Preliminary Evaluation of Moniliformin as a Potential Threat for Teleosts
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethics Statement on Animal Experiments
2.2. Experimental Model
2.3. Experimental Design
2.3.1. Assessment of the Effects on Operculum Mineralization in Zebrafish Larvae
2.3.2. Assessment of the Effects on the Incidence of Deformities in Zebrafish Larvae
2.3.3. Assessment of the Cytotoxic, Proliferative and Mineralogenic Effects In Vitro
2.3.4. RNA Extraction and Quantitative Real-Time PCR
2.4. Data Analysis
3. Results
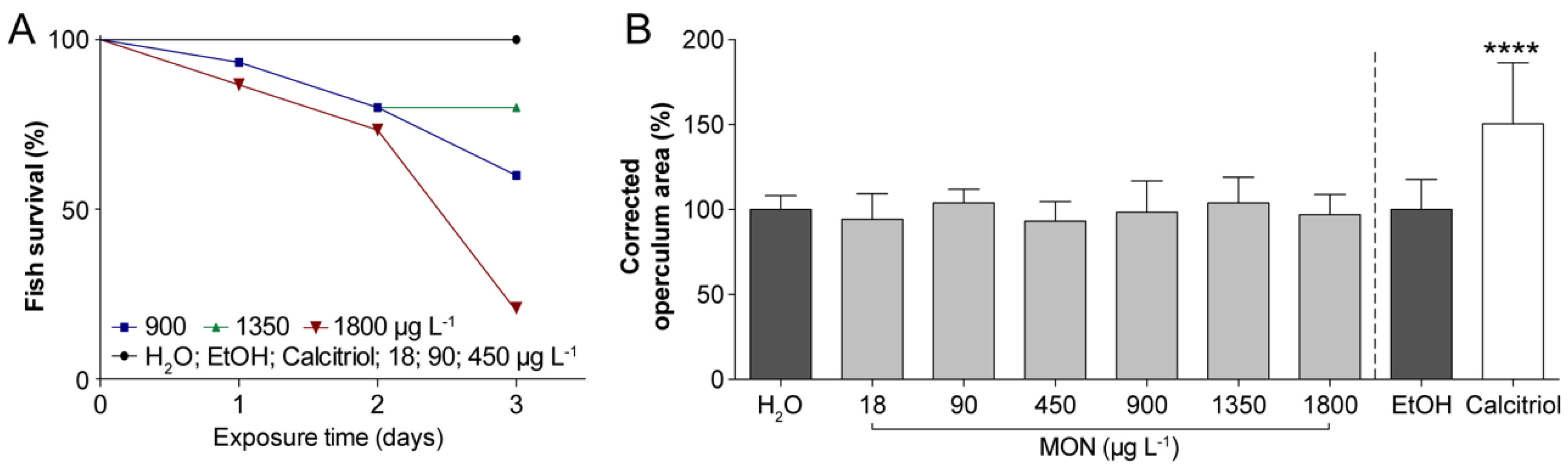
3.1. Assessment of the Effects on Operculum Mineralization in Zebrafish Larvae
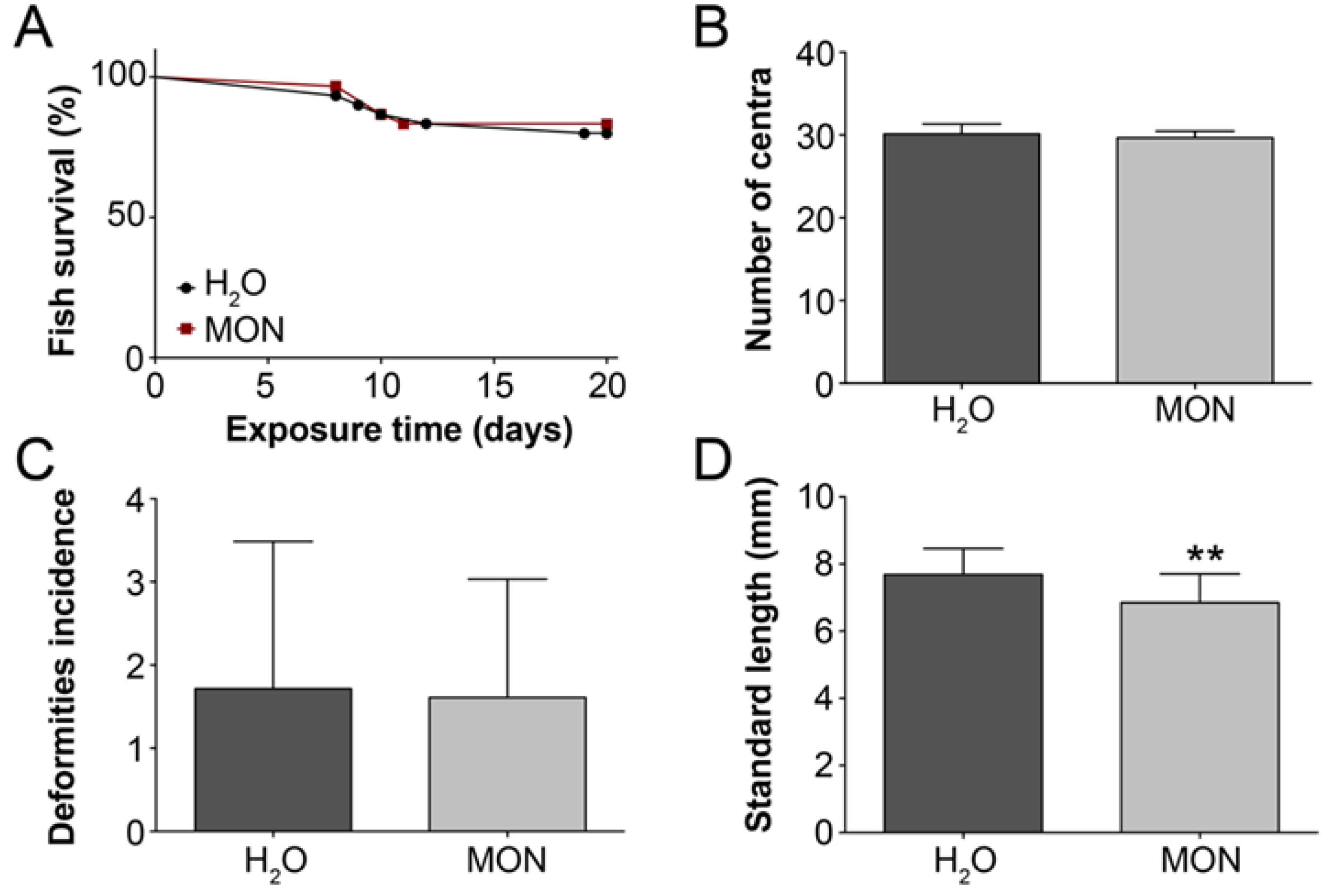
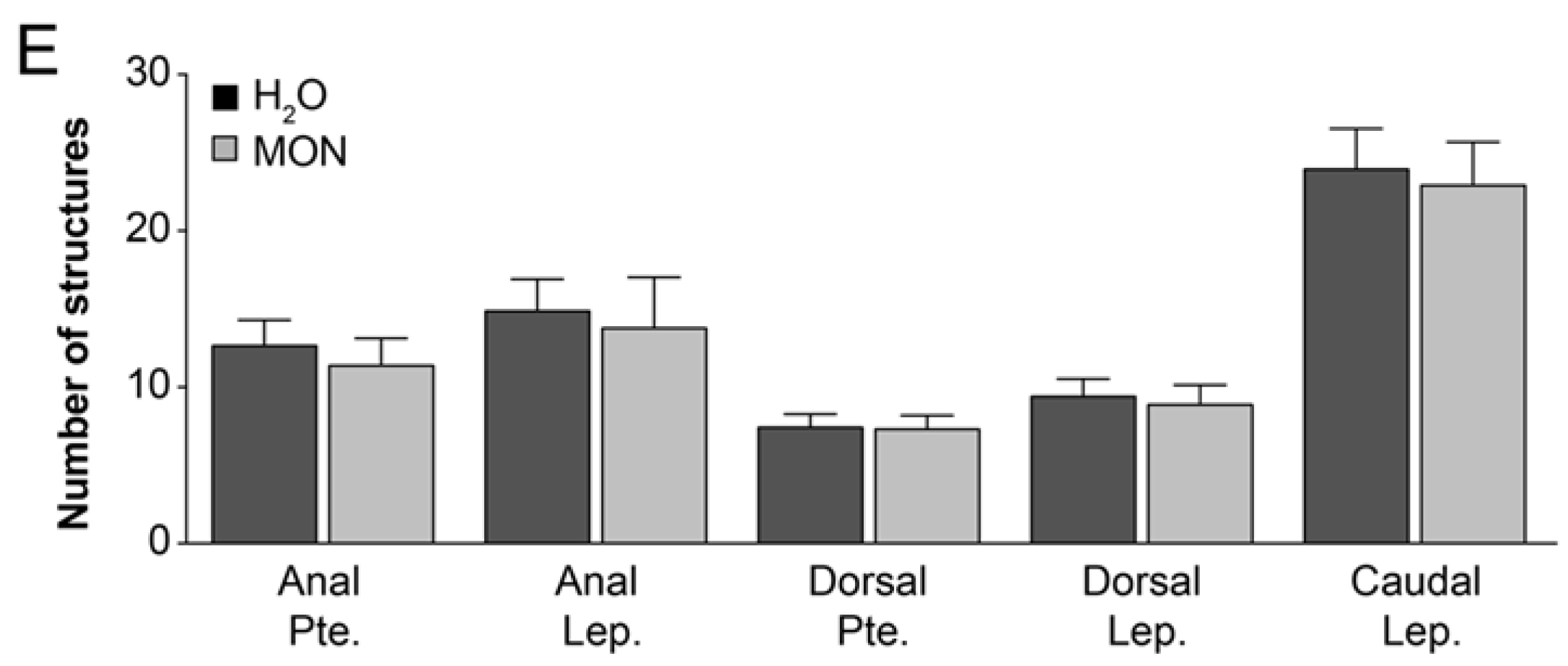
3.2. Assessment of the Effects on the Incidence of Deformities in Zebrafish Larvae
3.3. Assessment of the Cytotoxic, Proliferative and Mineralogenic Effects In Vitro
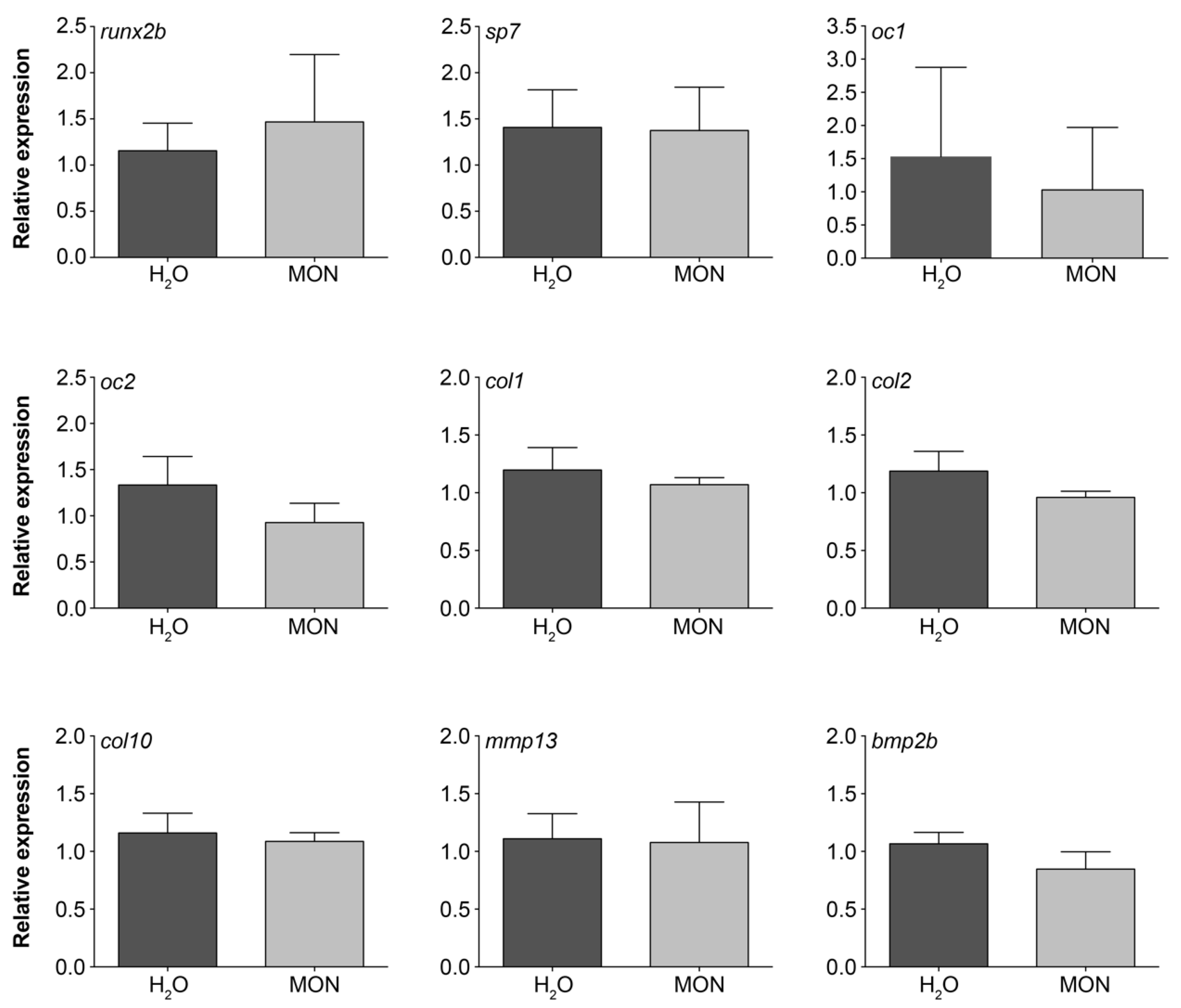
3.4. Assessment of the Effects on Gene Expression Levels in Zebrafish Larvae
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miraglia, M.; Marvin, H.J.P.; Kleter, G.A.; Battilani, P.; Brera, C.; Coni, E.; Cubadda, F.; Croci, L.; De Santis, B.; Dekkers, S.; et al. Climate change and food safety: An emerging issue with special focus on Europe. Food Chem. Toxicol. 2009, 47, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef] [Green Version]
