Intussusception in Young Children: Protocol for Multisite Hospital Sentinel Surveillance in India
Abstract
:1. Introduction
2. Experimental Design
2.1. Objectives
2.2. Design
3. Procedure
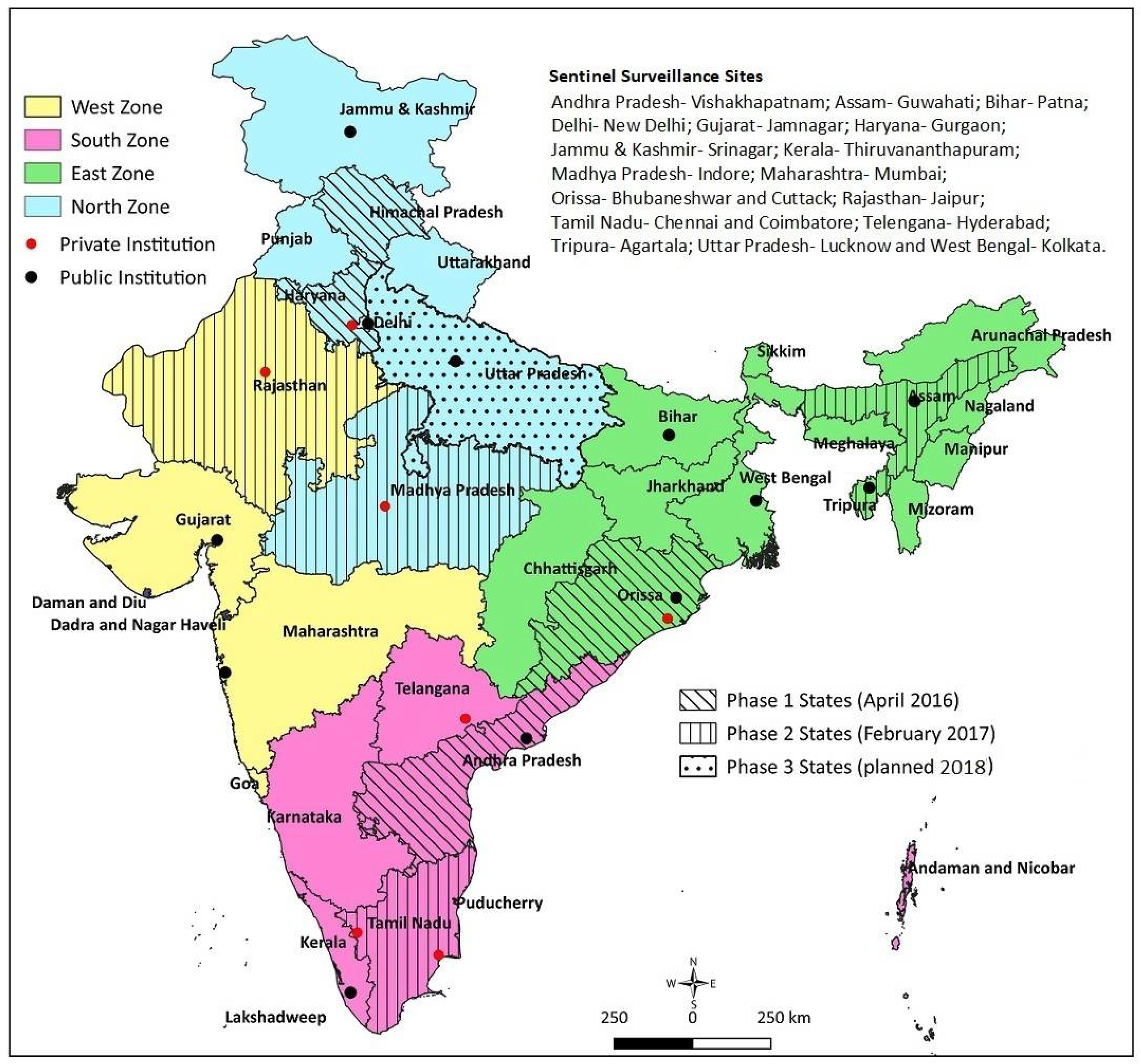
3.1. Selection of Sites and Institutions
3.2. Study Sites
3.3. Administrative and Regulatory Approvals
3.4. Study Period
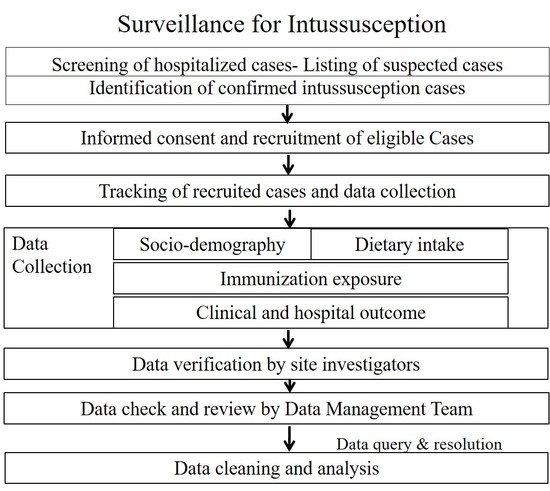
3.5. Case Identification and Enrollment
3.6. Data Collection
3.7. Site Research Teams
3.8. Case Adjudication and Labeling
3.9. Quality Assurance
3.10. Data Management
3.11. Project Management
3.12. Ethical Aspects
3.13. Linkage with the Public Health Surveillance
3.14. Dissemination
4. Expected Results
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Murphy, T.V.; Gargiullo, P.M.; Massoudi, M.S.; Nelson, D.B.; Jumaan, A.O.; Okoro, C.A.; Zanardi, L.R.; Setia, S.; Fair, E.; LeBaron, C.W.; et al. Intussusception among Infants Given an Oral Rotavirus Vaccine. N. Engl. J. Med. 2001, 344, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Matson, D.O.; Dennehy, P.; Van Damme, P.; Santosham, M.; Rodriguez, Z.; Dallas, M.J.; Heyse, J.F.; Goveia, M.G.; Black, S.B.; et al. Safety and Efficacy of a Pentavalent Human–Bovine (WC3) Reassortant Rotavirus Vaccine. N. Engl. J. Med. 2006, 354, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, G.M.; Pérez-Schael, I.; Velázquez, F.R.; Abate, H.; Breuer, T.; Clemens, S.C.; Cheuvart, B.; Espinoza, F.; Gillard, P.; Innis, B.L.; et al. Safety and Efficacy of an Attenuated Vaccine against Severe Rotavirus Gastroenteritis. N. Engl. J. Med. 2006, 354, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Tate, J.E.; Steiner, C.A.; Cortese, M.M.; Patel, M.M.; Parashar, U.D. Trends in Intussusception Hospitalizations among US Infants before and after Implementation of the Rotavirus Vaccination Program, 2000–2009. J. Infect. Dis. 2012, 206, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; López-Collada, V.R.; Bulhões, M.M.; De