Biomineralization of Polyelectrolyte-Functionalized Electrospun Fibers: Optimization and In Vitro Validation for Bone Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Cellulose Derivate
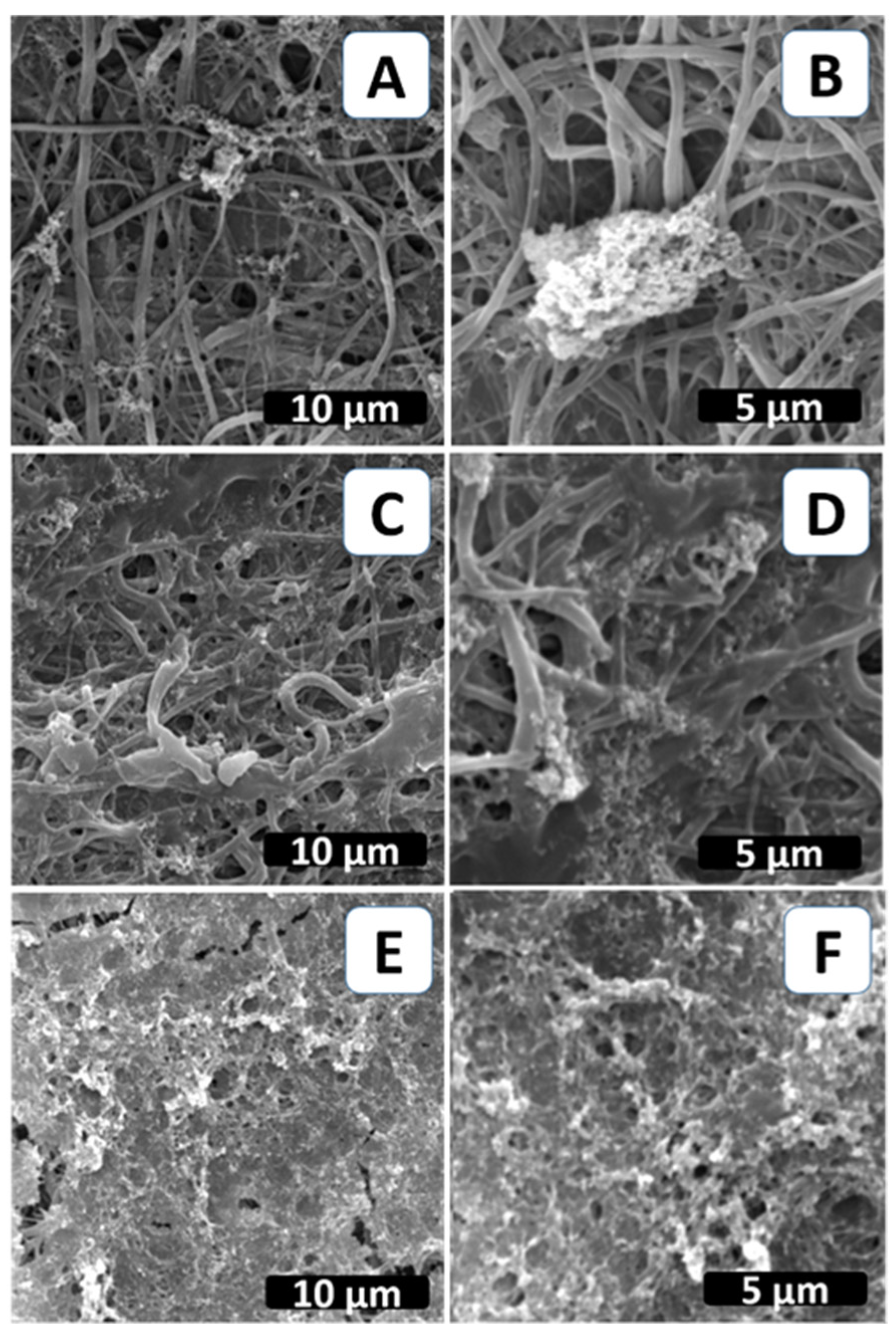
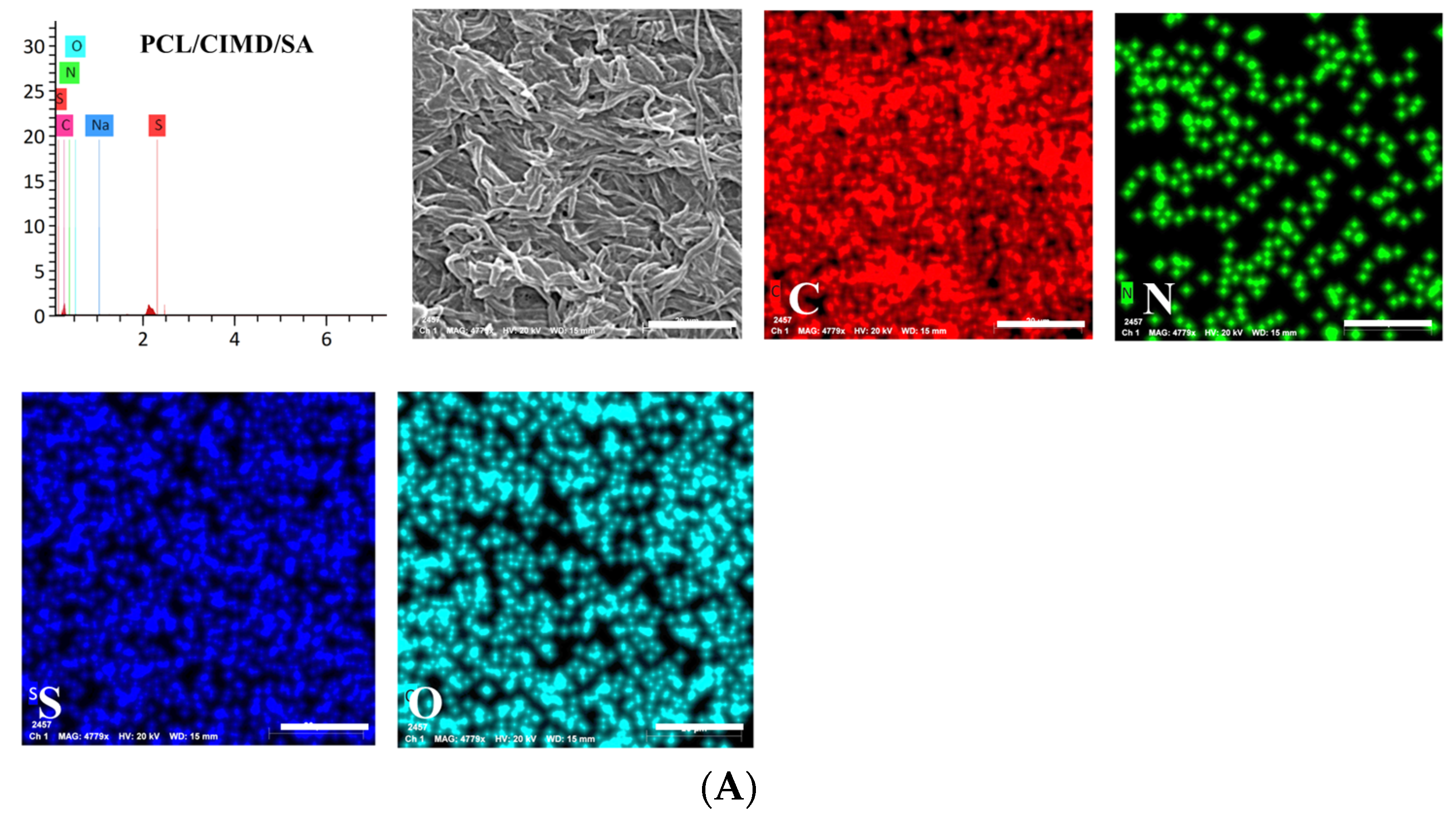
2.2. Biomimetic Mineralization of the Functionalized PCL Fiber Mats
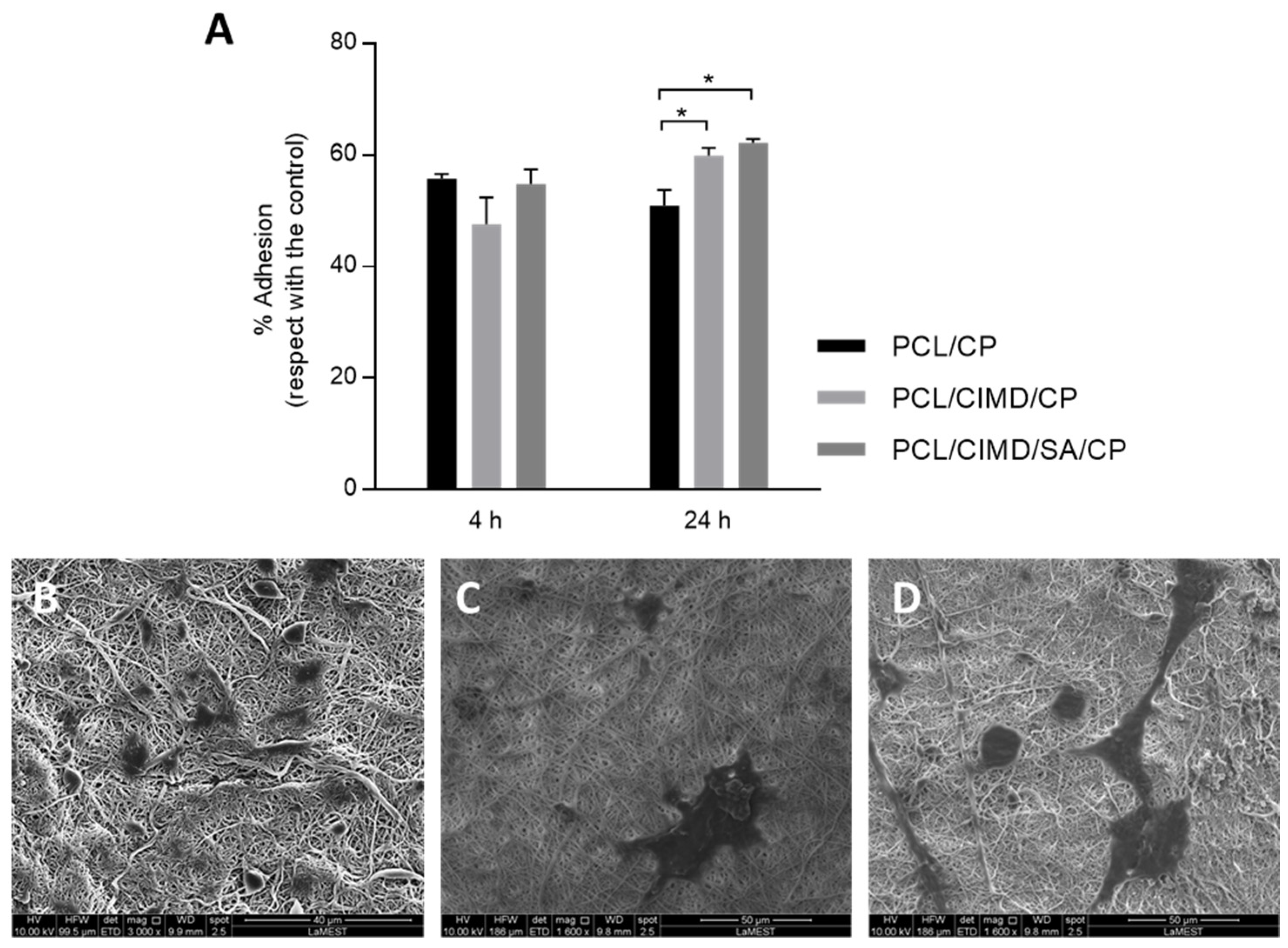
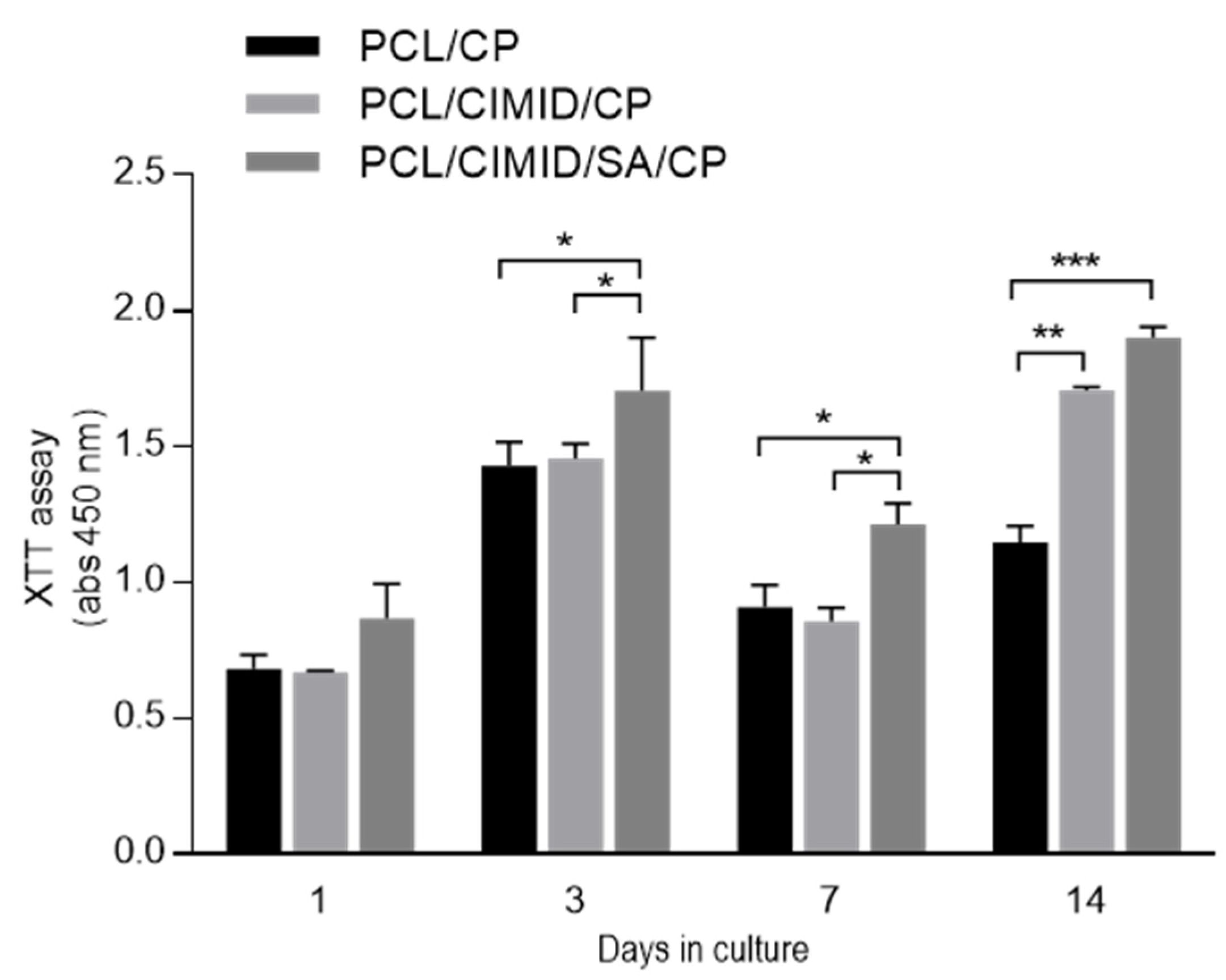
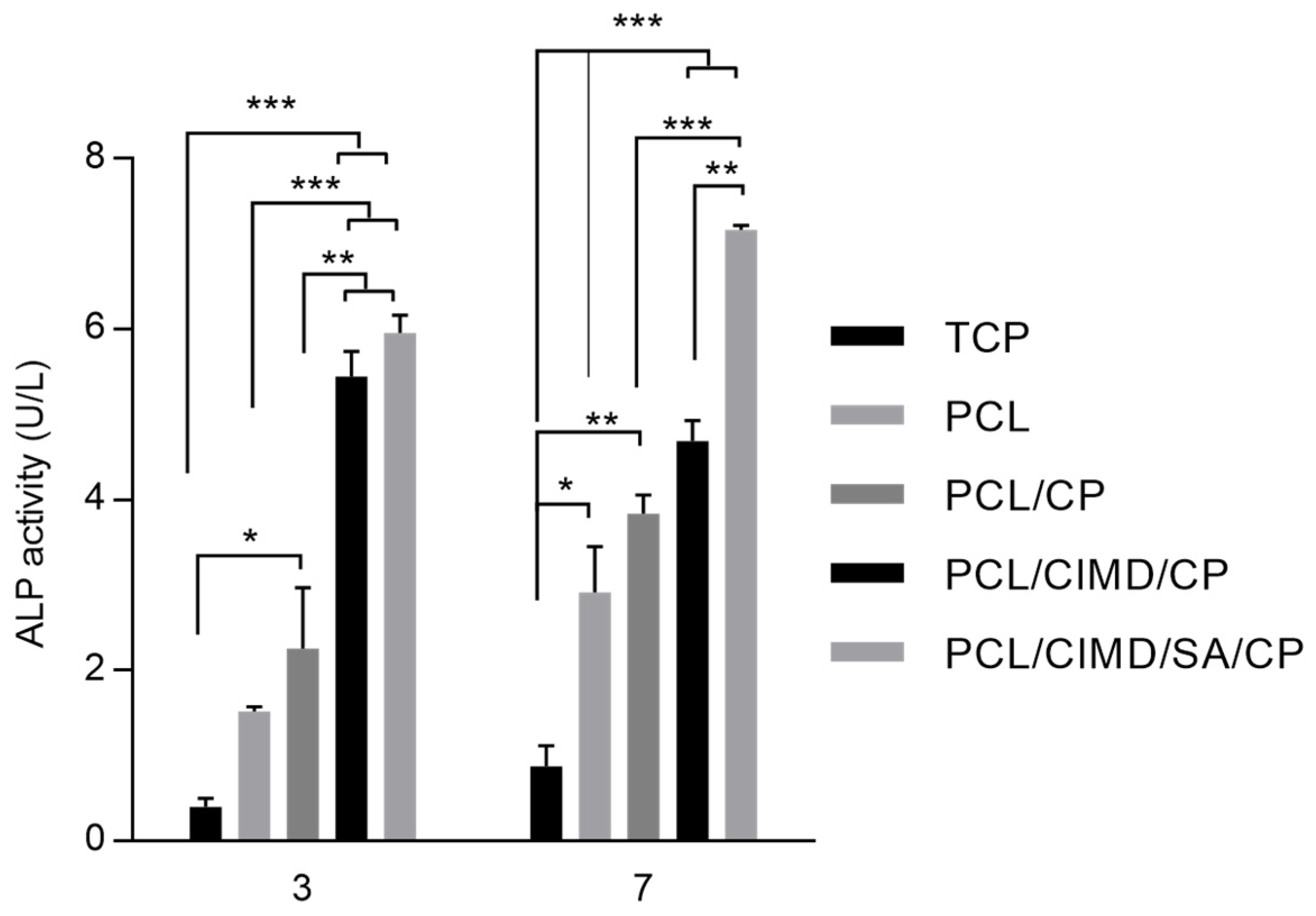
2.3. In Vitro Cell Results
2.4. Antimicrobial Evaluation of Composite Films
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Electrospun Fiber Preparation
4.3. Functionalization via Polyelectrolytes
4.4. Biomimetic Mineralization
4.5. Fiber Characterization
4.6. Biocompatibility Assays
4.6.1. Cell Culture
4.6.2. In Vitro Assays
4.7. Antimicrobial Evaluation by Disc Diffusion Method
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Pupilli, F.; Ruffini, A.; Dapporto, M.; Tavoni, M.; Tampieri, A.; Sprio, S. Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration. Biomimetics 2022, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Wang, N.; You, X.-G.; Zhang, A.-D.; Chen, X.-G.; Liu, Y. Collagen-Based Biocomposites Inspired by Bone Hierarchical Structures for Advanced Bone Regeneration: Ongoing Research and Perspectives. Biomater. Sci. 2022, 10, 318–353. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Leonor, I.B.; Azevedo, H.S.; Reis, R.L.; Mano, J.F. Designing Biomaterials Based on Biomineralization of Bone. J. Mater. Chem. 2010, 20, 2911. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Scaglione, S.; Sandri, M.; Alvarez-Perez, M.A.; Tampieri, A.; Quarto, R.; Ambrosio, L. MgCHA Particles Dispersion in Porous PCL Scaffolds: In Vitro Mineralization and In Vivo Bone Formation. J. Tissue Eng. Regen. Med. 2014, 8, 291–303. [Google Scholar] [CrossRef]
