Valorizing Tree-Nutshell Particles as Delivery Vehicles for a Natural Herbicide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plastic Bags
2.2. Plant Seeds
2.3. SA Herbicidal Efficacy in Soil
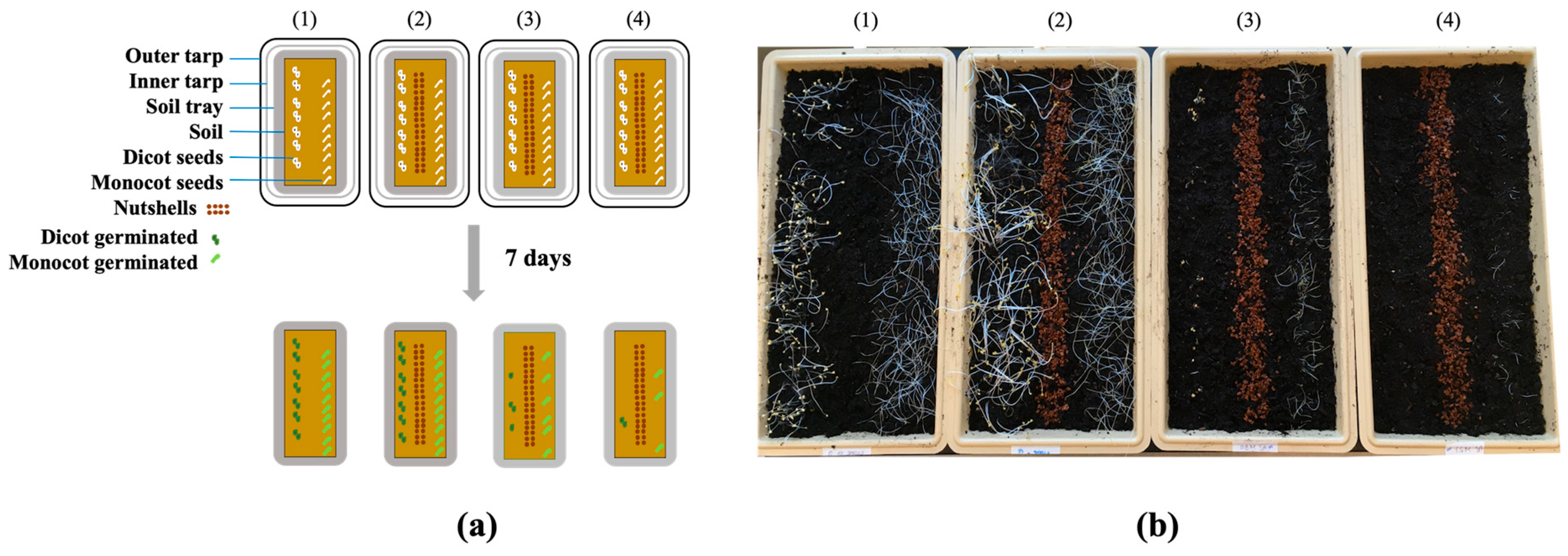
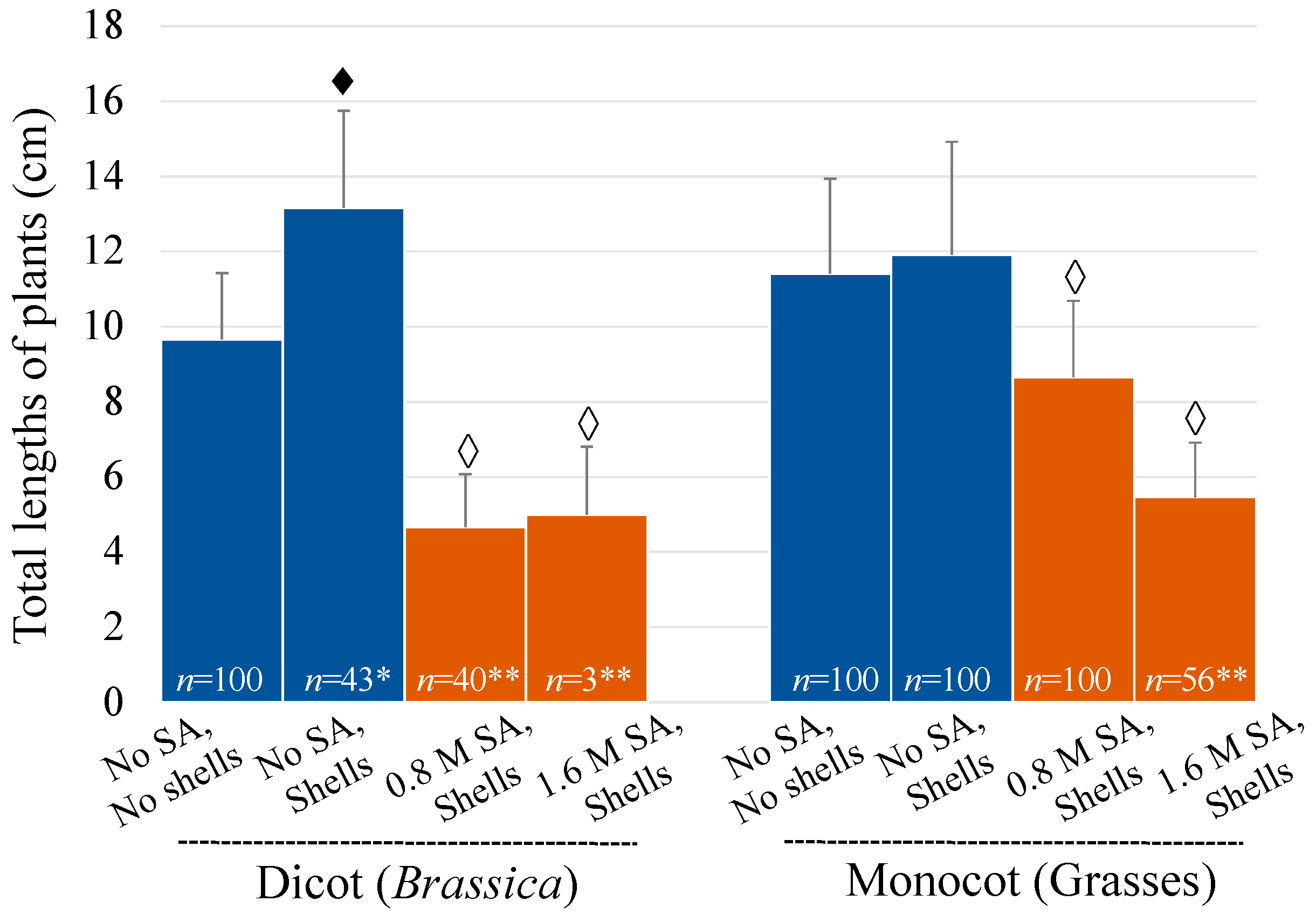
2.3.1. Pre-Emergent Herbicidal Efficacy of SA (with a Soil Covering and DMSO)
2.3.2. Post-Emergent Herbicidal Efficacy of SA (with a Soil Covering and DMSO; Method 1)
2.3.3. Post-Emergent Herbicidal Efficacy of SA (with a Soil Covering and DMSO; Method 2)
2.3.4. Post-Emergent Herbicidal Efficacy of SA (without a Soil Covering and with 60% Ethanol (v/v); Method 3)
2.4. SA Antifungal Efficacy on Tree Nutshells
2.5. Statistical Analysis
3. Results and Discussion
3.1. Pre-Emergent Herbicidal Efficacy of SA (w/ Soil Covering, DMSO)
3.2. Post-Emergent Herbicidal Efficacy of SA (w/ Soil Covering, DMSO; Method 1)
3.3. Post-Emergent Herbicidal Efficacy of SA (with a Soil Covering and DMSO; Method 2)
3.4. Post-Emergent Herbicidal Efficacy of SA (without a Soil Covering and with 60% Ethanol (v/v); Method 3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakroborty, S.; Pal, K.; Nath, N.; Singh, V.; Barik, A.; Soren, S.; Panda, P.; Asthana, N.; Kyzas, G.Z. Sustainable synthesis of multifunctional nanomaterials from rice wastes: A comprehensive review. Environ. Sci. Pollut. Res. 2023, 30, 95039–95053. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Sharma, S.; Kumar, U.; Sihag, P.; Balyan, P.; Singh, K.P.; Dhankher, O.P. Strategies for economic utilization of rice straw residues into value-added by-products and prevention of environmental pollution. Sci. Total Environ. 2024, 906, 167714. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, L.; Wang, K.; Renard, C.M.G.C.; Le Bourvellec, C.; Hu, Z.; Liu, X. Plant leaf proanthocyanidins: From agricultural production by-products to potential bioactive molecules. Crit. Rev. Food Sci. Nutr. 2023, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Almond Board of California. Emerging Markets and New Uses Generate Value for Hulls, Shells. Available online: https://www.almonds.com/almond-industry/industry-news/emerging-markets-and-new-uses-generate-value-hulls-shells (accessed on 20 September 2023).
- Almond Board of California. Whole Orchard Recycling. Available online: https://www.almonds.com/almond-industry/orchard-management/whole-orchard-recycling (accessed on 20 September 2021).
- Kim, J.H.; Chan, K.L. Natural salicylaldehyde for fungal and pre- and post-emergent weed control. Appl. Sci. 2022, 12, 3749. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Roufegarinejad, L.; Amarowicz, R. Recent advances in the use of walnut (Juglans regia L.) shell as a valuable plant-based bio-sorbent for the removal of hazardous materials. RSC Adv. 2020, 10, 7026–7047. [Google Scholar] [CrossRef] [PubMed]
- Tomishima, H.; Luo, K.; Mitchell, A.E. The almond (Prunus dulcis): Chemical properties, utilization, and valorization of coproducts. Annu. Rev. Food Sci. Technol. 2022, 13, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Mondragon, G.; Garrigós, M.C.; Eceiza, A.; Jiménez, A. Microwave-assisted extraction of cellulose nanocrystals from almond (Prunus amygdalus) shell waste. Front. Nutr. 2023, 9, 1071754. [Google Scholar] [CrossRef]
