Biocompatible Composite Filaments Printable by Fused Deposition Modelling Technique: Selection of Tuning Parameters by Influence of Biogenic Hydroxyapatite and Graphene Nanoplatelets Ratios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Precursor Materials
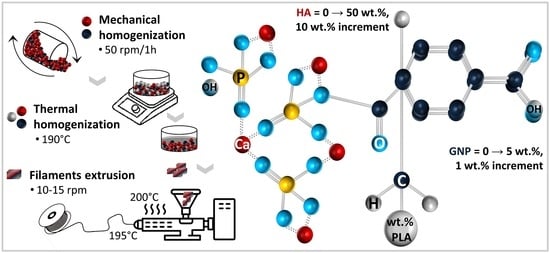
2.2. Preparation of PLA/HA/GNP Composite Filaments
2.2.1. Cylindrical Composite Pellets Destined for Wettability and Mechanical Investigations
2.2.2. Composite Filaments Extrusion
2.3. Experimental Characterization Techniques
3. Results and Discussion
3.1. FT–IR Evaluation
3.2. SEM/EDS Evaluation
3.3. Contact Angle and Surface Energy Investigations
3.4. Mechanical Features Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Al Abir, A.; Chakrabarti, D.; Trindade, B. Fused Filament Fabricated Poly (lactic acid) Parts Reinforced with Short Carbon Fiber and Graphene Nanoparticles with Improved Tribological Properties. Polymers 2023, 15, 2451. [Google Scholar] [CrossRef]
- You, X.; Zhang, Q.; Yang, J.; Dong, S. Review on 3D-printed graphene-reinforced composites for structural applications. Compos. Part A Appl. Sci. Manuf. 2023, 167, 107420. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef]
- Nejaim, Y.; Farias Gomes, A.; Queiroz, P.M.; da Silva Siqueira, A.; Muñoz, P.A.R.; Fechine, G.J.M.; Haiter-Neto, F. Artifact expression of polylactic acid/hydroxyapatite/graphene oxide nanocomposite in CBCT: A promising dental material. Clin. Oral. Investig. 2020, 24, 1695–1700. [Google Scholar] [CrossRef]
- Backes, E.H.; de Nóbile Pires, L.; Selistre-de-Araujo, H.S.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Development and characterization of printable PLA/β-TCP bioactive composites for bone tissue applications. J. Appl. Polym. Sci. 2021, 138, 49759. [Google Scholar] [CrossRef]
- Michael, F.M.; Shee, L.S.; Raju, G.; Rustagi, S.; Walvekar, R.; Chaudhary, V.; Khalid, M. Biocompatibility of poly-lactic acid/nanohydroxyapatite/graphene nanocomposites for load bearing bone implants. J. Electrochem. Soc. 2023, 170, 027502. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, D.M. Influence of deposition temperature on the properties of hydroxyapatite obtained by electrochemical assisted deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Stan, G.E.; Tite, T.; Popa, A.-C.; Chirica, I.M.; Negrila, C.C.; Besleaga, C.; Zgura, I.; Sergentu, A.C.; Popescu-Pelin, G.; Cristea, D. The Beneficial Mechanical and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings. Coatings 2020, 10, 1119. [Google Scholar] [CrossRef]
- Miculescu, F.; Miculescu, M.; Ciocan, L.; Ernuteanu, A.; Antoniac, I.; Pencea, I.; Matei, E. Comparative studies regarding heavy elements concentration in human cortical bone. Dig. J. Nanomater. Biostruct. 2011, 6, 1117–1127. [Google Scholar]
- Vladescu, A.; Badea, M.; Padmanabhan, S.C.; Paraschiv, G.; Floroian, L.; Gaman, L.; Morris, M.A.; Marty, J.-L.; Cotrut, C.M. Nanomaterials for medical applications and their antimicrobial advantages. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–431. [Google Scholar] [CrossRef]
- Ahmed, J.; Bher, A.; Auras, R.; Al-Zuwayed, S.A.; Joseph, A.; Mulla, M.F.; Alazemi, A. Morphological, thermo-mechanical, and barrier properties of coextruded multilayer polylactide composite films reinforced with graphene nanoplatelets and encapsulated thyme essential oil. Food Packag. Shelf Life 2023, 40, 101179. [Google Scholar] [CrossRef]
- Brancewicz-Steinmetz, E.; Sawicki, J. Bonding and strengthening the PLA biopolymer in multi-material additive manufacturing. Materials 2022, 15, 5563. [Google Scholar] [CrossRef]
