Assessment of SARS-CoV-2 (COVID-19) Clinical Mouthwash Protocol and Prevalence of the Oral Pathogen Scardovia wiggsiae: A Pilot Study of Antibacterial Effects
Abstract
:1. Introduction
2. Methods
2.1. Project Approval
2.2. Human Subjects
2.3. SARS-CoV-2 (COVID-19) Protocol
2.4. DNA Isolation
2.5. DNA Analysis
2.6. qPCR Screening
- Positive control, bacterial 16S rRNA
- Forward 16S rRNA primer: 5′-ACG CGT CGA CAG AGT TTG ATC CTG GCT-3′;
- Reverse 16S rRNA primer: 5′-GGG ACT ACC AGG GTA TCT AAT-3′;
- Scardovia wiggsiae (SW) primer
- SW Forward primer: 5′-GTG GAC TTT ATG AAT AAG C-3′;
- SW Reverse primer: 5′-CTA CCG TTA AGC AGT AAG-3′;
2.7. Statistical Analysis
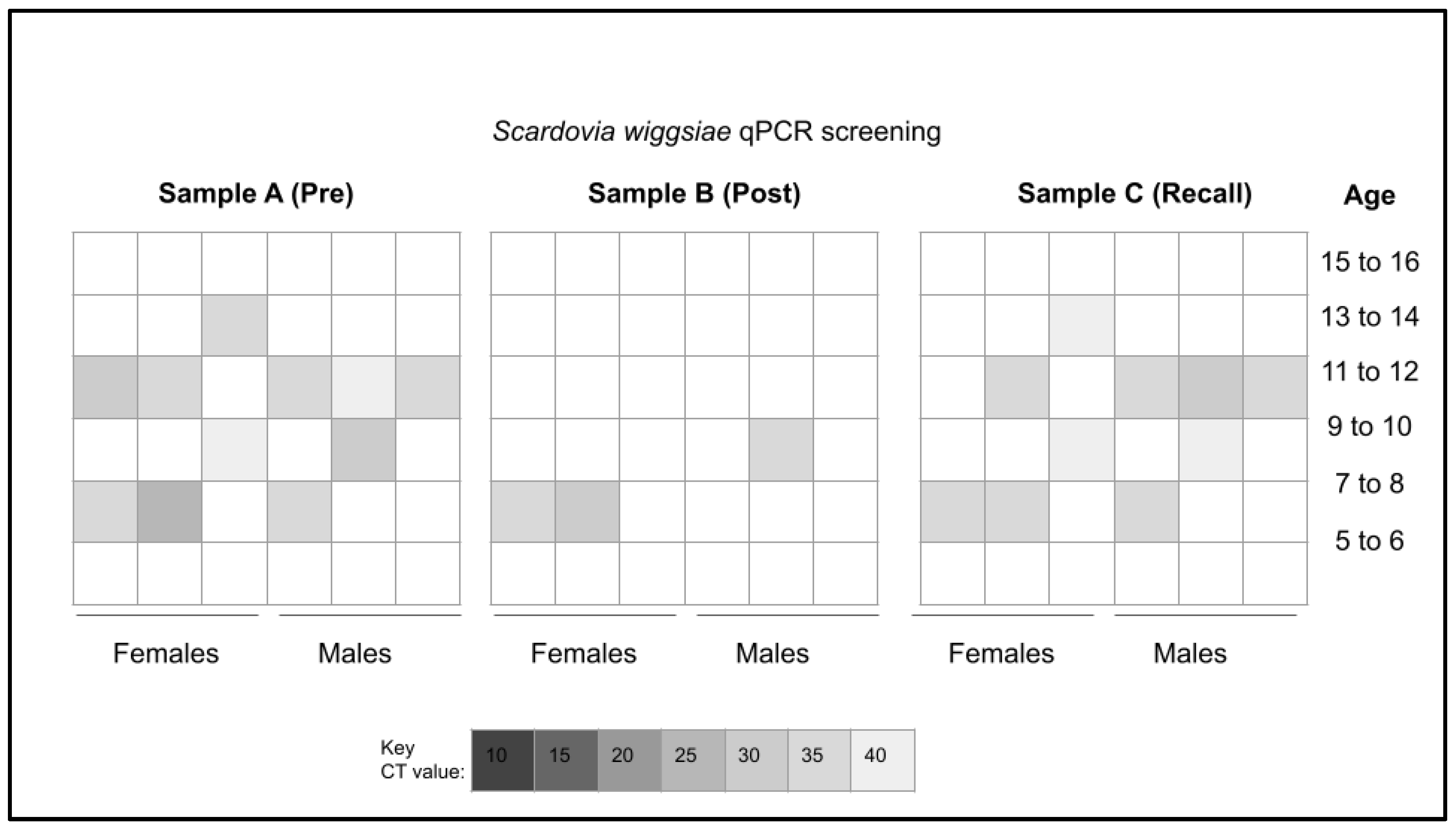
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basilicata, M.; Zarone, F.; Leone, R.; Guerriero, C.; Di Lauro, M.; Franco, R.; Bernardini, S.; Noce, A.; Bollero, P.; Sorrentino, R. Impact of SARS-CoV-2 on dentistry: A review of literature. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3386–3398. [Google Scholar] [CrossRef]
- Caggiano, M.; Acerra, A.; Martina, S.; Galdi, M.; D’ambrosio, F. Infection Control in Dental Practice during the COVID-19 Pandemic: What Is Changed? Int. J. Environ. Res. Public Health 2023, 20, 3903. [Google Scholar] [CrossRef]
- Hartig, M.; Stephens, C.; Foster, A.; Fontes, D.; Kinzel, M.; García-Godoy, F. Stopping the COVID-19 pandemic in dental offices: A review of SARS-CoV-2 transmission and cross-infection prevention. Exp. Biol. Med. 2021, 246, 2381–2390. [Google Scholar] [CrossRef]
- Patil, S.; Moafa, I.H.; Bhandi, S.; Jafer, M.A.; Khan, S.S.; Khan, S.; Carroll, W.B.; Awan, K.H. Dental care and personal protective measures for dentists and non-dental health care workers. Disease-a-Month 2020, 66, 101056. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Caggiano, M.; Amato, M.; Moccia, G.; Capunzo, M.; De Caro, F. Infection Control in Dental Practice During the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 4769. [Google Scholar] [CrossRef] [PubMed]
- Barrueco, A.S.; Mateos-Moreno, M.V.; Aubá, J.M.V.; González, A.C.; Castaño, A.B.; Yanguas, R.R.; Goñi, A.B.; Ferrero, J.Z.; Español, C.C.; Márquez, V.A.; et al. In vivo effect of mouthwashes on viable viral load of SARS-CoV-2 in saliva: A pilot study. J. Oral Microbiol. 2023, 15, 2198432. [Google Scholar] [CrossRef]
- Ziaeefar, P.; Bostanghadiri, N.; Yousefzadeh, P.; Gabbay, J.; Bonjar, A.H.S.; Ahsaie, M.G.; Centis, R.; Sabeti, M.; Sotgiu, G.; Migliori, G.B.; et al. The efficacy of mouthwashes in reducing SARS-CoV-2 viral loads in human saliva: A systematic review. New Microbes New Infect. 2022, 49–50, 101064. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.R.; Kazmi, S.M.R.; Iqbal, N.T.; Iqbal, J.; Ali, S.T.; Abbas, S.A. A quadruple blind, randomised controlled trial of gargling agents in reducing intraoral viral load among hospitalised COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 785. [Google Scholar] [CrossRef]
