Protocol for the Isolation of Stratum Corneum from Pig Ear Skin: Evaluation of the Trypsin Digestion Conditions
Abstract
:1. Introduction
2. Materials and Equipment
3. Experimental Design
3.1. Study Aims
- i.
- Design and evaluation of different experimental parameters, varying the trypsin concentration, the temperature, the time of digestion and the freezing of the skin prior to digestion;
- ii.
- Study of the influence of each set of conditions on the isolated SC regarding the visual aspect, histological features and permeability properties;
- iii.
- Characterization of the SC model isolated in the selected optimal conditions by assessment of the histological morphology and storage stability.
3.2. Preliminary Steps: Preparation of Pig Ear Skin
3.3. Design of the Methodologies and Visual Inspection of the Skin after Trypsin Digestion
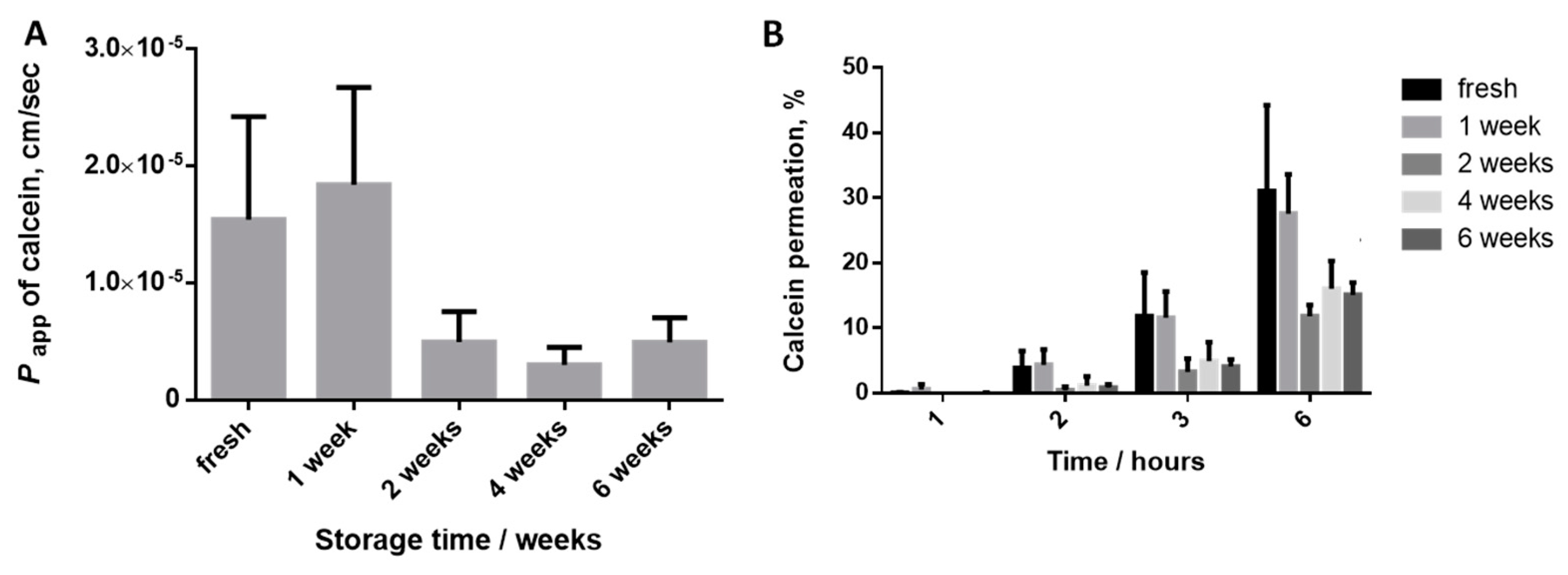
3.4. Evaluation of the Calcein Permeability
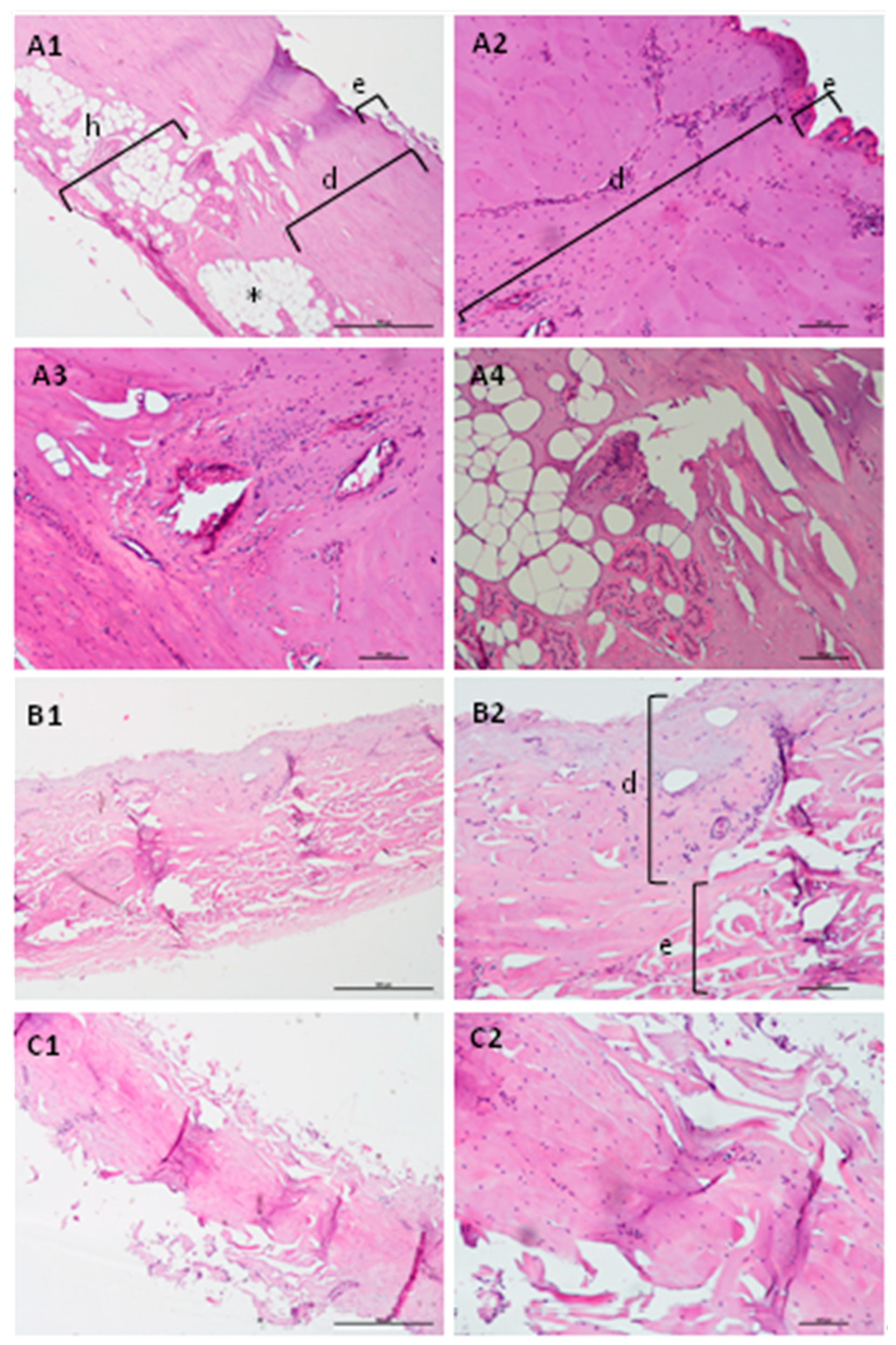
3.5. Histological Characterization
4. Results
4.1. Characterization of the Model Obtained in the Selected Conditions: Histological Analysis
4.2. Characterization of the Model Obtained in the Selected Conditions Storage Stability
5. Limitations
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Moniz, T.; Costa Lima, S.A.; Reis, S. Human skin models: From healthy to disease-mimetic systems; characteristics and applications. Br. J. Pharmacol. 2020, 177, 4314–4329. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Lau, W.M. Skin Deep: The Basics of Human Skin Structure and Drug Penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The applied anatomy of human skin: A model for regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Liu, X.; Cleary, J.; German, G.K. The global mechanical properties and multi-scale failure mechanics of heterogeneous human stratum corneum. Acta Biomater. 2016, 43, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Mendivil, M.F.; Page, A.; Bressloff, N.W.; Limbert, G. A mechanistic insight into the mechanical role of the stratum corneum during stretching and compression of the skin. J. Mech. Behav. Biomed. Mater. 2015, 49, 197–219. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.S.; Osdol, W.W.V.; Dauskardt, R.H. Mechanical and Microstructural Properties of Stratum Corneum. Mater. Res. Soc. Symp. Proc. 2002, 724, N2.7. [Google Scholar] [CrossRef]