- Zhou, C.; Ye, X.; Fan, Y.; Ma, L.; Tan, Y.; Qing, F.; Zhang, X. Biomimetic Fabrication of a Three-Level Hierarchical Calcium Phosphate/Collagen/Hydroxyapatite Scaffold for Bone Tissue Engineering. Biofabrication 2014, 6, 035013. [Google Scholar] [CrossRef] [PubMed]
- Dejob, L.; Toury, B.; Tadier, S.; Grémillard, L.; Gaillard, C.; Salles, V. Electrospinning of in Situ Synthesized Silica-Based and Calcium Phosphate Bioceramics for Applications in Bone Tissue Engineering: A Review. Acta Biomater. 2021, 123, 123–153. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Giavaresi, G.; Guarino, V.; Raucci, M.G.; Sandri, M.; Tampieri, A.; Ambrosio, L.; Fini, M. Bioactivity and Bone Healing Properties of Biomimetic Porous Composite Scaffold: In Vitro and In Vivo Studies. J. Biomed. Mater. Res. A 2015, 103, 2932–2941. [Google Scholar] [CrossRef]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. Using a Synthetic Body Fluid (SBF) Solution of 27 MM HCO3− to Make Bone Substitutes More Osteointegrative. Mater. Sci. Eng. C 2008, 28, 129–140. [Google Scholar] [CrossRef]
- Salama, A. Soy Protein Acid Hydrolysate/Silica Hybrid Material as Novel Adsorbent for Methylene Blue. Compos. Commun. 2019, 12, 101–105. [Google Scholar] [CrossRef]
- Bharati, S.; Sinha, M.K.; Basu, D. Hydroxyapatite Coating by Biomimetic Method on Titanium Alloy Using Concentrated SBF. Bull. Mater. Sci. 2005, 28, 617–621. [Google Scholar] [CrossRef]