- Yang, L.; Yungang, W.; Tao, L.; Li, Z.; Yanyuan, B.; Haoran, X. High-performance sorbents from ionic liquid activated walnut shell carbon: An investigation of adsorption and regeneration. RSC Adv. 2023, 13, 22744–22757. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific opinion on the safety and efficacy of benzyl alcohols, aldehydes, acids, esters and acetals (chemical group 23) when used as flavourings for all animal species. EFSA J. 2012, 10, 2785. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Substances Added to Food (Formerly EAFUS). Salicylaldehyde. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=FoodSubstances&id=SALICYLALDEHYDE (accessed on 24 November 2023).
- Kim, J.H.; Campbell, B.C.; Mahoney, N.; Chan, K.L.; Molyneux, R.J. Chemosensitization of aflatoxigenic fungi to antimycin A and strobilurin using salicylaldehyde, a volatile natural compound targeting cellular antioxidation system. Mycopathologia 2011, 171, 291–298. [Google Scholar] [CrossRef]
- Kim, J.H.; Chan, K.; Faria, N.C.G.; Martins, M.D.L.; Campbell, B. Targeting the oxidative stress response system of fungi with redox-potent chemosensitizing agents. Front. Microbiol. 2012, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhou, C.; Xie, Y.; Wang, M.; Zhou, C.; Li, X.; Du, Y.; Lu, F. A new method for simultaneous determination of 14 phenolic acids in agricultural soils by multiwavelength HPLC-PDA analysis. RSC Adv. 2022, 12, 14939–14944. [Google Scholar] [CrossRef] [PubMed]
- Real, M.; Facenda, G.; Celis, R. Sorption and dissipation of the allelochemicals umbelliferone and salicylic acid in a Mediterranean soil environment: Effect of olive-mill waste addition. Sci. Total Environ. 2021, 774, 145027. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6998, Salicylaldehyde. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/salicylaldehyde (accessed on 20 September 2023).