- Paterson, R.R.M.; Lima, N. Further mycotoxin effects from climate change. Food Res. Int. 2011, 44, 2555–2566. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Sardjono; Yamashita, A.; Yoshizawa, T. Natural co-occurrence of aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol, nivalenol and zearalenone) in corn from Indonesia. Food Addit. Contam. Part A 1998, 15, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize—An extensive survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Krska, R. How does climate change impact on the occurrence and the determination of natural toxins. In Proceedings of the 7th International Symposium on Recent Advances in Food Analysis, Prague, Czech Republic, 3–6 November 2015. [Google Scholar]
- Anater, A.; Manyes, L.; Meca, G.; Ferrer, E.; Luciano, F.B.; Pimpão, C.T.; Font, G. Mycotoxins and their consequences in aquaculture: A review. Aquaculture 2016, 451, 1–10. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Naehrer, K.; Santos, G.A. Occurrence of mycotoxins in commercial aquafeeds in Asia and Europe: A real risk to aquaculture? Rev. Aquac. 2016. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Schatzmayr, D.; Hofstetter, U.; Santos, G.A. Occurrence of mycotoxins in aquaculture: Preliminary overview of Asian and European plant ingredients and finished feeds. World Mycotoxin J. 2017, 10, 183–194. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Goertz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.W.; Waalwijk, C.; de Vries, I.; Oerke, E.C. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111. [Google Scholar] [CrossRef]