- Mendes, M.W.D.; Ágreda, C.G.; Bressiani, A.H.A.; Bressiani, J.C. A New Titanium Based Alloy Ti–27Nb–13Zr Produced by Powder Metallurgy with Biomimetic Coating for Use as a Biomaterial. Mater. Sci. Eng. C 2016, 63, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.F.C.; de Sousa, L.L.; Ferreira, C.C.; Gaspar de Souza, B.F.; da Rigo, E.C.S.; Mariano, N.A. Biomimetic Coating on Titanium: Evaluation of Bioactivity and Corrosion. Mater. Res. Express 2020, 6, 1265g5. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.; Deng, F.; Shen, L.; Zhao, Z.; Peng, S.; Shuai, C. A Bifunctional Bone Scaffold Combines Osteogenesis and Antibacterial Activity via in Situ Grown Hydroxyapatite and Silver Nanoparticles. Bio-Des. Manuf. 2021, 4, 452–468. [Google Scholar] [CrossRef]
- Liao, C.-T.; Ho, M.-H. The Fabrication of Biomimetic Chitosan Scaffolds by Using SBF Treatment with Different Crosslinking Agents. Membranes 2010, 1, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Hesemann, P. Recent Trends in Elaboration, Processing, and Derivatization of Cellulosic Materials Using Ionic Liquids. ACS Sustain. Chem. Eng. 2020, 8, 17893–17907. [Google Scholar] [CrossRef]
- Zuppolini, S.; Salama, A.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Cellulose Amphiphilic Materials: Chemistry, Process and Applications. Pharmaceutics 2022, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Abou-Zeid, R.E.; Cruz-Maya, I.; Guarino, V. Soy Protein Hydrolysate Grafted Cellulose Nanofibrils with Bioactive Signals for Bone Repair and Regeneration. Carbohydr. Polym. 2020, 229, 115472. [Google Scholar] [CrossRef]