- Uchida, Y.; Park, K. Stratum Corneum. In Immunology of the Skin: Basic and Clinical Sciences in Skin Immune Responses; Kabashima, K., Ed.; Springer: Tokyo, Japan, 2016; pp. 15–30. [Google Scholar]
- Sinko, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takacs-Novak, K. Skin-PAMPA: A new method for fast prediction of skin penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef]
- Sinkó, B.; Vizserálek, G.; Takács-Novák, K. Skin PAMPA: Application in practice. ADMET DMPK 2014, 2, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Engesland, A.; Skar, M.; Hansen, T.; Skalko-Basnet, N.; Flaten, G.E. New applications of phospholipid vesicle-based permeation assay: Permeation model mimicking skin barrier. J. Pharm. Sci. 2013, 102, 1588–1600. [Google Scholar] [CrossRef]
- Di Cagno, M.; Bibi, H.A.; Bauer-Brandl, A. New biomimetic barrier Permeapad™ for efficient investigation of passive permeability of drugs. Eur. J. Pharm. Sci. 2015, 73, 29–34. [Google Scholar] [CrossRef]
- Jacobsen, A.-C.; Nielsen, S.; Brandl, M.; Bauer-Brandl, A. Drug Permeability Profiling Using the Novel Permeapad® 96-Well Plate. Pharm. Res. 2020, 37, 93. [Google Scholar] [CrossRef]
- Abraham, W.; Downing, D.T. Preparation of model membranes for skin permeability studies using stratum corneum lipids. J. Invest. Dermatol. 1989, 93, 809–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftsson, T.; Konrádsdóttir, F.; Másson, M. Development and evaluation of an artificial membrane for determination of drug availability. Int. J. Pharm. 2006, 326, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Tsinman, K.; Sinkó, B. A high throughput method to predict skin penetration and screen topical formulations. Cosmet. Toilet. 2013, 128, 192–199. [Google Scholar]
- Flaten, G.E.; Dhanikula, A.B.; Luthman, K.; Brandl, M. Drug permeability across a phospholipid vesicle based barrier: A novel approach for studying passive diffusion. Eur. J. Pharm. Sci. 2006, 27, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Flaten, G.E.; Bunjes, H.; Luthman, K.; Brandl, M. Drug permeability across a phospholipid vesicle-based barrier 2. Characterization of barrier structure, storage stability and stability towards pH changes. Eur. J. Pharm. Sci. 2006, 28, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Berben, P.; Bauer-Brandl, A.; Brandl, M.; Faller, B.; Flaten, G.E.; Jacobsen, A.