- Environmental Protection Agency (EPA). Salicylaldehyde; Exemption from the Requirement of a Tolerance. 81 FR 17611. Available online: https://www.federalregister.gov/d/2016-07085 (accessed on 20 September 2023).
- Sinclair, T.R.; Chase, C.A.; Chellemi, D.O.; Fornari, F. Noxious Weed Control by Soil Solarization. U.S. Patent US-6,189,466-B1, 20 February 2001. [Google Scholar]
- Stapleton, J.J.; Wilen, C.A.; Molinar, R.H. Soil Solarization for Gardens & Landscapes; University of California, Agricultural and Natural Resources: Davis, CA, USA, 2019; Available online: http://ipm.ucanr.edu/PMG/PESTNOTES/pn74145.html (accessed on 21 September 2023).
- Environmental Protection Agency (EPA). Dimethyl Sulfoxide; Exemption from the Requirement of a Tolerance. 87 FR 61531. Available online: https://www.federalregister.gov/d/2022-22129 (accessed on 20 September 2023).
- Niemczak, M.; Stachowiak, W.; Kaczmarek, D.K.; Grzanka, M.; Sobiech, Ł. A comprehensive study demonstrating the influence of the solvent composition on the phytotoxicity of compounds, as exemplified by 2,4-D-based ILs with a choline-type cation. Pest Manag. Sci. 2023, 79, 3602–3610. [Google Scholar] [CrossRef]
- United Seeds, Inc. United Seeds Mixes & Blends. Available online: https://unitedseeds.com/ (accessed on 25 November 2023).
- Oregon State University. Tall Fescue. Available online: https://agsci.oregonstate.edu/beaverturf/tall-fescue (accessed on 25 November 2023).
- USDA, Agricultural Research Service, National Plant Germplasm System. Germplasm Resources Information Network (GRIN Taxonomy); National Germplasm Resources Laboratory: Beltsville, MD, USA, 2023. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=319636 (accessed on 21 November 2023).
- Kim, J.H.; Chan, K.L.; Mahoney, N.; Campbell, B.C. Antifungal activity of redox-active benzaldehydes that target cellular antioxidation. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 23. [Google Scholar] [CrossRef]
- Kirkman, T.W. Statistics to Use. Available online: http://www.physics.csbsju.edu/stats/ (accessed on 18 September 2023).
- Young-Matthews, A. Plant Guide for Field Mustard (Brassica Rapa var. Rapa); USDA-Natural Resources Conservation Service, Corvallis Plant Materials Center: Corvallis, OR, USA, 2012. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_brrar.pdf (accessed on 18 September 2023).
- California Invasive Plant Council. Brassica Rapa. Available online: https://www.cal-ipc.org/plants/profile/brassica-rapa-profile/ (accessed on 25 November 2023).
- Missouri Botanical Garden. Brassica Juncea. Available online: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=278099 (accessed on 25 November 2023).
