Combination of a Synthetic Bioceramic Associated with a Polydioxanone-Based Membrane as an Alternative to Autogenous Bone Grafting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Calculation
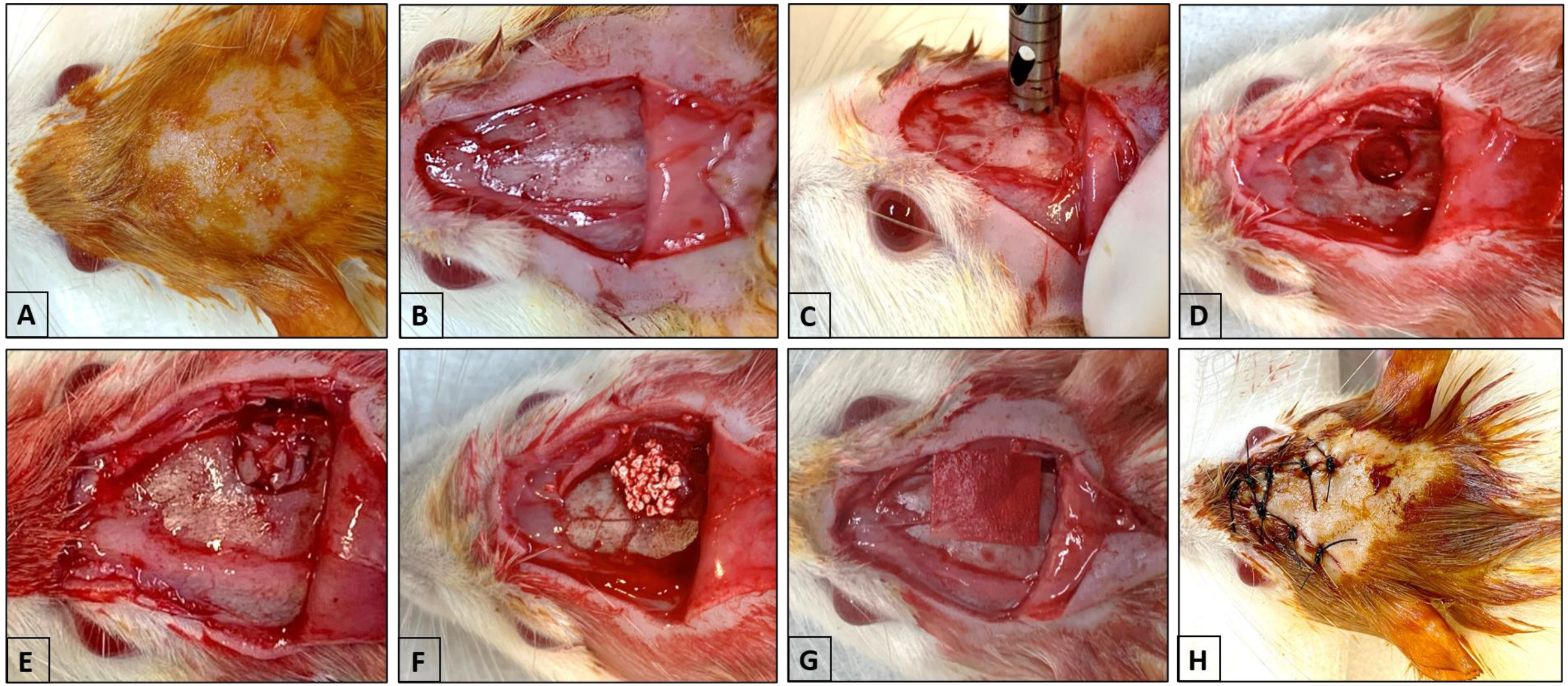
2.3. Surgical Procedure (Critical Calvaria Defect)
2.4. Distribution of Samples and Laboratory Processing
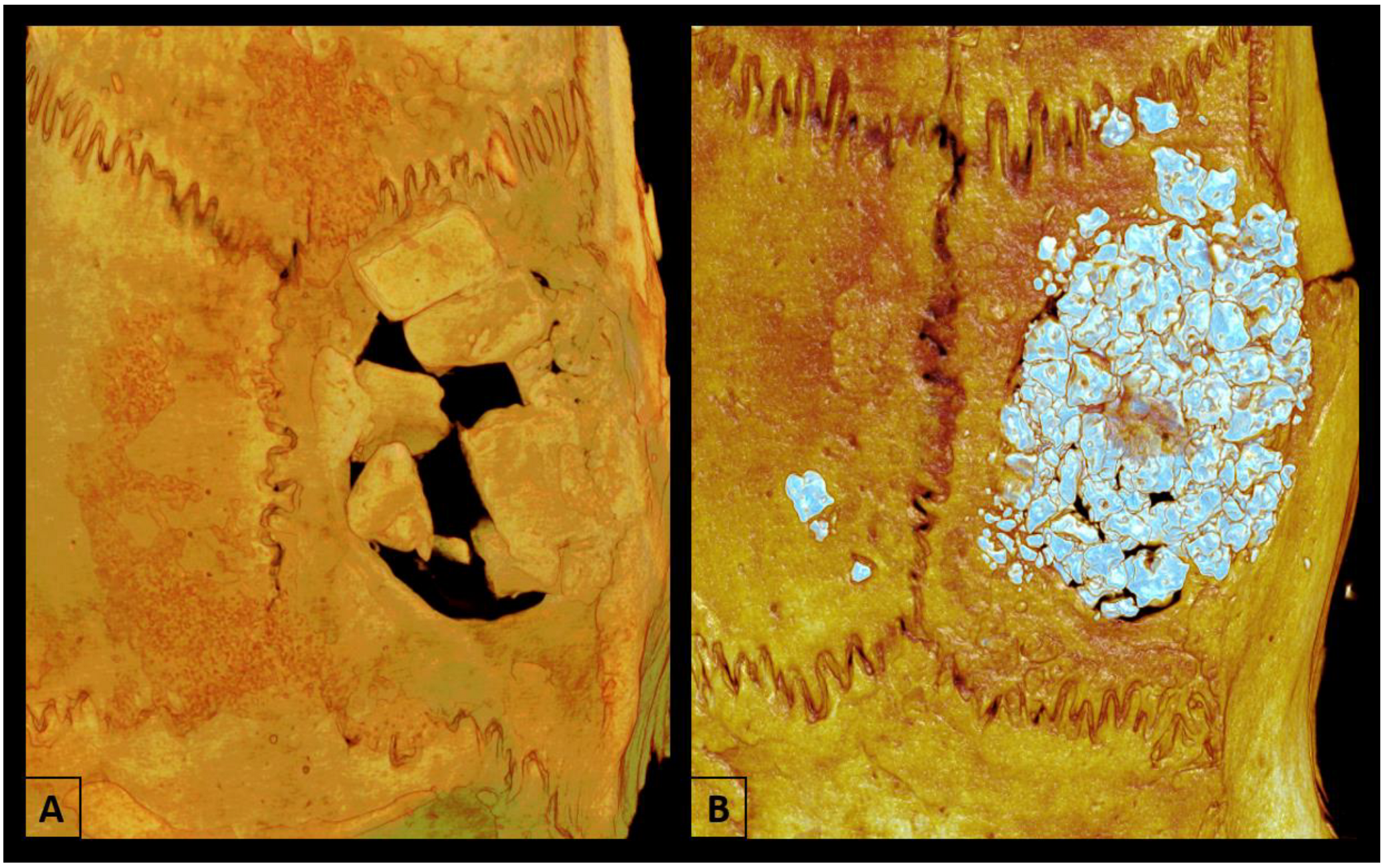
2.5. Microtomographic Analysis (Micro-CT)
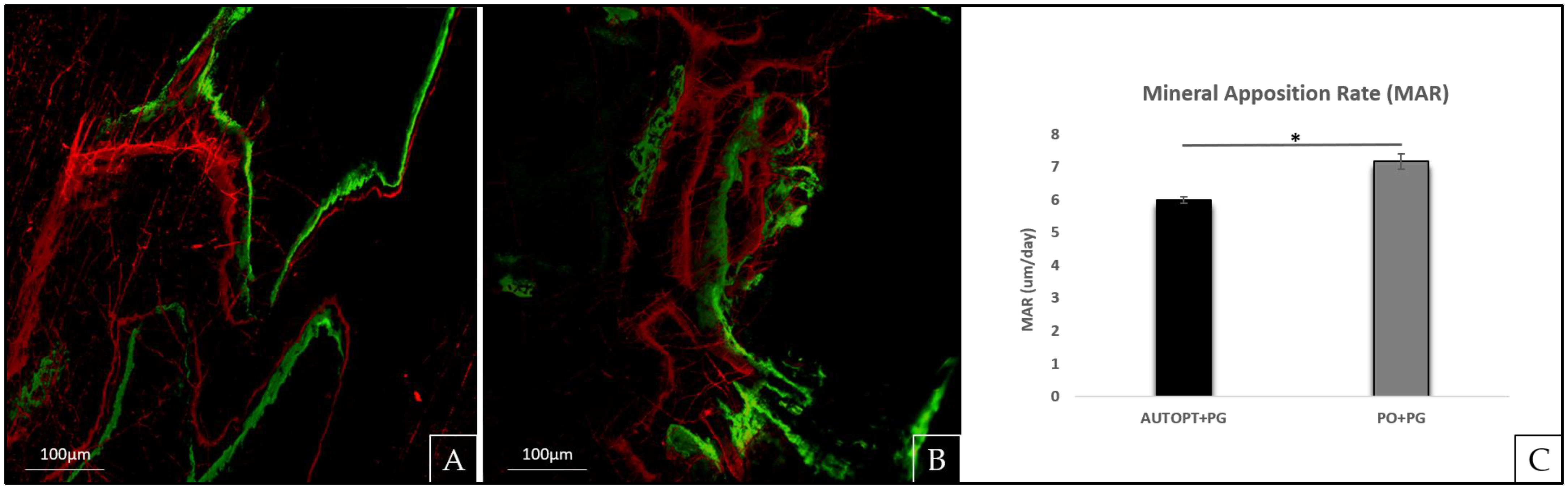
2.6. Laser Confocal Microscopy Analysis
2.7. Gene Expression (RT-PCR)
2.8. Histometry Analysis (H.E)
2.9. Immunolabeling Analysis
2.10. Statistical Analysis
3. Results
3.1. Microtomographic Analysis (Micro-CT)
3.1.1. Bone Volume Percentage (BV.TV)
3.1.2. Trabecular Thickness (Tb.Th)
3.1.3. Trabecular Number (Tb.N)
3.1.4. Trabecular Separation (Tb.Sp)
3.1.5. Intersection Surface (i.S)
3.1.6. Total Porosity (Po.Tot)
3.2. Laser Confocal Microscopy Analysis
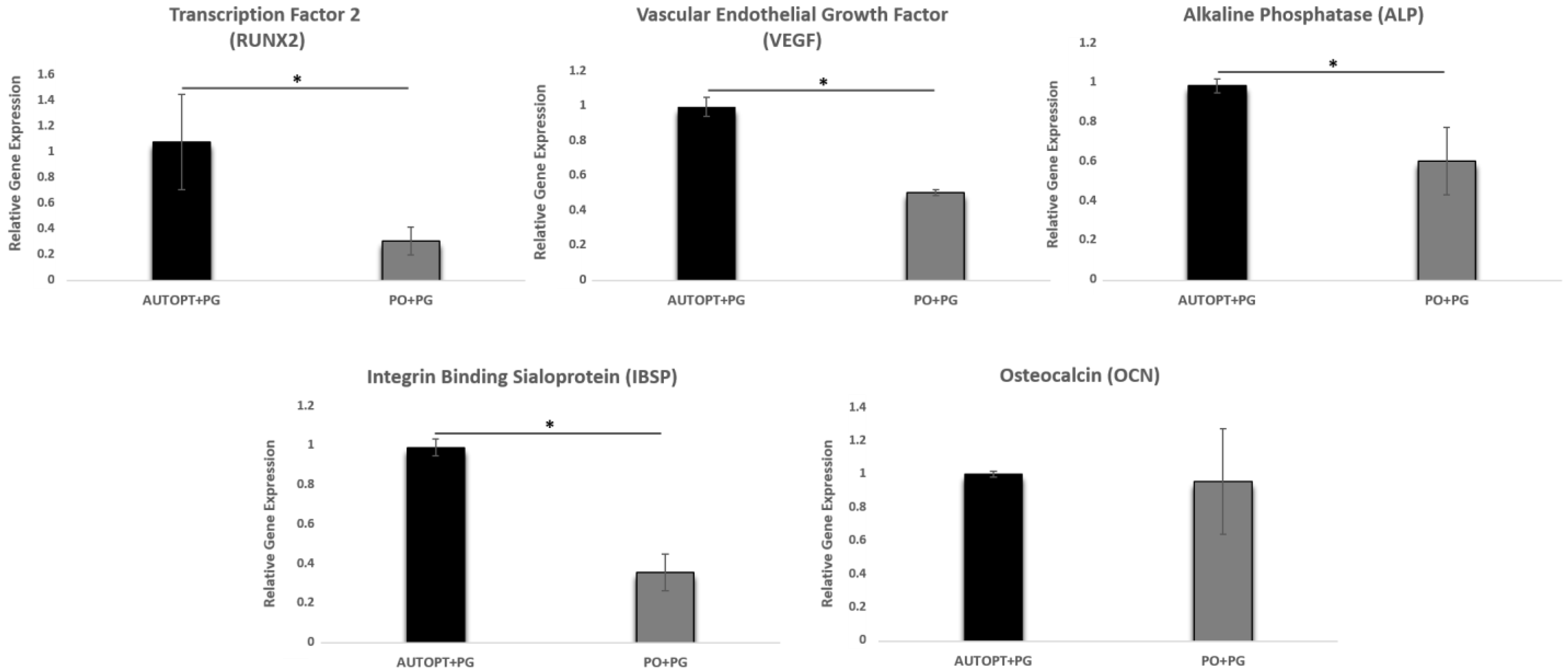
3.3. Gene Expression Analysis (RT-PCR)
3.3.1. Transcription Factor 2 (RUNX2)
3.3.2. Vascular Endothelial Growth Factor (VEGF)
3.3.3. Alkaline Phosphatase (ALP)
3.3.4. Osteocalcin (OCN)
3.3.5. Integrin Binding Sialoprotein (IBSP)
3.4. Histological Analysis
3.4.1. Bone Repair Process at 7 Days
3.4.2. Bone Repair Process at 30 Days
3.5. Immunolabeling Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, K.B.; Vasconcelos, B.C.D.E.; Júnior, F.d.A.L.; de Sousa, F.B.; Andrade, E.S.d.S.; Vasconcellos, R.J.d.H. Histomorphometric evaluation of calcium phosphate bone grafts on bone repair. Braz. J. Otorhinolaryngol. 2015, 77, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Escoda-Francolí, J.; Sánchez-Garcés, M.Á.; Gimeno-Sandig, Á.; Muñoz-Guzón, F.; Barbany-Cairó, J.R.; Badiella-Busquets, L.; Gay-Escoda, C. Guided bone regeneration using beta-tricalcium phosphate with and without fibronectin-An experimental study in rats. Clin. Oral Implants Res. 2018, 29, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Herten, M.; Ferrari, D.; Wieland, M.; Schmitz, L.; Engelhardt, E.; Becker, J. Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite+beta tricalcium phosphate (Bone Ceramic®) or a collagen-coated natural bone mineral (BioOss Collagen®): An immunohistochemical study in dogs. Int. J. Oral Maxillofac. Surg. 2007, 36, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.S.; Cestari, T.M.; Paulin, J.B.; Martins, R.; Rocha, C.A.; Arantes, R.V.N.; Costa, B.C.; dos Santos, C.M.; Assis, G.F.; Taga, R. Osteoinductive porous biphasic calcium phosphate ceramic as an alternative to autogenous bone grafting in the treatment of mandibular bone critical-size defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1546–1557. [Google Scholar] [CrossRef]
- Bouler, J.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Owen, G.R.; Dard, M.; Larjava, H. Hydoxyapatite/beta--tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 2493–2512. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Hallman, M.; Thor, A. Bone substitutes and growth factors as an alternative/complement to autogenous bone for grafting in implant dentistry. Periodontology 2000 2008, 47, 172–192. [Google Scholar] [CrossRef]
- Xiao, W.; Fu, H.; Rahaman, M.N.; Liu, Y.; Bal, B.S. Hollow hydroxyapatite microspheres: A novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration. Acta Biomater. 2013, 9, 8374–8383. [Google Scholar] [CrossRef] [PubMed]
- Macedo, R.M.; Lacerda, S.A.; Thomazini, J.A.; Brentegani, L.G. Bone integration behavior of hydroxyapatite/β-tricalcium phosphate graft implanted in dental alveoli: A histomorphometric and scanning electron microscopy study. Implant. Dent. 2014, 23, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Bagher, Z.; Rajaei, F.; Shokrgozar, M. Comparative Study of Bone Repair Using Porous Hydroxyapatite/ β-Tricalcium Phosphate and Xenograft Scaffold in Rabbits with Tibia Defect. Iran. Biomed. J. 2012, 16, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Del Fabbro, M. The Impact of Bioceramic Scaffolds on Bone Regeneration in Preclinical In Vivo Studies: A Systematic Review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef] [PubMed]
- da Silva Brum, I.; Frigo, L.; Goncalo Pinto dos Santos, P.; Nelson Elias, C.; da Fonseca, G.A.M.D.; Jose de Carvalho, J. Performance of Nano-Hydroxyapatite/Beta-Tricalcium Phosphate and Xenogenic Hydroxyapatite on Bone Regeneration in Rat Calvarial Defects: Histomorphometric, Immunohistochemical and Ultrastructural Analysis. Int. J. Nanomed. 2021, 16, 3473–3485. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic Material for Bone, Periodontal, and Dental Tissue Regeneration: Where Are We Now, and Where Are We Heading Next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Pitol-Palin, L.; Frigério, P.B.; Moura, J.; Pilatti, L.; de Oliveira, L.M.J.; Matsubara, E.Y.; Tunchel, S.; Shibli, J.A.; Blay, A.; Saska, S.; et al. Performance of Polydioxanone-Based Membrane in Association with 3D-Printed Bioceramic Scaffolds in Bone Regeneration. Polymers 2022, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Jordana, F.; Le Visage, C.; Weiss, P. Substituts osseux. Med. Sci. 2017, 33, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Frigério, P.B.; Quirino, L.C.; Gabrielli, M.A.C.; Carvalho, P.H.d.A.; Júnior, I.R.G.; Pereira-Filho, V.A. Evaluation of Bone Repair Using a New Biphasic Synthetic Bioceramic (Plenum® Osshp) in Critical Calvaria Defect in Rats. Biology 2023, 12, 1417. [Google Scholar] [CrossRef]
- Dos Santos, V.I.; Merlini, C.; Aragones, Á.; Cesca, K.; Fredel, M.C. In vitro evaluation of bilayer membranes of PLGA/hydroxyapatite/β-tricalcium phosphate for guided bone regeneration. Mater. Sci. Eng. C 2020, 112, 110849. [Google Scholar] [CrossRef]
- Hoornaert, A.; D’arros, C.; Heymann, M.-F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef]
- Saska, S.; Pilatti, L.; Silva, E.S.d.S.; Nagasawa, M.A.; Câmara, D.; Lizier, N.; Finger, E.; Konwińska, M.D.; Kempisty, B.; Tunchel, S.; et al. Polydioxanone-Based Membranes for Bone Regeneration. Polymers 2021, 13, 1685. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, K.A.; Sindet-Pedersen, S.; Hoepffner, H.-J. Clinical and histological findings in guided bone regeneration (GBR) around titanium dental implants with autogeneous bone chips using a new resorbable membrane. J. Biomed. Mater. Res. 2000, 53, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Quirino, L.C.; Carvalho, P.H.d.A.; Neto, R.T.A.; Comachio, C.A.; Monteiro, N.G.; Ervolino-Silva, A.C.; Okamoto, R.; Pereira-Filho, V.A. Polydioxanone Membrane Compared with Collagen Membrane for Bone Regeneration. Polymers 2023, 15, 868. [Google Scholar] [CrossRef] [PubMed]
- Medical Devices. Available online: https://scc-medical-devices.com/biological-evaluation-biocompatibility-iso-10993 (accessed on 10 December 2021).