- Qi, P.; Ohba, S.; Hara, Y.; Fuke, M.; Ogawa, T.; Ohta, S.; Ito, T. Fabrication of Calcium Phosphate-Loaded Carboxymethyl Cellulose Non-Woven Sheets for Bone Regeneration. Carbohydr. Polym. 2018, 189, 322–330. [Google Scholar] [CrossRef]
- Siddiqui, N.; Kishori, B.; Rao, S.; Anjum, M.; Hemanth, V.; Das, S.; Jabbari, E. Electropsun Polycaprolactone Fibres in Bone Tissue Engineering: A Review. Mol. Biotechnol. 2021, 63, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Can-Herrera, L.A.; Oliva, A.I.; Dzul-Cervantes, M.A.A.; Pacheco-Salazar, O.F.; Cervantes-Uc, J.M. Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage. Polymers 2021, 13, 662. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Song, Q.; Liu, S.; Pei, W.; Wang, P.; Zheng, L.; Huang, C.; Ma, M.; Jiang, Q.; Zhang, K. Advanced Applications of Cellulose-Based Composites in Fighting Bone Diseases. Compos. B Eng. 2022, 245, 110221. [Google Scholar] [CrossRef]
- Tolba, E.; Salama, A.; Saleh, A.K.; Cruz-Maya, I.; Guarino, V. Sodium Alginate- and Cationic Cellulose-Functionalized Polycaprolactone Nanofibers for In Vitro and Antibacterial Applications. Molecules 2023, 28, 7305. [Google Scholar] [CrossRef] [PubMed]
- Tommila, M.; Jokilammi, A.; Terho, P.; Wilson, T.; Penttinen, R.; Ekholm, E. Hydroxyapatite Coating of Cellulose Sponges Attracts Bone-Marrow-Derived Stem Cells in Rat Subcutaneous Tissue. J. R. Soc. Interface 2009, 6, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Alloisio, M.; Castellano, M.; Vicini, S. Multilayer Alginate–Polycaprolactone Electrospun Membranes as Skin Wound Patches with Drug Delivery Abilities. ACS Appl. Mater. Interfaces 2020, 12, 31162–31171. [Google Scholar] [CrossRef] [PubMed]