- Hossain, M.I.; Chowdhury, M.A.; Zahid, M.S.; Sakib-Uz-Zaman, C.; Rahaman, M.L.; Kowser, M.A. Development and analysis of nanoparticle infused plastic products manufactured by machine learning guided 3D printer. Polym. Test. 2022, 106, 107429. [Google Scholar] [CrossRef]
- Babilotte, J.; Guduric, V.; Le Nihouannen, D.; Naveau, A.; Fricain, J.C.; Catros, S. 3D printed polymer–mineral composite biomaterials for bone tissue engineering: Fabrication and characterization. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2579–2595. [Google Scholar] [CrossRef]
- Singhvi, M.; Zinjarde, S.; Gokhale, D. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Laput, O.; Vasenina, I.; Salvadori, M.C.; Savkin, K.; Zuza, D.; Kurzina, I. Low-temperature plasma treatment of polylactic acid and PLA/HA composite material. J. Mater. Sci. 2019, 54, 11726–11738. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.; Ghazali, S.; Heim, H.P.; Feldmann, M. Impact modified PLA-hydroxyapatite composites–Thermo-mechanical properties. Compos. Part A Appl. Sci. Manuf. 2018, 107, 326–333. [Google Scholar] [CrossRef]
- Mohammadi Zerankeshi, M.; Sayedain, S.S.; Tavangarifard, M.; Alizadeh, R. Developing a novel technique for the fabrication of PLA-graphite composite filaments using FDM 3D printing process. Ceram. Int. 2022, 48, 31850–31858. [Google Scholar] [CrossRef]
- Ananth, K.P.; Guo, B.; Zhang, C.; Wang, W.; Zhou, P.; Bai, J. Investigation of biphasic calcium phosphate (BCp)/polyvinylpyrrolidone (PVp)/graphene oxide (GO) composite for biomedical implants. Ceram. Int. 2020, 46, 24413–24423. [Google Scholar] [CrossRef]
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Ioan Voicu, S.; Ciocan, L.T. Synthesis and characterization of pla-micro-structured hydroxyapatite composite films. Materials 2020, 13, 274. [Google Scholar] [CrossRef]
- Stan, G.; Popa, A.; Bojin, D. Bioreactivity evaluation in simulated body fluid of magnetron sputtered glass and glass-ceramic coatings: A FTIR spectroscopy study. Dig. Dig. J. Nanomater. Biostruct. 2010, 5, 557–566. [Google Scholar]
- Mocanu, A.-C.; Miculescu, M.; Machedon-Pisu, T.; Maidaniuc, A.; Ciocoiu, R.C.; Ioniță, M.; Pasuk, I.; Stan, G.E.; Miculescu, F. Internal and external surface features of newly developed porous ceramics with random interconnected 3D channels by a fibrous sacrificial porogen method. Appl. Surf. Sci. 2019, 489, 226–238. [Google Scholar] [CrossRef]
- Miculescu, F.; Ciocan, L.; Miculescu, M.; Ernuteanu, A. Effect of heating process on micro structure level of cortical bone prepared for compositional analysis. Dig. J. Nanomater. Biostruct. 2011, 6, 225–233. [Google Scholar]
- Popa, A.; Stan, G.; Husanu, M.; Mercioniu, I.; Santos, L.; Fernandes, H.; Ferreira, J. Bioglass implant-coating interactions in synthetic physiological fluids with varying degrees of biomimicry. Int. J. Nanomed. 2017, 12, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, F.; Maidaniuc, A.; Miculescu, M.; Dan Batalu, N.; Cătălin Ciocoiu, R.; Voicu, S.t.I.; Stan, G.E.; Thakur, V.K. Synthesis and characterization of jellified composites from bovine bone-derived hydroxyapatite and starch as precursors for robocasting. ACS Omega 2018, 3, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Maidaniuc, A.; Miculescu, F.; Voicu, S.I.; Andronescu, C.; Miculescu, M.; Matei, E.; Mocanu, A.C.; Pencea, I.; Csaki, I.; Machedon-Pisu, T. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2018, 438, 158–166. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, M.; Voicu, S.; Ciocan, L.; Niculescu, M.; Corobea, M.; Rada, M.; Miculescu, F. Effect of micron sized silver particles concentration on the adhesion induced by sintering and antibacterial properties of hydroxyapatite microcomposites. J. Adhes. Sci. Technol. 