- Ministério da Ciência, Tecnologia e Inovação. Available online: https://www.gov.br/mcti/pt-br/acompanhe-o-mcti/concea/arquivos/pdf/legislacao/resolucao-normativa-no-33-de-18-de-novembro-de-2016.pdf/view (accessed on 10 December 2021).
- Lisboa-Filho, P.N.; Gomes-Ferreira, P.H.S.; Batista, F.R.S.; Momesso, G.A.C.; Faverani, L.P.; Okamoto, R. Bone repair with raloxifene and bioglass nanoceramic composite in animal experiment. Connect. Tissue Res. 2018, 59, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Ariza, N.M.; Clarke, B.L. Bone biology, signaling pathways, and therapeutic targets for osteoporosis. Maturitas 2015, 82, 245–255. [Google Scholar] [CrossRef]
- Frigério, P.B.; Gomes-Ferreira, P.H.S.; Batista, F.R.d.S.; Moura, J.; Júnior, I.R.G.; Botticelli, D.; Lisboa-Filho, P.N.; Okamoto, R. Effect of Topical PTH 1-34 Functionalized to Biogran® in the Process of Alveolar Repair in Rats Submitted to Orchiectomy. Materials 2021, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Manrique, N.; Pereira, C.C.S.; Luvizuto, E.R.; Sánchez, M.D.P.R.; Okamoto, T.; Okamoto, R.; Sumida, D.H.; Antoniali, C. Hypertension modifies OPG, RANK, and RANKL expression during the dental socket bone healing process in spontaneously hypertensive rats. Clin. Oral Investig. 2015, 19, 1319–1327. [Google Scholar] [CrossRef]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef]
- Pedrosa, W.F.; Okamoto, R.; Faria, P.E.P.; Arnez, M.F.M.; Xavier, S.P.; Salata, L.A. Immunohistochemical, tomographic and histological study on onlay bone graft remodeling. Part II: Calvarial bone. Clin. Oral Implant. Res. 2009, 20, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Hampel, G.A.; Yilmaz, E.; Massrey, C.; Clifton, W.; Iwanaga, J.; Loukas, M.; Tubbs, R.S. History of Bone Grafts in Spine Surgery. Cureus 2022, 14, e24655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, X.; Qi, Y.; Ma, X.; Qiao, S.; Cai, H.; Zhao, B.C.; Jiang, H.B.; Lee, E.