- Thula, T.T.; Svedlund, F.; Rodriguez, D.E.; Podschun, J.; Pendi, L.; Gower, L.B. Mimicking the Nanostructure of Bone: Comparison of Polymeric Process-Directing Agents. Polymers 2011, 3, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Nardecchia, S.; Gutiérrez, M.C.; Serrano, M.C.; Dentini, M.; Barbetta, A.; Ferrer, M.L.; del Monte, F. In Situ Precipitation of Amorphous Calcium Phosphate and Ciprofloxacin Crystals during the Formation of Chitosan Hydrogels and Its Application for Drug Delivery Purposes. Langmuir 2012, 28, 15937–15946. [Google Scholar] [CrossRef]
- Salazar-Noratto, G.E.; Luo, G.; Denoeud, C.; Padrona, M.; Moya, A.; Bensidhoum, M.; Bizios, R.; Potier, E.; Logeart-Avramoglou, D.; Petite, H. Understanding and Leveraging Cell Metabolism to Enhance Mesenchymal Stem Cell Transplantation Survival in Tissue Engineering and Regenerative Medicine Applications. Stem Cells 2020, 38, 22–33. [Google Scholar] [CrossRef]
- Feng, Z.; Jin, M.; Liang, J.; Kang, J.; Yang, H.; Guo, S.; Sun, X. Insight into the Effect of Biomaterials on Osteogenic Differentiation of Mesenchymal Stem Cells: A Review from a Mitochondrial Perspective. Acta Biomater. 2023, 164, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, W.; Gao, Y.; Hao, P.; Shang, J.; Duan, H.; Yang, Z.; Li, X. Differentiation of Bone Marrow Mesenchymal Stem Cells into Neural Lineage Cells Induced by BFGF-Chitosan Controlled Release System. BioMed Res. Int. 2019, 2019, 5086297. [Google Scholar] [CrossRef]