2016, 30, 1829–1841. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, F.; Ciocoiu, R.C.; Butte, T.M.; Pasuk, I.; Stan, G.E.; Voicu, S.I.; Ciocan, L.T. Effect of the processing parameters on surface, physico-chemical and mechanical features of bioceramics synthesized from abundant carp fish bones. Ceram. Int. 2020, 46, 10159–10171. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Stan, G.E.; Maidaniuc, A.; Miculescu, M.; Antoniac, I.V.; Ciocoiu, R.-C.; Voicu, Ș.I.; Mitran, V.; Cîmpean, A.; Miculescu, F. Naturally-Derived Biphasic Calcium Phosphates through Increased Phosphorus-Based Reagent Amounts for Biomedical Applications. Materials 2019, 12, 381. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Miculescu, F.; Miculescu, M.; Ciocoiu, R.C.; Pandele, A.M.; Stan, G.E.; Cîmpean, A.; Voicu, Ș.I.; Ciocan, L.-T. Comprehensive analysis of compatible natural fibre as sacrificial porogen template for tailored ceramic 3D bioproducts destined for hard tissue reconstruction. Ceram. Int. 2021, 47, 5318–5334. [Google Scholar] [CrossRef]
- Miculescu, F.; Bojin, D.; Ciocan, L.; Antoniac, I.; Miculescu, M.; Miculescu, N. Experimental researches on biomaterial-tissue interface interactions. J. Optoelectron. Adv. Mater. 2007, 9, 3303–3306. [Google Scholar]
- Nadarajan, V.; Phang, S.W.; Choo, H.L. Fabrication of 3D-printed bone scaffold of natural hydroxyapatite from fish bones in polylactic acid composite. AIP Conf. Proc. 2020, 2233, 040004. [Google Scholar] [CrossRef]
- Michael, F.M.; Khalid, M.; Chantara Thevy, R.; Raju, G.; Shahabuddin, S.; Walvekar, R.; Mubarak, N.M. Graphene/Nanohydroxyapatite hybrid reinforced polylactic acid nanocomposite for load-bearing applications. Polym.-Plast. Technol. Mater. 2022, 61, 803–815. [Google Scholar] [CrossRef]
- Nevado, P.; Lopera, A.; Bezzon, V.; Fulla, M.; Palacio, J.; Zaghete, M.; Biasotto, G.; Montoya, A.; Rivera, J.; Robledo, S. Preparation and in vitro evaluation of PLA/biphasic calcium phosphate filaments used for fused deposition modelling of scaffolds. Mater. Sci. Eng. C 2020, 114, 111013. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Custodio, C.L.; Broñola, P.J.M.; Cayabyab, S.R.; Lagura, V.U.; Celorico, J.R.; Basilia, B.A. Powder Loading Effects on the Physicochemical and Mechanical Properties of 3D Printed Poly Lactic Acid/Hydroxyapatite Biocomposites. Int. J. Bioprint. 2021, 7, 326. [Google Scholar] [CrossRef]
- Sahmani, S.; Khandan, A.; Esmaeili, S.; Saber-Samandari, S.; Nejad, M.G.; Aghdam, M. Calcium phosphate-PLA scaffolds fabricated by fused deposition modeling technique for bone tissue applications: Fabrication, characterization and simulation. Ceram. Int. 2020, 46, 2447–2456. [Google Scholar] [CrossRef]
- Quodbach, J.; Bogdahn, M.; Breitkreutz, J.; Chamberlain, R.; Eggenreich, K.; Elia, A.G.; Gottschalk, N.; Gunkel-Grabole, G.; Hoffmann, L.; Kapote, D. Quality of FDM 3D printed medicines for pediatrics: Considerations for formulation development, filament extrusion, printing process and printer design. Ther. Innov. Regul. Sci. 2022, 56, 910–928. [Google Scholar] [CrossRef]
- Sotorrío, G.; Alonso, J.; Olsson, N.; Tenorio, J. Printability of materials for extrusion 3D printing technologies: A review of material requirements and testin. Mater. Construcción 2021, 71, e267. [Google Scholar] [CrossRef]
- Bayart, M.; Dubus, M.; Charlon, S.; Kerdjoudj, H.; Baleine, N.; Benali, S.; Raquez, J.-M.; Soulestin, J. Pellet-Based fused filament fabrication (FFF)-derived process for the development of polylactic acid/hydroxyapatite scaffolds dedicated to bone regeneration. Materials 2022, 15, 5615. [Google Scholar] [CrossRef] [PubMed]