-S. Comparison of Autogenous Tooth Materials and Other Bone Grafts. Tissue Eng. Regen. Med. 2021, 18, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Shue, L.; Yufeng, Z.; Mony, U. Biomaterials for periodontal regeneration. Biomatter 2012, 2, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Paderni, S.; Terzi, S.; Amendola, L. Major bone defect treatment with an osteoconductive bone substitute. Musculoskelet. Surg. 2009, 93, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Botticelli, D.; Canullo, L.; Baba, S.; Xavier, S.P. New bone ingrowth into β-TCP/HA graft activated with argon plasma: A histomorphometric study on sinus lifting in rabbits. Int. J. Implant. Dent. 2020, 6, 36. [Google Scholar] [CrossRef]
- Khan, R.; Witek, L.; Breit, M.; Colon, D.; Tovar, N.; Janal, M.N.; Jimbo, R.; Coelho, P.G. Bone Regenerative Potential of Modified Biphasic Graft Materials. Implant. Dent. 2015, 24, 149–154. [Google Scholar] [CrossRef]
- Kowalczewski, J.B.; Milecki, M.; Wielopolski, A.; Slósarczyk, A.; Marczak, D.; Okoń, T. Usefulness of HA+beta-TCP in bone defects repair during revision hip and knee arthroplasty. Chir. Narzadow Ruchu Ortop. Pol. 2010, 75, 348–352. [Google Scholar]
- Pripatnanont, P.; Praserttham, P.; Suttapreyasri, S.; Leepong, N.; Monmaturapoj, N. Bone Regeneration Potential of Biphasic Nanocalcium Phosphate with High Hydroxyapatite/Tricalcium Phosphate Ratios in Rabbit Calvarial Defects. Int. J. Oral Maxillofac. Implant. 2016, 31, 294–303. [Google Scholar] [CrossRef]
- Kang, H.-J.; Makkar, P.; Padalhin, A.R.; Lee, G.-H.; Im, S.-B.; Lee, B.-T. Comparative study on biodegradation and biocompatibility of multichannel calcium phosphate based bone substitutes. Mater. Sci. Eng. C 2020, 110, 110694. [Google Scholar] [CrossRef] [PubMed]
- Arunjaroensuk, S.; Nampuksa, K.; Monmaturapoj, N.; Thunyakitpisal, P.; Porntaveetus, T.; Mattheos, N.; Pimkhaokham, A. Gene expression, micro-CT and histomorphometrical analysis of sinus floor augmentation with biphasic calcium phosphate and deproteinized bovine bone mineral: A randomized controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2024, 26, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Gallinetti, S.; Canal, C.; Ginebra, M. Development and Characterization of Biphasic Hydroxyapatite/β-TCP Cements. J. Am. Ceram. Soc. 2014, 97, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Basu, B. Unravelling Doped Biphasic Calcium Phosphate: Synthesis to Application. ACS Appl. Bio Mater. 2019, 2, 5263–5297. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Shin, H.