- Ressler, A.; Antunović, M.; Teruel-Biosca, L.; Ferrer, G.G.; Babić, S.; Urlić, I.; Ivanković, M.; Ivanković, H. Osteogenic Differentiation of Human Mesenchymal Stem Cells on Substituted Calcium Phosphate/Chitosan Composite Scaffold. Carbohydr. Polym. 2022, 277, 118883. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Z.; Alemán, M.E.; Donate, R. Nanofibers and Microfibers for Osteochondral Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1058, 97–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, W.; Chou, J.; Wen, S.; Sun, Y.; Zhang, H. Electrospun Nanosilicates-Based Organic/Inorganic Nanofibers for Potential Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2018, 172, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic Composite Scaffold of Hydroxyapatite/Gelatin-Chitosan Core-Shell Nanofibers for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 97, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.; Tahmasebi Birgani, Z.; Habibovic, P. Biomaterial-Induced Pathway Modulation for Bone Regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef] [PubMed]
- Montrezor, L.H.; Benevenuto, L.G.D.; Antunes, B.F.; Amaral, A.C.; Novo, L.P.; Carvalho, A.J.F.; Trovatti, E. The Influence of Chitosan, Cellulose and Alginate Chemical Nature on Mineral Matrix Formation. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 875–885. [Google Scholar] [CrossRef]
- Toworfe, G.K.; Composto, R.J.; Shapiro, I.M.; Ducheyne, P. Nucleation and Growth of Calcium Phosphate on Amine-, Carboxyl- and Hydroxyl-Silane Self-Assembled Monolayers. Biomaterials 2006, 27, 631–642. [Google Scholar] [CrossRef]