-C.; Brouwers, J.; Augustijns, P. Drug permeability profiling using cell-free permeation tools: Overview and applications. Eur. J. Pharm. Sci. 2018, 119, 219–233. [Google Scholar] [CrossRef]
- Engesland, A.; Škalko-Basnet, N.; Flaten, G.E. Phospholipid vesicle-based permeation assay and EpiSkin® in assessment of drug therapies destined for skin administration. J. Pharm. Sci. 2015, 104, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Engesland, A.; Škalko-Basnet, N.; Flaten, G.E. In vitro models to estimate drug penetration through the compromised stratum corneum barrier. Drug Dev. Ind. Pharm. 2016, 42, 1742–1751. [Google Scholar] [CrossRef]
- Ma, M.; Di, H.-J.; Zhang, H.; Yao, J.-H.; Dong, J.; Yan, G.-J.; Qiao, H.-Z.; Chen, J. Development of phospholipid vesicle-based permeation assay models capable of evaluating percutaneous penetration enhancing effect. Drug Dev. Ind. Pharm. 2017, 43, 2055–2063. [Google Scholar] [CrossRef]
- Palac, Z.; Engesland, A.; Flaten, G.E.; Skalko-Basnet, N.; Filipovic-Grcic, J.; Vanic, Z. Liposomes for (trans)dermal drug delivery: The skin-PVPA as a novel in vitro stratum corneum model in formulation development. J. Liposome Res. 2014, 24, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhu, X.; Shen, J.; Xu, H.; Ma, M.; Gu, W.; Jiang, Q.; Chen, J.; Duan, J. Characterization of a liposome-based artificial skin membrane for in vitro permeation studies using Franz diffusion cell device. J. Liposome Res. 2017, 27, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Jurek, I.; Góral, I.; Mierzyńska, Z.; Moniuszko-Szajwaj, B.; Wojciechowski, K. Effect of synthetic surfactants and soapwort (Saponaria officinalis L.) extract on skin-mimetic model lipid monolayers. Biochim. Biophys. Acta 2019, 1861, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Shakel, Z.; Nunes, C.; Costa Lima, S.A.; Reis, S. Development of a novel human stratum corneum model, as a tool in the optimization of drug formulations. Int. J. Pharm. 2019, 569, 118571. [Google Scholar] [CrossRef] [PubMed]