- Baudín, C.; Benet, T.; Pena, P. Effect of graphene on setting and mechanical behaviour of tricalcium phosphate bioactive cements. J. Mech. Behav. Biomed. Mater. 2019, 89, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, L.; Kedziora, S.; Museyibov, E.; Schlienz, M.; Szatkowski, P.; Szatkowska, M.; Gralewski, J. Influence of UV Ageing on Properties of Printed PLA Containing Graphene Nanopowder. Materials 2022, 15, 8135. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.-C.; Miculescu, F.; Dascălu, C.-A.; Voicu, Ș.I.; Pandele, M.-A.; Ciocoiu, R.-C.; Batalu, D.; Dondea, S.; Mitran, V.; Ciocan, L.-T. Influence of Ceramic Particles Size and Ratio on Surface—Volume Features of the Naturally Derived HA-Reinforced Filaments for Biomedical Applications. J. Funct. Biomater. 2022, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.-C.; Miculescu, F.; Constantinescu, A.E.; Pandele, M.-A.; Voicu, Ș.I.; Cîmpean, A.; Miculescu, M.; Negrescu, A.M. Selection Route of Precursor Materials in 3D Printing Composite Filament Development for Biomedical Applications. Materials 2023, 16, 2359. [Google Scholar] [CrossRef] [PubMed]
- Ferri, J.; Jordá, J.; Montanes, N.; Fenollar, O.; Balart, R. Manufacturing and characterization of poly (lactic acid) composites with hydroxyapatite. J. Thermoplast. Compos. Mater. 2018, 31, 865–881. [Google Scholar] [CrossRef]
- Negrescu, A.-M.; Mocanu, A.-C.; Miculescu, F.; Mitran, V.; Constantinescu, A.-E.; Cimpean, A. In Vitro Studies on 3D-Printed PLA/HA/GNP Structures for Bone Tissue Regeneration. Biomimetics 2024, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 14778:2021; Paper and Board—Measurement of Water Contact Angle by Optical Methods. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 604:2002; Plastics—Determination of Compressive Properties. International Organization for Standardization: Geneva, Switzerland, 2002.
- Huang, F.; Motealleh, B.; Wang, D.; Cornelius, C.J. Tailoring intrinsic hydrophobicity and surface energy on rough surface via low-T Cassie–Wenzel wetting transition method. AIChE J. 2023, 69, e17908. [Google Scholar] [CrossRef]
- Kalsoom, U.; Nesterenko, P.N.; Paull, B. Recent developments in 3D printable composite materials. RSC Adv. 2016, 6, 60355–60371. [Google Scholar] [CrossRef]
- Baldan, A. Adhesion phenomena in bonded joints. Int. J. Adhes. Adhes. 2012, 38, 95–116. [Google Scholar] [CrossRef]
- Miculescu, F.; Jepu, I.; Porosnicu, C.; Lungu, C.; Miculescu, M.; Burhala, B. A study on the influence of the primary electron beam on nanodimensional layers analysis. Dig. J. Nanomater. Biostruct. 2011, 6, 335–345. [Google Scholar]
- Zhu, Y.q.; Yu, C.x.; Li, Y.; Zhu, Q.q.; Zhou, L.; Cao, C.; Yu, T.t.; Du, F.p. Research on the changes in wettability of rice (Oryza sativa.) leaf surfaces at different development stages using the OWRK method. Pest. Manag. Sci. 2014, 70, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Senturk Parreidt, T.; Schmid, M.; Hauser, C. Validation of a novel technique and evaluation of the surface free energy of food. Foods 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Song, L.; Nie, S.; Hu, Y. Combustion properties and thermal degradation behavior of polylactide with an effective intumescent flame retardant. Polym. Degrad. Stab. 2009, 94, 291–296. [Google Scholar] [CrossRef]
- Manafi, P.; Ghasemi, I.; Karrabi, M.; Azizi, H.; Ehsaninamin, P. Effect of graphene nanoplatelets on crystallization kinetics of poly (lactic acid). Soft Mater. 2014, 12, 433–444. [Google Scholar] [CrossRef]