- Zhu, P.; Masuda, Y.; Yonezawa, T.; Koumoto, K. Investigation of Apatite Deposition onto Charged Surfaces in Aqueous Solutions Using a Quartz-Crystal Microbalance. J. Am. Ceram. Soc. 2003, 86, 782–790. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Wang, R.; Kong, X.; Wang, X. Mineralization of Calcium Phosphate Controlled by Biomimetic Self-Assembled Peptide Monolayers via Surface Electrostatic Potentials. Bioact. Mater. 2020, 5, 387–397. [Google Scholar] [CrossRef]
- Ghazagh, P.; Frounchi, M. Hydroxyapatite/Alginate/Polyvinyl Alcohol/Agar Composite Double-Network Hydrogels as Injectable Drug Delivery Microspheres. Chem. Pap. 2024, 78, 2967–2976. [Google Scholar] [CrossRef]
- Abdellatif Soliman, S.M.; Sanad, M.F.; Shalan, A.E. Synthesis, Characterization and Antimicrobial Activity Applications of Grafted Copolymer Alginate-g-Poly(N-Vinyl Imidazole). RSC Adv. 2021, 11, 11541–11548. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Saleh, A.K.; Cruz-maya, I.; Guarino, V. Bacterial Cellulose/Cellulose Imidazolium Bio-Hybrid Membranes for In Vitro and Antimicrobial Applications. J. Funct. Biomater. 2023, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.S.; Lee, J.-S.; Han, J. Development of a Biodegradable Polycaprolactone Film Incorporated with an Antimicrobial Agent via an Extrusion Process. Sci. Rep. 2019, 9, 20236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Zhou, D.; Xia, X.; Zhou, H.; Wang, Y.; Ke, H. Preparation and Characterization of Polycaprolactone (PCL) Antimicrobial Wound Dressing Loaded with Pomegranate Peel Extract. ACS Omega 2023, 8, 20323–20331. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Sobaszkiewicz, K.; Mirska, I. Anti-Microbial Activities of Ionic Liquids. Green Chem. 2003, 5, 52–56. [Google Scholar] [CrossRef]