- Moniz, T.; Costa Lima, S.A.; Reis, S. Application of the human stratum corneum lipid-based mimetic model in assessment of drug-loaded nanoparticles for skin administration. Int. J. Pharm. 2020, 591, 119960. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Kligman, A.M.; Christophers, E. Preparation of isolated sheets of human stratum corneum. Arch. Dermatol. 1963, 88, 702–705. [Google Scholar] [CrossRef]
- Gray, G.M.; Yardley, H.J. Different populations of pig epidermal cells: Isolation and lipid composition. J. Lipid Res. 1975, 16, 441–447. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Gooris, G.S.; Bras, W.; Downing, D.T. Lipid organization in pig stratum corneum. J. Lipid Res. 1995, 36, 685–695. [Google Scholar] [CrossRef]
- Foerster, A.C.; Neubert, R.H.H. Dermal Peptide Delivery Using Enhancer Molecules and Colloidal Carrier Systems—Part IV: Search for an Alternative Model Membrane for Future ATR Permeation Studies Using PKEK as the Model Substance. Skin Pharmacol. Physiol. 2019, 32, 151–161. [Google Scholar] [CrossRef]
- Grayson, S.; Elias, P.M. Isolation and lipid biochemical characterization of stratum corneum membrane complexes: Implications for the cutaneous permeability barrier. J. Investig. Dermatol. 1982, 78, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Elias, P.M.; Williams, M.L.; Maloney, M.E.; Bonifas, J.A.; Brown, B.E.; Grayson, S.; Epstein, E.H., Jr. Stratum corneum lipids in disorders of cornification. Steroid sulfatase and cholesterol sulfate in normal desquamation and the pathogenesis of recessive X-linked ichthyosis. J. Clin. Investig. 1984, 74, 1414–1421. [Google Scholar] [CrossRef]
- Barba, C.; Alonso, C.; Martí, M.; Manich, A.; Coderch, L. Skin barrier modification with organic solvents. Biochim. Biophys. Acta 2016, 1858, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Quatela, A.; Tfayli, A.; Baillet-Guffroy, A. Examination of the effect of Stratum Corneum isolation process on the integrity of the barrier function: A confocal Raman spectroscopy study. Skin Res. Technol. 2016, 22, 75–80. [Google Scholar] [CrossRef]
- Liu, Y.; Lunter, D.J. Optimal configuration of confocal Raman spectroscopy for precisely determining stratum corneum thickness: Evaluation of the effects of polyoxyethylene stearyl ethers on skin. Int. J. Pharm. 2021, 597, 120308. [Google Scholar] [CrossRef]