- Ratih, D.; Siburian, R. The performance of graphite/N-graphene and graphene/N-graphene as electrode in primary cell batteries. J. Phys. Conf. Ser. 2018, 1116, 042034. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 465. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Hołysz, L.; Chodkowski, M.; Clemente Guinarte, D. What Can You Learn about Apparent Surface Free Energy from the Hysteresis Approach? Colloids Interfaces 2021, 5, 4. [Google Scholar] [CrossRef]
- Vafaei, S.; Tuck, C.; Ashcroft, I.; Wildman, R. Surface microstructuring to modify wettability for 3D printing of nano-filled inks. Chem. Eng. Res. Des. 2016, 109, 414–420. [Google Scholar] [CrossRef]
- Banjo, A.D.; Agrawal, V.; Auad, M.L.; Celestine, A.-D.N. Moisture-induced changes in the mechanical behavior of 3D printed polymers. Compos. Part C Open Access 2022, 7, 100243. [Google Scholar] [CrossRef]
- Nakamura, M.; Hori, N.; Ando, H.; Namba, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, F.; Zadpoor, A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B 2017, 5, 6175–6192. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhang, K.; He, R.; Ding, G.; Xia, M.; Jin, X.; Xie, C. Additive manufacturing of hydroxyapatite bioceramic scaffolds: Dispersion, digital light processing, sintering, mechanical properties, and biocompatibility. J. Adv. Ceram. 2020, 9, 360–373. [Google Scholar] [CrossRef]
- Basirun, W.J.; Nasiri-Tabrizi, B.; Baradaran, S. Overview of hydroxyapatite–graphene nanoplatelets composite as bone graft substitute: Mechanical behavior and in-vitro biofunctionality. Crit. Rev. Solid State Mater. Sci. 2018, 43, 177–212. [Google Scholar] [CrossRef]
- Dascalu, C.-A.; Miculescu, F.; Mocanu, A.-C.; Constantinescu, A.E.; Butte, T.M.; Pandele, A.M.; Ciocoiu, R.-C.; Voicu, S.I.; Ciocan, L.T. Novel synthesis of core-shell biomaterials from polymeric filaments with a bioceramic coating for biomedical applications. Coatings 2020, 10, 283. [Google Scholar] [CrossRef]
- Dee, P.; You, H.Y.; Teoh, S.-H.; Le Ferrand, H. Bioinspired approaches to toughen calcium phosphate-based ceramics for bone repair. J. Mech. Behav. Biomed. Mater. 2020, 112, 104078. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, F.; Mocanu, A.C.; Stan, G.E.; Miculescu, M.; Maidaniuc, A.; Cîmpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T.; Ciocan, L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018, 438, 147–157. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocanu, A.-C.; Constantinescu, A.-E.; Pandele, M.-A.; Voicu, Ș.I.; Ciocoiu, R.-C.; Batalu, D.; Semenescu, A.; Miculescu, F.; Ciocan, L.-T. Biocompatible Composite Filaments Printable by Fused Deposition Modelling Technique: Selection of Tuning Parameters by Influence of Biogenic Hydroxyapatite and Graphene Nanoplatelets Ratios. Biomimetics 2024, 9, 189. https://doi.org/10.3390/biomimetics9030189
Mocanu A-C, Constantinescu A-E, Pandele M-A, Voicu ȘI, Ciocoiu R-C, Batalu D, Semenescu A, Miculescu F, Ciocan L-T. Biocompatible Composite Filaments Printable by Fused Deposition Modelling Technique: Selection of Tuning Parameters by Influence of Biogenic Hydroxyapatite and Graphene Nanoplatelets Ratios. Biomimetics. 2024; 9(3):189. https://doi.org/10.3390/biomimetics9030189
Chicago/Turabian StyleMocanu, Aura-Cătălina, Andreea-Elena Constantinescu, Mădălina-Andreea Pandele, Ștefan Ioan Voicu, Robert-Cătălin Ciocoiu, Dan Batalu, Augustin Semenescu, Florin Miculescu, and Lucian-Toma Ciocan. 2024. "Biocompatible Composite Filaments Printable by Fused Deposition Modelling Technique: Selection of Tuning Parameters by Influence of Biogenic Hydroxyapatite and Graphene Nanoplatelets Ratios" Biomimetics 9, no. 3: 189. https://doi.org/10.3390/biomimetics9030189