- Shinde, Y.A.; Jagtap, M.P.; Patil, M.G.; Khatri, N. Experimental investigation on the effect of soil solarization incorporating black, silver, and transparent polythene, and straw as mulch, on the microbial population and weed growth. Chemosphere 2023, 336, 139263. [Google Scholar] [CrossRef]
- Briassoulis, D. Agricultural plastics as a potential threat to food security, health, and environment through soil pollution by microplastics: Problem definition. Sci. Total Environ. 2023, 892, 164533. [Google Scholar] [CrossRef]
- Khalid, A.R.; Shah, T.; Asad, M.; Ali, A.; Samee, E.; Adnan, F.; Bhatti, M.F.; Marhan, S.; Kammann, C.I.; Haider, G. Biochar alleviated the toxic effects of PVC microplastic in a soil-plant system by upregulating soil enzyme activities and microbial abundance. Environ. Pollut. 2023, 332, 121810. [Google Scholar] [CrossRef]
- Khan, A.; Jie, Z.; Wang, J.; Nepal, J.; Ullah, N.; Zhao, Z.-Y.; Wang, P.-Y.; Ahmad, W.; Khan, A.; Wang, W.; et al. Ecological risks of microplastics contamination with green solutions and future perspectives. Sci. Total Environ. 2023, 899, 165688. [Google Scholar] [CrossRef]
- Sa’adu, I.; Farsang, A. Plastic contamination in agricultural soils: A review. Environ. Sci. Eur. 2023, 35, 13. [Google Scholar] [CrossRef]
- Ndikuryayo, F.; Yang, W.-C. New insights into the interactions between herbicides: Trends from recent studies. J. Agric. Food Chem. 2023, 71, 10970–10981. [Google Scholar] [CrossRef] [PubMed]
- Hazra, D.K.; Mondal, P.; Purkait, A.; Mandal, S.; Bhattacharyya, S.; Karmakar, R.; Roy, S.; Banerjee, T.; Banerjee, H. Determination of quizalofop-p-ethyl in onion: Residual dissipation pattern, weed control efficiency, and food safety assessment under field conditions. Environ. Monit. Assess. 2023, 195, 1067. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.P.; Clay, S.A.; Swanton, C.J.; Anderson, J.V.; Chao, W.S. Weed-induced crop yield loss: A new paradigm and new challenges. Trends Plant Sci. 2023, 28, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Schouteten, J.J.; Degieter, M.; Krupanek, J.; Jarosz, W.; Areta, A.; Emmi, L.; De Steur, H.; Gellynck, X. European stakeholders’ perspectives on implementation potential of precision weed control: The case of autonomous vehicles with laser treatment. Precis. Agric. 2023, 24, 2200–2222. [Google Scholar] [CrossRef]
- Dong, F.; Li, Y.; Chen, X.; Wu, J.; Wang, S.; Zhang, X.; Ma, G.; Lee, Y.-W.; Mokoena, M.P.; Olaniran, A.O.; et al. Analysis of the Fusarium graminearum species complex from gramineous weeds near wheat fields in Jiangsu province, China. Plant Dis. 2021, 105, 3269–3275. [Google Scholar] [CrossRef]
- Janaviciene, S.; Venslovas, E.; Kadziene, G.; Matelioniene, N.; Berzina, Z.; Bartkevics, V.; Suproniene, S. Diversity of mycotoxins produced by Fusarium strains infecting weeds. Toxins 2023, 15, 420. [Google Scholar] [CrossRef]
- Reboud, X.; Eychenne, N.; Délos, M.; Folcher, L. Withdrawal of maize protection by herbicides and insecticides increases mycotoxins contamination near maximum thresholds. Agron. Sustain. Dev. 2016, 36, 43. [Google Scholar] [CrossRef]
- USDA National Agricultural Statistics Service (NASS). 2022 California Almond Acreage Report; USDA, NASS: Sacramento, CA, USA, 2022. Available online: https://www.nass.usda.gov/Statistics_by_State/California/Publications/Specialty_and_Other_Releases/Almond/Acreage/202304almac.pdf (accessed on 24 September 2023).
- Sosnoskie, L.M. On the Hunt for Alkaliweed (Cressa truxillensis). Available online: https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=29855 (accessed on 23 November 2023).
- Suszkiw, J. DDGs—Ethanol Byproduct Fights Weeds, Boosts Crop Yields; USDA, ARS: Washington, DC, USA, 2007; p. 18. Available online: https://agresearchmag.ars.usda.gov/2007/may/weeds/ (accessed on 24 September 2023).