- Ferguson, J. A method to facilitate the isolation and handling of stratum corneum. Br. J. Dermatol. 1977, 96, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wu, Y.; Zhang, H.; Liu, P.; Yao, J.; Yao, P.; Chen, J.; Duan, J. Development of essential oils as skin permeation enhancers: Penetration enhancement effect and mechanism of action. Pharm. Biol. 2017, 55, 1592–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowser, P.A.; White, R.J. Isolation, barrier properties and lipid analysis of stratum compactum, a discrete region of the stratum corneum. Br. J. Dermatol. 1985, 112, 1–14. [Google Scholar] [CrossRef]
- Groen, D.; Gooris, G.S.; Ponec, M.; Bouwstra, J.A. Two new methods for preparing a unique stratum corneum substitute. Biochim. Biophys. Acta 2008, 1778, 2421–2429. [Google Scholar] [CrossRef] [Green Version]
- Swartzendruber, D.C.; Kitko, D.J.; Wertz, P.W.; Madison, K.C.; Downing, D.T. Isolation of corneocyte envelopes from porcine epidermis. Arch. Dermatol. Res. 1988, 280, 424–429. [Google Scholar] [CrossRef]
- Berkers, T.; Visscher, D.; Gooris, G.S.; Bouwstra, J.A. Degree of Skin Barrier Disruption Affects Lipid Organization in Regenerated Stratum Corneum. Acta Derm. Venereol. 2018, 98, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Aasen, T.; Izpisúa Belmonte, J.C. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat. Protoc. 2010, 5, 371–382. [Google Scholar] [CrossRef]
- Liu, Z.; Wen, J.; Leng, X.; Zhou, Q.; Zhou, C.; Zhao, H.; Wu, X. A Simplified and Efficient Method to Isolate Primary Human Keratinocytes from Adult Skin Tissue. JoVE 2018, e57784. [Google Scholar] [CrossRef] [Green Version]
- Baccarin, T.; Lemos-Senna, E. Potential Application of Nanoemulsions for Skin Delivery of Pomegranate Peel Polyphenols. AAPS PharmSciTech 2017, 18, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Kalia, Y.N.; Alberti, I.; Naik, A.; Guy, R.H. Assessment of topical bioavailability in vivo: The importance of stratum corneum thickness. Skin Pharmacol. Appl. Skin Physiol. 2001, 14 (Suppl. 1), 82–86. [Google Scholar] [CrossRef] [PubMed]