- Allen, N.K.; Burmeister, H.R.; Weaver, G.A.; Mirocha, C.J. Toxicity of dietary and intravenously administered moniliformin to broiler chickens. Poult. Sci. 1981, 60, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Mirocha, C.J.; Vesonder, R.F.; Gunther, R. Acute toxic effects of an isolate of moniliformin-producing Fusarium oxysporum and purified moniliformin on rats. Arch. Environ. Contam. Toxicol. 1990, 19, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, J.A.; Carlton, W.W.; Tuite, J.F. Toxicity of Fusarium moniliforme var. subglutinans for chicks, ducklings and Turkey poults. Avian Dis. 1989, 33, 357–360. [Google Scholar] [PubMed]
- Yildirim, M.; Manning, R.; Lovell, J.; Grizzle, R. Toxicity of moniliformin and fumonisin B1 fed singly and in combination in diets for channel catfish Ictalurus punctatus. J. World Aquac. Soc. 2000, 31, 599–608. [Google Scholar] [CrossRef]
- Tuan, N.A.; Manning, B.B.; Lovell, R.T.; Rottinghaus, G.E. Responses of nile tilapia (Oreochromis niloticus) fed diets containing different concentrations of moniliformin or fumonisin B1. Aquaculture 2003, 217, 515–528. [Google Scholar] [CrossRef]
- Zhang, A.; Cao, J.-L.; Yang, B.; Chen, J.-H.; Zhang, Z.-T.; Li, S.-Y.; Fu, Q.; Hugnes, C.E.; Caterson, B. Effects of moniliformin and selenium on human articular cartilage metabolism and their potential relationships to the pathogenesis of Kashin-Beck disease. J. Zhejiang Univ. Sci. B 2010, 11, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, S.; Shi, Z.; Yue, Y.; Sun, J.; Chen, J.; Fu, Q.; Hughes, C.E.; Caterson, B. Articular cartilage metabolism in patients with Kashin-Beck Disease: An endemic osteoarthropathy in China. Osteoarthr. Cartil. 