Oliveira, L.H.; Márquez, A.B.; Flannery, B.; Esparza-Aguilar, M.; Montenegro Renoiner, E.I.; Luna-Cruz, M.E.; Sato, H.K.; et al. Intussusception Risk and Health Benefits of Rotavirus Vaccination in Mexico and Brazil. N. Engl. J. Med. 2011, 364, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Buttery, J.P.; Danchin, M.H.; Lee, K.J.; Carlin, J.B.; McIntyre, P.B.; Elliott, E.J.; Booy, R.; Bines, J.E.; PAEDS/APSU Study Group. Intussusception following rotavirus vaccine administration: Post-marketing surveillance in the National Immunization Program in Australia. Vaccine 2011, 29, 3061–3066. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Kawade, A.; Rongsen-Chandola, T.; Bavdekar, A.; Bhandari, N.; Taneja, S.; Antony, K.; Bhatnagar, V.; Gupta, A.; Kabra, M.; et al. Active surveillance for intussusception in a phase III efficacy trial of an oral monovalent rotavirus vaccine in India. Vaccine 2014, 32, A104–A109. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, N.; Rongsen-Chandola, T.; Bavdekar, A.; John, J.; Antony, K.; Taneja, S.; Goyal, N.; Kawade, A.; Kang, G.; Rathore, S.S.; et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: A randomised, double-blind, placebo-controlled trial. Lancet 2014, 383, 2136–2143. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Desai, S.; Tewari, T.; Kawade, A.; Goyal, N.; Garg, B.S.; Kumar, D.; Kanungo, S.; Kamat, V.; Kang, G.; et al. A randomized Phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine 2017, 35, 6228–6237. [Google Scholar] [CrossRef] [PubMed]
- Operational Guidelines—Introduction of Rotavirus Vaccine in the Universal Immunization Program in India. Ministry of Health and Family Welfare, Government of India, December 2015. Available online: https://www.itsu.org.in/repository-resources/Operational-guidelines-Introduction-of-RVV-in-the-UIP-in-India.pdf (accessed on 18 September 2017).