- Carrouel, F.; Viennot, S.; Valette, M.; Cohen, J.-M.; Dussart, C.; Bourgeois, D. Salivary and Nasal Detection of the SARS-CoV-2 Virus After Antiviral Mouthrinses (BBCovid): A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 906. [Google Scholar] [CrossRef]
- Mendoza, J.P.I.M.; Ubillús, B.P.T.; Bolívar, G.T.S.; Palacios, R.D.P.C.; Lopez, P.S.G.H.; Rodríguez, D.A.P.; Koecklin, K.H.U. Antiviral effect of mouthwashes against SARS-COV-2: A systematic review. Saudi Dent. J. 2022, 34, 167–193. [Google Scholar] [CrossRef]
- Rahman, G.S.; Alshetan, A.A.N.; Alotaibi, S.S.O.; Alaskar, B.M.I.; Baseer, M.A. Is chlorhexidine mouthwash effective in lowering COVID-19 viral load? A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 366–377. [Google Scholar] [CrossRef]
- Elmahgoub, F.; Coll, Y. Could certain mouthwashes reduce transmissibility of COVID-19? Evidence Based Dent. 2021, 22, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Leão, B.L.; de Araujo, C.M.; Basso, I.B.; Schroder, A.G.; Guariza-Filho, O.; Ravazzi, G.C.; Gonçalves, F.M.; Zeigelboim, B.S.; Santos, R.S.; Stechman-Neto, J. Is there scientific evidence of the mouthwashes effectiveness in reducing viral load in Covid-19? A systematic review. J. Clin. Exp. Dent. 2021, 13, e179–e189. [Google Scholar] [CrossRef]
- Ortega, K.L.; Rech, B.O.; El Haje, G.L.C.; Gallo, C.B.; Pérez-Sayáns, M.; Braz-Silva, P.H. Do hydrogen peroxide mouthwashes have a virucidal effect? A systematic review. J. Hosp. Infect. 2020, 106, 657–662. [Google Scholar] [CrossRef]
- Alves, P.; Gryson, L.; Hajjar, J.; Lepelletier, D.; Reners, M.; Salazar, J.R.; Simon, A. Role of antiseptics in the prevention and treatment of infections in nursing homes. J. Hosp. Infect. 2022, 131, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.; Barrois, B.; Lambert, J.; Malhotra-Kumar, S.; Santos-Fernandes, V.; Monstrey, S. Addressing the challenges in antisepsis: Focus on povidone iodine. Int. J. Antimicrob. Agents 2020, 56, 106064. [Google Scholar] [CrossRef] [PubMed]
- Kanagalingam, J.; Feliciano, R.; Hah, J.H.; Labib, H.; Le, T.A.; Lin, J.C. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int. J. Clin. Pract. 2015, 69, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, K.S.; Ngo, H.C.; Samaranayake, L.P. Cariogenic microbiome and microbiota of the early primary dentition: A contemporary overview. Oral Dis. 2018, 25, 982–995. [Google Scholar] [CrossRef]