2015, 16, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Boglione, C.; Gisbert, E.; Gavaia, P.; Witten, P.E.; Moren, M.; Fontagné, S.; Koumoundouros, G. Skeletal anomalies in reared European fish larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Rev. Aquac. 2013, 5, S121–S167. [Google Scholar] [CrossRef]
- Subasinghe, R.P.; Arthur, J.R.; Shariff, M. Health Management in Asian Aquaculture: Proceedings of the Regional Expert Consultation on Aquaculture Health Management in Asia and the Pacific, Serdang, Malaysia, 22–24 May 1995; FAO Fisheries Technical Paper. No. 360; FAO: Rome, Italy, 1996; 142p, ISBN 92-5-103917-8. [Google Scholar]
- Tarasco, M.; Laizé, V.; Cardeira, J.; Cancela, M.L.; Gavaia, P.J. The zebrafish operculum: A powerful system to assess osteogenic bioactivities of molecules with pharmacological and toxicological relevance. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 197, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gavaia, P.J.; Sarasquete, C.; Cancela, M.L. Detection of mineralized structures in early stages of development of marine teleostei using a modified alcian blue-alizarin red double staining technique for bone and cartilage. Biotech. Histochem. 2000, 75, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, G.; Campos, C.; Valente, L.M.P.; Conceição, L.E.C.; Cancela, M.L.; Gavaia, P.J. Effect of egg incubation temperature on the occurrence of skeletal deformities in solea senegalensis. J. Appl. Ichthyol. 2012, 28, 471–476. [Google Scholar] [CrossRef]