- Curdy, C.; Kalia, Y.N.; Naik, A.; Guy, R.H. Piroxicam delivery into human stratum corneum in vivo: Iontophoresis versus passive diffusion. J. Control. Release 2001, 76, 73–79. [Google Scholar] [CrossRef]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.J.; Fluhr, J.W. The tape stripping procedure—Evaluation of some critical parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.; Merino, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar, D.; Ganem, A. The Tape-Stripping Technique as a Method for Drug Quantification in Skin. J. Pharm. Pharm. Sci. 2008, 11, 104–130. [Google Scholar] [CrossRef] [Green Version]
- Esposito, L.; Barbosa, A.I.; Moniz, T.; Costa Lima, S.; Costa, P.; Celia, C.; Reis, S. Design and Characterization of Sodium Alginate and Poly(vinyl) Alcohol Hydrogels for Enhanced Skin Delivery of Quercetin. Pharmaceutics 2020, 12, 1149. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Canadas, A.; Romão, P.; Gärtner, F. Multiple Cutaneous Metastasis of a Malignant Leydig Cell Tumour in a Dog. J. Comp. Pathol. 2016, 155, 181–184. [Google Scholar] [CrossRef]
- Matos, R.; Fonseca, K.L.; Mereiter, S.; Maceiras, A.R.; Gomes, J.; Vilaplana, C.; Gartner, F.; Rodrigues, P.N.S.; Reis, C.A.; Saraiva, M.; et al. Mycobacterium tuberculosis Infection Up-Regulates Sialyl Lewis X Expression in the Lung Epithelium. Microorganisms 2021, 9, 99. [Google Scholar] [CrossRef] [PubMed]


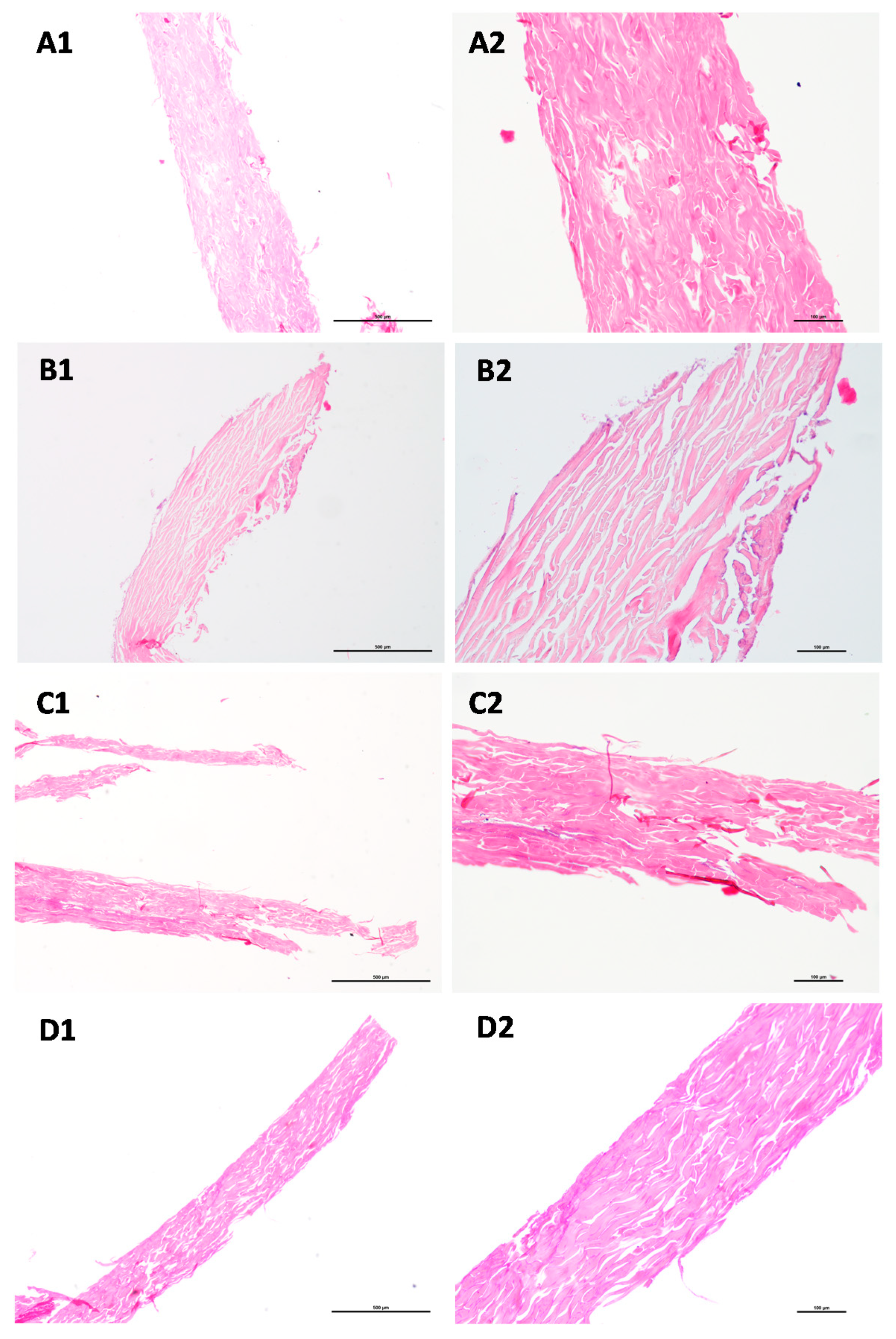

| Condition | Incubation Time | Temperature | Trypsin Concentration | Pre- Freezing |
|---|---|---|---|---|
| A | 4 h + O/N 1 (total ~ 20 h) | 4 °C | 0.1% | No |
| B | 4 h + O/N 1 (total ~ 20 h) | 4 °C | 0.1% | Yes, 48 h |
| C | 3 h | 37 °C | 0.1% | No |
| D | 3 h | 37 °C | 0.05% | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moniz, T.; Lima, S.A.C.; Reis, S. Protocol for the Isolation of Stratum Corneum from Pig Ear Skin: Evaluation of the Trypsin Digestion Conditions. Methods Protoc. 2021, 4, 80. https://doi.org/10.3390/mps4040080
Moniz T, Lima SAC, Reis S. Protocol for the Isolation of Stratum Corneum from Pig Ear Skin: Evaluation of the Trypsin Digestion Conditions. Methods and Protocols. 2021; 4(4):80. https://doi.org/10.3390/mps4040080
Chicago/Turabian StyleMoniz, Tânia, Sofia A. Costa Lima, and Salette Reis. 2021. "Protocol for the Isolation of Stratum Corneum from Pig Ear Skin: Evaluation of the Trypsin Digestion Conditions" Methods and Protocols 4, no. 4: 80. https://doi.org/10.3390/mps4040080