- Isaac, R.D.; Sanjeev, K.; Subbulakshmi, C.L.; Amirtharaj, L.V.; Sekar, M. Identification of a novel bacterium Scardovia wiggsiae in high caries risk adolescence: A metagenomic and melt curve analysis. J. Conserv. Dent. 2022, 25, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Tantikalchan, S.; Mitrakul, K. Association between Bifidobacterium and Scardovia wiggsiae and caries-related factors in severe early childhood caries and caries-free Thai children: A quantitative real-time PCR analysis and a questionnaire cross-sectional study. Eur. Arch. Paediatr. Dent. 2022, 23, 437–447. [Google Scholar] [CrossRef]
- Zhan, L. Rebalancing the Caries Microbiome Dysbiosis: Targeted Treatment and Sugar Alcohols. Adv. Dent. Res. 2018, 29, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Sette-de-Souza, P.H.; Soares Martins, J.C.; Martins-de-Barros, A.V.; Rodrigues Vieira, B.; Fernandes Costa, M.J.; da Costa Araújo, F.A. A critical appraisal of evidence in the use of preprocedural mouthwash to avoid SARS-CoV-2 transmission during oral interventions. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10222–10224. [Google Scholar] [CrossRef] [PubMed]
- Carrouel, F.; Gonçalves, L.S.; Conte, M.P.; Campus, G.; Fisher, J.; Fraticelli, L.; Gadea-Deschamps, E.; Ottolenghi, L.; Bourgeois, D. Antiviral Activity of Reagents in Mouth Rinses against SARS-CoV-2. J. Dent. Res. 2020, 100, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.G.M.; Hashizume, L.N.; Maltz, M. The effect of different formulations of chlorhexidine in reducing levels of mutans streptococci in the oral cavity: A systematic review of the literature. J. Dent. 2007, 35, 359–370. [Google Scholar] [CrossRef]
- Silvestri, L.; Weir, I.; Gregori, D.; Taylor, N.; Zandstra, D.; Van Saene, J.J.; Van Saene, H.K. Effectiveness of oral chlorhexidine on nosocomial pneumonia, causative micro-organisms and mortality in critically ill patients: A systematic review and meta-analysis. Minerva Anestesiol. 2013, 80, 805–820. [Google Scholar]
- Prasad, M.; Patthi, B.; Singla, A.; Gupta, R.; Jankiram, C.; Kumar, J.K.; Vashishtha, V.; Malhi, R. The Clinical Effectiveness of Post-Brushing Rinsing in Reducing Plaque and Gingivitis: A Systematic Review. J. Clin. Diagn. Res. 2016, 10, ZE01–ZE07. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.R.; Thomas, A.; Shetty, S.B. Anti-microbial efficacy of green tea and chlorhexidine mouth rinses against Streptococcus mutans, Lactobacilli spp. and Candida albicans in children with severe early childhood caries: A randomized clinical study. J. Indian Soc. Pedod. Prev. Dent. 2016, 34, 65. [Google Scholar] [CrossRef]
- Thomas, A.; Thakur, S.; Habib, R. Comparison of Antimicrobial Efficacy of Green Tea, Garlic with Lime, and Sodium Fluoride Mouth Rinses against Streptococcus mutans, Lactobacilli species, and Candida albicans in Children: A Randomized Double-blind Controlled Clinical Trial. Int. J. Clin. Pediatr. Dent. 2017, 10, 234–239. [Google Scholar] [CrossRef]