-L.; Kim, B.-S.; Kim, H.-J.; Ryoo, H.-M. RUNX2-modifying enzymes: Therapeutic targets for bone diseases. Exp. Mol. Med. 2020, 52, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Liu, L.; Cheng, L.; Chen, X.; Xi, S.; Jiang, T. Calcium-to-phosphorus releasing ratio affects osteoinductivity and osteoconductivity of calcium phosphate bioceramics in bone tissue engineering. Biomed. Eng. Online 2023, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Hassumi, J.S.; Mulinari-Santos, G.; Fabris, A.L.d.S.; Jacob, R.G.M.; Gonçalves, A.; Rossi, A.C.; Freire, A.R.; Faverani, L.P.; Okamoto, R. Alveolar bone healing in rats: Micro-CT, immunohistochemical and molecular analysis. J. Appl. Oral Sci. 2018, 26, e20170326. [Google Scholar] [CrossRef]
- Komori, T. Molecular Mechanism of Runx2-Dependent Bone Development. Mol. Cells 2020, 43, 168–175. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B.; van Wijnen, A.J.; Stein, J.L.; Montecino, M.; Javed, A.; Zaidi, S.K.; Young, D.W.; Choi, J.-Y.; Pockwinse, S.M. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 2004, 23, 4315–4329. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.-P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; DeGuzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Emad, B.; Sherif, E.-M.; Basma, G.M.; Wong, R.W.; Bendeus, M.; Rabie, A.B.M. Vascular endothelial growth factor augments the healing of demineralized bone matrix grafts. Int. J. Surg. 2006, 4, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Macedo, R.M.; Lacerda, S.A.; Okamoto, R.; Shahdad, S.; Brentegani, L.G. Vital bone formation after grafting of autogenous bone and biphasic calcium phosphate bioceramic in extraction sockets of rats: Histological, histometric, and immunohistochemical evaluation. Implant. Dent. 2018, 27, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Zhu, X.; Tang, Z.; Yang, X.; Tan, Y.; Fan, Y.; Zhang, X. Enhanced effect of β-tricalcium phosphate phase on neovascularization of porous calcium phosphate ceramics: In vitro and in vivo evidence. Acta Biomater. 2015, 11, 435–448. [Google Scholar] [CrossRef]
