390/biomimetics9030184
Chicago/Turabian StyleMercer, Christopher, and Naoe Hosoda. 2024. "Analysis of Structure and Function of Ladybird Leg and Subsequent Design and Fabrication of a Simplified Leg Structure for Robotic Applications" Biomimetics 9, no. 3: 184. https://doi.org/10.3390/biomimetics9030184