- World Health Organisation. Rotavirus vaccines WHO position paper: January 2013—Recommendations. Vaccine 2013, 31, 6170–6171. [Google Scholar]
- Jiang, J.; Jiang, B.; Parashar, U.; Nguyen, T.; Bines, J.; Patel, M.M. Childhood Intussusception: A Literature Review. PLoS ONE 2013, 8, e68482. [Google Scholar] [CrossRef] [PubMed]
- Bines, J.E.; Kohl, K.S.; Forster, J.; Zanardi, L.R.; Davis, R.L.; Hansen, J.; Murphy, T.M.; Music, S.; Niu, M.; Varricchio, F.; et al. Acute intussusception in infants and children as an adverse event following immunization: Case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Bahl, R.; Saxena, M.; Bhandari, N.; Taneja, S.; Mathur, M.; Parashar, U.D.; Gentsch, J.; Shieh, W.J.; Zaki, S.R.; Glass, R.; et al. Population-Based Incidence of Intussusception and a Case-Control Study to Examine the Association of Intussusception with Natural Rotavirus Infection among Indian Children. J. Infect. Dis. 2009, 200 (Suppl. 1), S277–S281. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, K.; Kang, G.; Bose, A.; Chacko, J.; Boudville, I.; Datta, S.K.; Bock, H.L. Retrospective surveillance for intussusception in children aged less than five years in a South Indian tertiary-care hospital. J. Health Popul. Nutr. 2009, 27, 660–665. [Google Scholar] [PubMed]
- Jehangir, S.; John, J.; Rajkumar, S.; Mani, B.; Srinivasan, R.; Kang, G. Intussusception in southern India: Comparison of retrospective analysis and active surveillance. Vaccine 2014, 32, A99–A103. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.V.; Kamath, V.; Shetty, R.; Kumar, V.; Prasad, R.; Saluja, T.; Dhingra, M.S. Retrospective surveillance for intussusception in children aged less than five years at two tertiary care centers in India. Vaccine 2014, 32, A95–A98. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. International statistical classification of diseases and related health problems. Available online: http://www.who.int/classifications/icd/en/ (accessed on 18 September 2017).
- Koch, J.; Harder, T.; von Kries, R.; Wichmann, O. The risk of intussusception after rotavirus vaccination—a systematic literature review and meta-analysis. Dtsch. Arztebl. Int. 2017, 114, 255–262. [Google Scholar] [PubMed]
- World Health Organisation. Global Vaccine Safety Blueprint; World Health Organisation: Geneva, Switzerland, 2012; Available online: http://apps.who.int/iris/bitstream/10665/70919/1/WHO_IVB_12.07_eng.pdf (accessed on 29 October 2017).
- World Health Organisation. Rotavirus vaccines: WHO position paper—January 2013. Wkly. Epidemiol. Rec. 2013, 88, 49–64. [Google Scholar]
- World Health Organisation. Post-Marketing Surveillance of Rotavirus Vaccine Safety; World Health Organisation: Geneva, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/10665/70017/1/WHO_IVB_09.01_eng.pdf (accessed on 18 September 2017).
| Clinical Conditions Considered as Suspected Cases | Codes | |
|---|---|---|
| ICD 10 | ICD 9 | |
| Intussusception | K56.1 | 560.0 |
| Volvulus | K56.2 | 560.2 |
| Gallstone ileus | K56.3 | 560.31 |
| Other impaction of intestine | K56.4 | 560.30 |
| Intestinal adhesions with obstruction | K56.5 | 560.81 |
| Other and unspecified intestinal obstruction | K56.6 | 560.9 |
| Ileus, unspecified | K56.7 | 560.1 |
| Paralytic ileus | K56.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, M.K.; Arora, N.K.; Bonhoeffer, J.; Zuber, P.L.F.; Maure, C.G. Intussusception in Young Children: Protocol for Multisite Hospital Sentinel Surveillance in India. Methods Protoc. 2018, 1, 11. https://doi.org/10.3390/mps1020011
Das MK, Arora NK, Bonhoeffer J, Zuber PLF, Maure CG. Intussusception in Young Children: Protocol for Multisite Hospital Sentinel Surveillance in India. Methods and Protocols. 2018; 1(2):11. https://doi.org/10.3390/mps1020011
Chicago/Turabian StyleDas, Manoja Kumar, Narendra Kumar Arora, Jan Bonhoeffer, Patrick L. F. Zuber, and Christine G. Maure. 2018. "Intussusception in Young Children: Protocol for Multisite Hospital Sentinel Surveillance in India" Methods and Protocols 1, no. 2: 11. https://doi.org/10.3390/mps1020011