- Lomelino, R.d.O.; Castro-Silva, I.I.; Linhares, A.B.R.; Alves, G.G.; Santos, S.R.d.A.; Gameiro, V.S.; Rossi, A.M.; Granjeiro, J.M. The association of human primary bone cells with biphasic calcium phosphate (βTCP/HA 70:30) granules increases bone repair. J. Mater. Sci. Mater. Med. 2011, 23, 781–788. [Google Scholar] [CrossRef]
- Chen, J.; Shapiro, H.S.; Wrana, J.L.; Reimers, S.; Heersche, J.N.; Sodek, J. Localization of bone sialoprotein (BSP) expression to sites of mineralized tissue formation in fetal rat tissues by in situ hybridization. Matrix 1991, 11, 133–143. [Google Scholar] [CrossRef]
- Nagata, T.; Bellows, C.G.; Kasugai, S.; Butler, W.T.; Sodek, J. Biosynthesis of bone proteins [SPP-1 (secreted phosphoprotein-1, osteopontin), BSP (bone sialoprotein) and SPARC (osteonectin)] in association with mineralized-tissue formation by fetal-rat calvarial cells in culture. Biochem. J. 1991, 274, 513–520. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, J.L.; Stein, G.S.; Van Wijnen, A.J.; Montecino, M.; Javed, A.; Gutierrez, S.; Shen, J.; Zaidi, S.K.; Drissi, H. Runx2/Cbfa1 functions: Diverse regulation of gene transcription by chromatin remodeling and co-regulatory protein interactions. Connect. Tissue Res. 2003, 44 (Suppl. S1), 141–148. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Noda, M. Gene expression in osteoblastic cells. Crit. Rev. Eukaryot. Gene Expr. 1991, 1, 85–98. [Google Scholar] [PubMed]
- de Oliveira, P.T.; Zalzal, S.F.; Irie, K.; Nanci, A. Early Expression of Bone Matrix Proteins in Osteogenic Cell Cultures. J. Histochem. Cytochem. 2003, 51, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, H.A.; Warner, K.J.; Li, M.C.; Hunter, G.K. Binding of Bone Sialoprotein, Osteopontin and Synthetic Polypeptides to Hydroxyapatite. Connect. Tissue Res. 2001, 42, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Uetanabaro, L.; Claudino, M.; Mobile, R.; Zielak, J.; Garlet, G.; de Araujo, M. Osteoconductivity of Biphasic Calcium Phosphate Ceramic Improves New Bone Formation: A Histologic, Histomorphometric, Gene Expression, and Microcomputed Tomography Study. Int. J. Oral Maxillofac. Implant. 2020, 35, 70–78. [Google Scholar] [CrossRef]

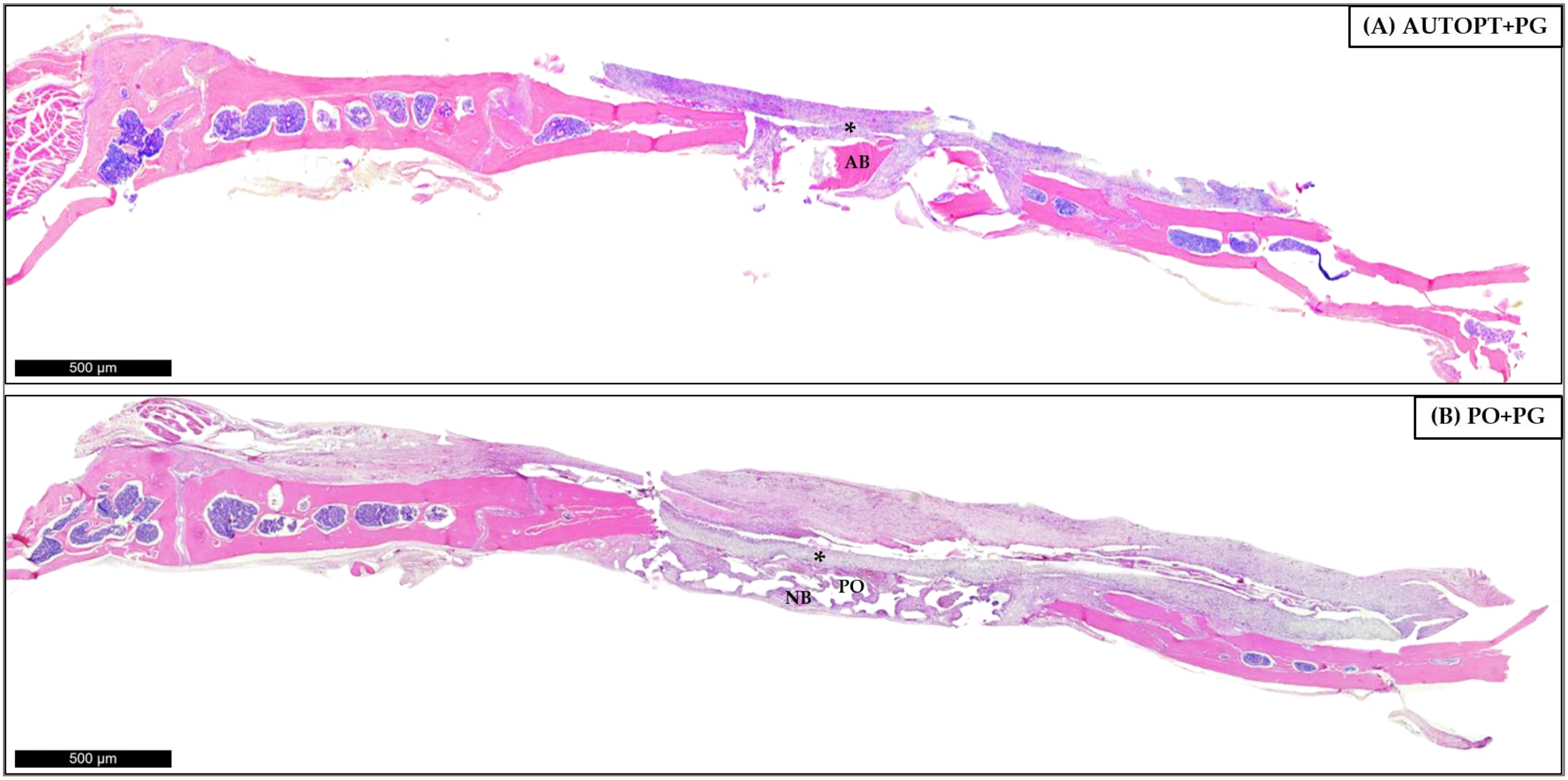

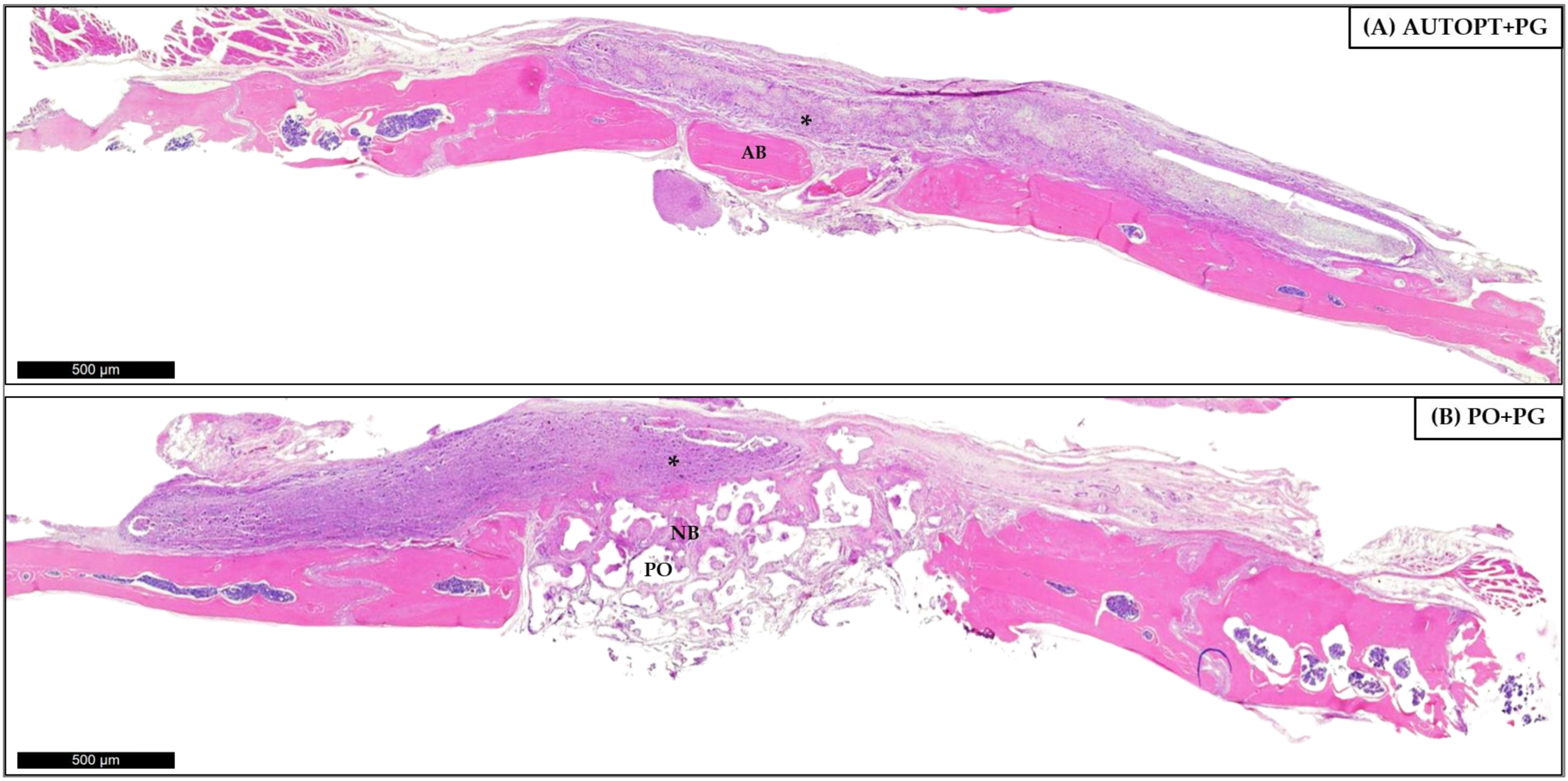
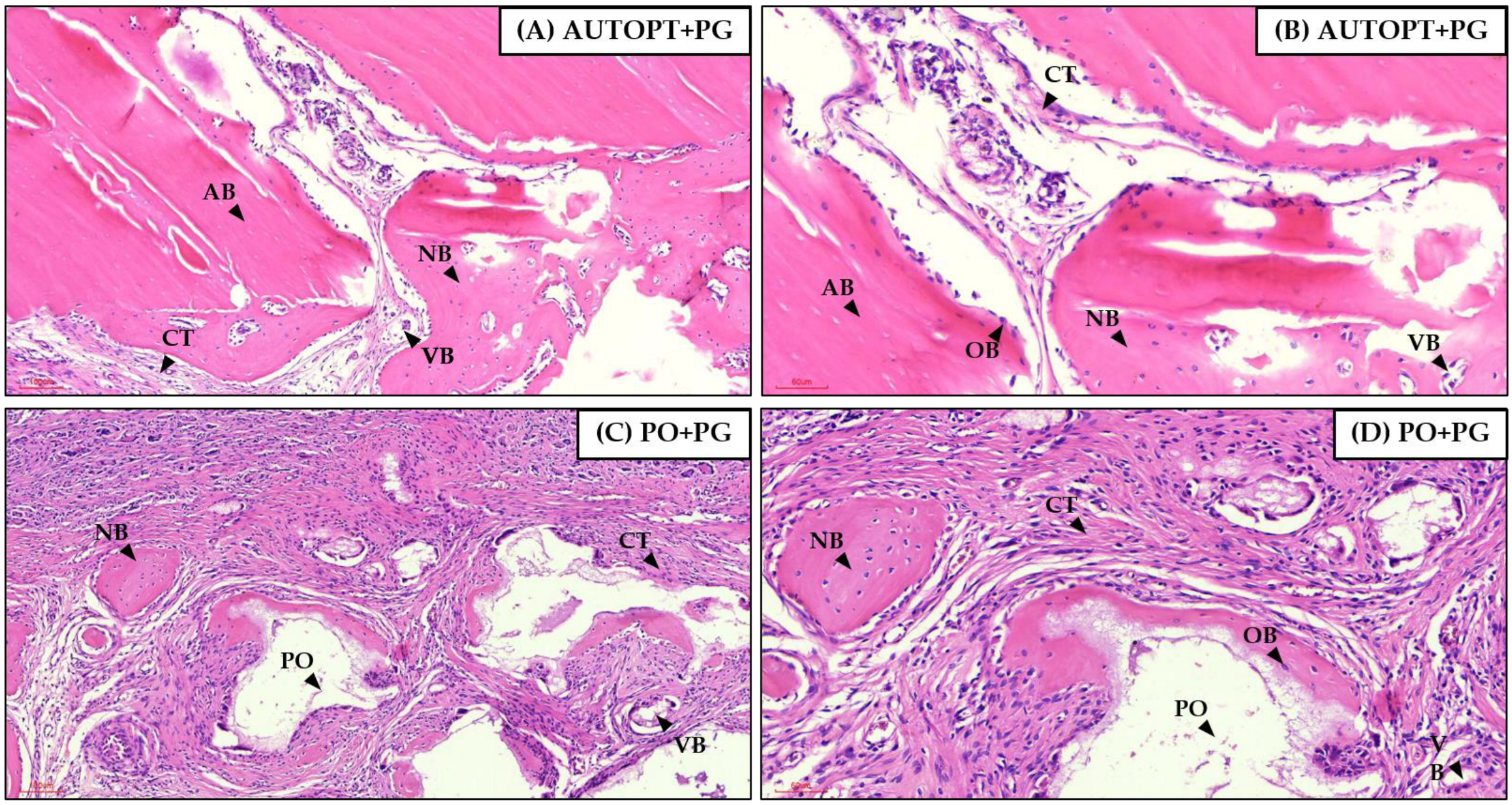

| Particulate Autogenous Bone + Plenum® Guide (AUTOPT+PG) | Plenum® Osshp + Plenum® Guide (PO+PG) | |