- Haviland, D.R.; Baldwin, R.A.; Hembree, K.J.; Michailides, T.J.; Westerdahl, B.B.; Beede, R.H.; Daane, K.M.; Fukuda, T.A.; Kallsen, C.E.; Shrestha, A.; et al. UC IPM Pest Management Guidelines: Pistachio; Publication 3461; UC ANR: Davis, CA, USA; Available online: https://ipm.ucanr.edu/agriculture/pistachio/ (accessed on 24 September 2023).



| Methods | Plastic Tarps | Days of Treatments | Solvents | Results |
|---|---|---|---|---|
| Pre-emergent herbicidal efficacy | Two layers for SA treatment (0.8 and 1.6 M) | 7 days * 7 days of SA treatment | DMSO *** | Inhibition of seed germination |
| Post-emergent herbicidal efficacy (Method 1) | Two layers for SA treatment (0.8 and 1.6 M) | 14 days * 7 days of germination and 7 days of SA treatment | DMSO *** | Inhibition of seedling elongation |
| Post-emergent herbicidal efficacy (Method 2) | One layer for SA treatment (1.6 M) | 26 days * 21 days of germination and growth and 5 days of SA treatment | DMSO *** | Wilting of seedlings (death) |
| Post-emergent herbicidal efficacy (Method 3) | No plastic-covering (0.8 and 1.6 M) | 36 days ** 21 days of germination and growth and 3 to 15 days of SA treatment | Ethanol (60%, v/v) | Wilting of seedlings (death) |
| Fungi | Mycotoxins | Crops | Weeds | References |
|---|---|---|---|---|
| Fusarium graminearum and Fusarium asiaticum | 3-acetyldeoxynivalenol (3ADON), 15-acetyldeoxynivalenol (15ADON), and nivalenol (NIV) | Wheat | Gramineous weeds surrounding wheat fields | [40] |
| F. graminearum and Fusarium avenaceum | deoxynivalenol (DON), 3-acetyldeoxynivalenol (3ADON), 15-acetyldeoxynivalenol (15ADON), NIV, zearalenone (ZEA), neosolaniol (NEO), enniatin A (ENN A), enniatin A1 (ENN A1), enniatin B (ENN B), enniatin B1 (ENN B1), T-2 toxin (T-2), HT-2 toxin (HT-2), and moniliformin (MON) | Spring wheat | Weeds surrounding spring- wheat fields | [41] |
| F. graminearum, F. culmorum, F. tricinctum, etc. | Fumonisins, Trichothecenes, and NIV | Maize (seeds) | Major weeds (sixteen): Chenopodium album L., Convolvulus arvensis L., Echinochloa crus-galli (L.) P. Beauvois, etc. Less common and seldom found weeds (eighteen): Capsella bursa-pastoris (L.) Medicus, Sonchus arvensis L., Amaranthus retroflexus L., etc. | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Chan, K.L.; Hart-Cooper, W.M.; Ford, D.; Orcutt, K.; Palumbo, J.D.; Tam, C.C.; Orts, W.J. Valorizing Tree-Nutshell Particles as Delivery Vehicles for a Natural Herbicide. Methods Protoc. 2024, 7, 1. https://doi.org/10.3390/mps7010001
Kim JH, Chan KL, Hart-Cooper WM, Ford D, Orcutt K, Palumbo JD, Tam CC, Orts WJ. Valorizing Tree-Nutshell Particles as Delivery Vehicles for a Natural Herbicide. Methods and Protocols. 2024; 7(1):1. https://doi.org/10.3390/mps7010001
Chicago/Turabian StyleKim, Jong H., Kathleen L. Chan, William M. Hart-Cooper, DeAngela Ford, Kaydren Orcutt, Jeffrey D. Palumbo, Christina C. Tam, and William J. Orts. 2024. "Valorizing Tree-Nutshell Particles as Delivery Vehicles for a Natural Herbicide" Methods and Protocols 7, no. 1: 1. https://doi.org/10.3390/mps7010001