- Agarwal, P.; Nagesh, L. Comparative evaluation of efficacy of 0.2% Chlorhexidine, Listerine and Tulsi extract mouth rinses on salivary Streptococcus mutans count of high school children—RCT. Contemp. Clin. Trials 2011, 32, 802–808. [Google Scholar] [CrossRef]
- Neeraja, R.; Anantharaj, A.; Praveen, P.; Karthik, V.; Vinitha, M. The effect of povidone-iodine and chlorhexidine mouth rinses on plaque Streptococcus mutans count in 6- to 12-year-old school children: An in vivo study. J. Indian Soc. Pedod. Prev. Dent. 2008, 26 (Suppl. S1), S14–S18. [Google Scholar] [PubMed]
- Li, Y.; Tanner, A. Effect of Antimicrobial Interventions on the Oral Microbiota Associated with Early Childhood Caries. Pediatr. Dent. 2015, 37, 226–244. [Google Scholar]
- Garcia, R.; Borrelli, B.; Dhar, V.; Douglass, J.; Gomez, F.R.; Hieftje, K.; Horowitz, A.; Li, Y.; Ng, M.W.; Twetman, S.; et al. Progress in Early Childhood Caries and Opportunities in Research, Policy, and Clinical Management. Dent. Traumatol. 2015, 37, 294–299. [Google Scholar]
- Coelho, A.S.E.C.; Paula, A.B.P.; Carrilho, T.M.P.; Da Silva, M.J.R.F.; Botelho, M.F.R.R.; Carrilho, E.V.V.F. Chlorhexidine mouthwash as an anticaries agent: A systematic review. Quintessence Int. 2017, 48, 585–591. [Google Scholar] [CrossRef]
- Marinho, V.C.; Chong, L.-Y.; Worthington, H.V.; Walsh, T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2016, 2021, CD002284. [Google Scholar] [CrossRef]
- Kameda, M.; Abiko, Y.; Washio, J.; Tanner, A.C.R.; Kressirer, C.A.; Mizoguchi, I.; Takahashi, N. Sugar Metabolism of Scardovia wiggsiae, a Novel Caries-Associated Bacterium. Front. Microbiol. 2020, 11, 479. [Google Scholar] [CrossRef]
- Philip, N.; Leishman, S.J.; Bandara, H.M.H.N.; Walsh, L.J. Casein Phosphopeptide-Amorphous Calcium Phosphate Attenuates Virulence and Modulates Microbial Ecology of Saliva-Derived Polymicrobial Biofilms. Caries Res. 2019, 53, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Alroudhan, I.E.; Gamal, M.; Ganji, K.K.; Khan, A.M.; Alsharari, K.N.; Alruwaili, M.K.; Al Waqdani, N.H. The Effectiveness of Mouthwashes with Various Ingredients in Plaque Control: A Systematic Review and Meta-Analysis. Altern. Ther. Health Med. 2021, 27, 52–57. [Google Scholar]
- Van der Weijden, F.A.; Van der Sluijs, E.; Ciancio, S.G.; Slot, D.E. Can Chemical Mouthwash Agents Achieve Plaque/Gingivitis Control? Dent. Clin. North Am. 2015, 59, 799–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavi, J.; Kingsley, K. Analysis of a Pediatric Dental School Patient Population Revealed Increasing Trends of Limited English Proficiency (LEP) Patients: Implications for Pediatric Dental Public Health and Access to Care. Pediatr. Rep. 2022, 14, 276–287. [Google Scholar] [CrossRef]