|---|---|---|
| BV.TV (%) | 49.29 ± 8.376 a | 56.26 ± 6.245 a |
| Tb.Th (mm) | 0.28 ± 0.010 a | 0.21 ± 0.013 b |
| Tb.N (mm) | 1.69 ± 0.242 a | 2.76 ± 0.471 b |
| Tb.Sp (mm) | 0.32 ± 0.008 a | 0.20 ± 0.052 b |
| i.S (mm2) | 25.04 ± 2.512 a | 26.76 ± 4.736 a |
| Po.Tot (%) | 59.15 ± 6.370 a | 43.79 ± 6.285 b |
| MAR (µm/day) | 5.99 ± 0.105 a | 7.16 ± 0.235 b |
| Expression Genes | Particulate Autogenous Bone + Plenum® Guide (AUTOPT+PG) | Plenum® Osshp + Plenum® Guide (PO+PG) |
|---|---|---|
| RUNX2 | 1.06 ± 0.365 a | 0.30 ± 0.107 b |
| VEGF | 0.99 ± 0.056 a | 0.50 ± 0.015 b |
| ALP | 0.99 ± 0.034 a | 0.60 ± 0.172 b |
| IBSP | 0.99 ± 0.042 a | 0.35 ± 0.094 b |
| OCN | 0.99 ± 0.017 a | 0.95 ± 0.157 a |
| Particulate Autogenous Bone + Plenum® Guide (AUTOPT+PG) | Plenum® Osshp + Plenum® Guide (PO+PG) | |
|---|---|---|
| RUNX2 | ++ | ++ |
| OPN | + | ++ |
| OCN | + | +++ |
| TRAP | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigério, P.B.; de Moura, J.; Pitol-Palin, L.; Monteiro, N.G.; Mourão, C.F.; Shibli, J.A.; Okamoto, R. Combination of a Synthetic Bioceramic Associated with a Polydioxanone-Based Membrane as an Alternative to Autogenous Bone Grafting. Biomimetics 2024, 9, 284. https://doi.org/10.3390/biomimetics9050284
Frigério PB, de Moura J, Pitol-Palin L, Monteiro NG, Mourão CF, Shibli JA, Okamoto R. Combination of a Synthetic Bioceramic Associated with a Polydioxanone-Based Membrane as an Alternative to Autogenous Bone Grafting. Biomimetics. 2024; 9(5):284. https://doi.org/10.3390/biomimetics9050284
Chicago/Turabian StyleFrigério, Paula Buzo, Juliana de Moura, Letícia Pitol-Palin, Naara Gabriela Monteiro, Carlos Fernando Mourão, Jamil Awad Shibli, and Roberta Okamoto. 2024. "Combination of a Synthetic Bioceramic Associated with a Polydioxanone-Based Membrane as an Alternative to Autogenous Bone Grafting" Biomimetics 9, no. 5: 284. https://doi.org/10.3390/biomimetics9050284
APA StyleFrigério, P. B., de Moura, J., Pitol-Palin, L., Monteiro, N. G., Mourão, C. F., Shibli, J. A., & Okamoto, R. (2024). Combination of a Synthetic Bioceramic Associated with a Polydioxanone-Based Membrane as an Alternative to Autogenous Bone Grafting. Biomimetics, 9(5), 284. https://doi.org/10.3390/biomimetics9050284