- Salama, A.; Saleh, A.K. Enhancement of Antimicrobial Response against Human Pathogens by a Novel Cationic Starch Derivative. Starch—Stärke 2022, 74, 2100286. [Google Scholar] [CrossRef]
- Demberelnyamba, D.; Kim, K.-S.; Choi, S.; Park, S.-Y.; Lee, H.; Kim, C.-J.; Yoo, I.-D. Synthesis and Antimicrobial Properties of Imidazolium and Pyrrolidinonium Salts. Bioorganic Med. Chem. 2004, 12, 853–857. [Google Scholar] [CrossRef]


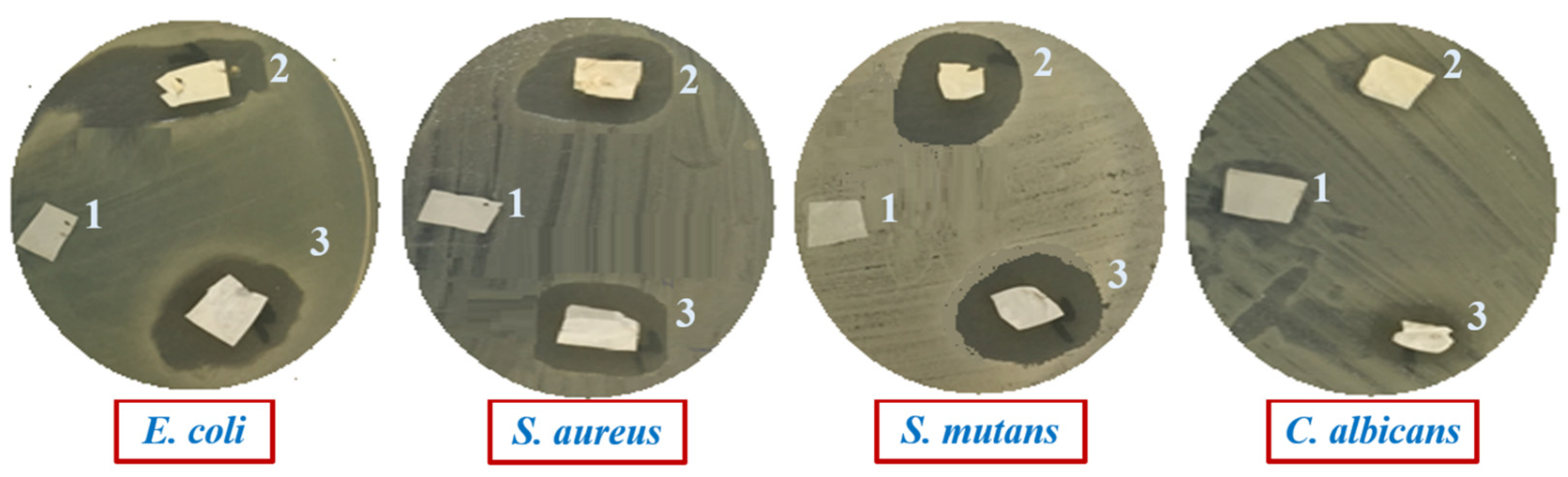
| Pathogenic Microbes | Diameters of Inhibition Zone (mm) | ||
|---|---|---|---|
| PCL/CP | PCL/CIMD/CP | PCL/CIMD/SA/CP | |
| E. coli | 0.0 ± 0.0 | 19 ± 1.17 | 18 ± 1.07 |
| S. aureus | 0.0 ± 0.0 | 20 ± 1.21 | 19 ± 1.17 |
| S. mutans | 0.0 ± 0.0 | 22 ± 1.19 | 20 ± 1.13 |
| C. albicans | 0.0 ± 0.0 | 7 ± 0.06 | 8 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, A.; Tolba, E.; Saleh, A.K.; Cruz-Maya, I.; Alvarez-Perez, M.A.; Guarino, V. Biomineralization of Polyelectrolyte-Functionalized Electrospun Fibers: Optimization and In Vitro Validation for Bone Applications. Biomimetics 2024, 9, 253. https://doi.org/10.3390/biomimetics9040253
Salama A, Tolba E, Saleh AK, Cruz-Maya I, Alvarez-Perez MA, Guarino V. Biomineralization of Polyelectrolyte-Functionalized Electrospun Fibers: Optimization and In Vitro Validation for Bone Applications. Biomimetics. 2024; 9(4):253. https://doi.org/10.3390/biomimetics9040253
Chicago/Turabian StyleSalama, Ahmed, Emad Tolba, Ahmed K. Saleh, Iriczalli Cruz-Maya, Marco A. Alvarez-Perez, and Vincenzo Guarino. 2024. "Biomineralization of Polyelectrolyte-Functionalized Electrospun Fibers: Optimization and In Vitro Validation for Bone Applications" Biomimetics 9, no. 4: 253. https://doi.org/10.3390/biomimetics9040253