- Emett, J.; David, R.; McDaniel, J.; McDaniel, S.; Kingsley, K. Comparison of DNA Extracted from Pediatric Saliva, Gingival Crevicular Fluid and Site-Specific Biofilm Samples. Methods Protoc. 2020, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Row, L.; Repp, M.R.; Kingsley, K. Screening of a Pediatric and Adult Clinic Population for Caries Pathogen Scardovia wiggsiae. J. Clin. Pediatr. Dent. 2016, 40, 438–444. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, S.; McDaniel, J.; Howard, K.M.; Kingsley, K. Molecular Screening and Analysis Reveal Novel Oral Site-Specific Locations for the Cariogenic Pathogen Scardovia wiggsiae. Dent. J. 2021, 9, 73. [Google Scholar] [CrossRef] [PubMed]
| Demographic | Study Sample | Clinic | Statistics |
|---|---|---|---|
| Sex | |||
| Males | 47.20% | 52.10% | X2 = 0.446, d.f. = 1 |
| Females | 52.80% | 47.90% | p = 0.5043 |
| Race and Ethnicity | |||
| White | 22.20% | 24.70% | X2 = 0.148, d.f. = 1 |
| Minority | 77.80% | 75.30% | p = 0.7003 |
| Hispanic | 61.10% | 52.40% | |
| Black | 11.10% | 12.20% | |
| Asian | 2.80% | 3.80% | |
| Age | |||
| Average | 9.16 years | 9.04 years | Two tailed t-test, p = 0.884 |
| Range | 5 to 16 years | 1 to 17 years |
| Study Sample | DNA Concentration | DNA Purity |
|---|---|---|
| A260:A280 Ratio | ||
| Pre-mouthwash (Sample A) Time (T) 1, n = 36 | Average: 1141.74 ng/uL +/− 38.5 | Average: 1.71 Range: 1.65–1.84 |
| Range: 629.1–1847.3 ng/uL | ||
| Post-mouthwash (Sample B) Time (T) 2, n = 36 | Average: 883.94 ng/uL +/− 41.7 | Average: 1.75 Range: 1.62–1.88 |
| Range: 663.1–1110.1 ng/uL | ||
| Two-tailed t-test T1:T2, p = 0.004 | ||
| Recall follow-up (Sample C) Time (T) 3, n = 36 | Average: 1350.85 ng/uL +/− 41.7 | Average: 1.76 Range: 1.61–1.85 |
| Range: 737.1–1207.0 ng/uL | ||
| Two-tailed t-test T1:T3, p = 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shayegh, M.; Sorenson, C.; Downey, J.; Lin, S.; Jiang, Y.; Sodhi, P.; Sullivan, V.; Howard, K.M.; Kingsley, K. Assessment of SARS-CoV-2 (COVID-19) Clinical Mouthwash Protocol and Prevalence of the Oral Pathogen Scardovia wiggsiae: A Pilot Study of Antibacterial Effects. Methods Protoc. 2023, 6, 65. https://doi.org/10.3390/mps6040065
Shayegh M, Sorenson C, Downey J, Lin S, Jiang Y, Sodhi P, Sullivan V, Howard KM, Kingsley K. Assessment of SARS-CoV-2 (COVID-19) Clinical Mouthwash Protocol and Prevalence of the Oral Pathogen Scardovia wiggsiae: A Pilot Study of Antibacterial Effects. Methods and Protocols. 2023; 6(4):65. https://doi.org/10.3390/mps6040065
Chicago/Turabian StyleShayegh, Melika, Chase Sorenson, Jackson Downey, Summer Lin, Yuxin Jiang, Praneeti Sodhi, Victoria Sullivan, Katherine M. Howard, and Karl Kingsley. 2023. "Assessment of SARS-CoV-2 (COVID-19) Clinical Mouthwash Protocol and Prevalence of the Oral Pathogen Scardovia wiggsiae: A Pilot Study of Antibacterial Effects" Methods and Protocols 6, no. 4: 65. https://doi.org/10.3390/mps6040065