- Pombinho, A.R.; Laizé, V.; Molha, D.M.; Marques, S.M.P.; Cancela, M.L. Development of two bone-derived cell lines from the marine teleost Sparus aurata; evidence for extracellular matrix mineralization and cell-type-specific expression of matrix Gla protein and osteocalcin. Cell Tissue Res. 2004, 315, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Valdes, G.; Bullerman, L.B. Thermal stability of moniliformin at varying temperature, pH, and time in an aqueous environment. J. Food Prot. 2000, 63, 1598–1601. [Google Scholar] [CrossRef] [PubMed]
- Hilgenfeld, R.; Saenger, W. Structural chemistry of natural and synthetic ionophores and their complexes with cations. In Topics in Current Chemistry; Boschke, F.L., Ed.; Springer: Berlin, Germany, 1982; Volume 101, pp. 1–82. [Google Scholar]

| Genes | Sequence (5′-3′) | NCBI Accession # | |
|---|---|---|---|
| runx2b | Fw: | TCAGGAATGCCTCAGGGGTTATG | NM_212862 |
| Rev: | CTTGCGGTGGGTTTGTGAATACT | ||
| sp7 | Fw: | GCTAAGTCCAGGGCAGGCTCAG | NM_212863 |
| Rev: | CAATGGCGTGAAATCAGGAGTGTAAC | ||
| bglap | Fw: | TTTATAGGCGGCGATGATTCC | NM_001083857 |
| Rev: | GAAGCGAACATGAAGAGTCTGACAGTCC | ||
| bglapl | Fw: | CCAACTCCGCATCAGACTCCGCATCA | NM_001291889 |
| Rev: | AGCAACACTCCGCTTCAGCAGCACAT | ||
| col1a1a | Fw: | GCTTCATTGCCCAGCCACAGGA | NM_199214 |
| Rev: | GCAGGGTTCTTCTTGGTGCCGTCT | ||
| col10a1a | Fw: | CAGGAAGAGTTTGGCGGCTGT | NM_131292 |
| Rev: | GACACGGCACGGTTCTGGTT | ||
| col10a1a | Fw: | ATACCCAGTTCTTGTCAAAAGTCCA | NM_001083827 |
| Rev: | GATCATAATGCTGCTCTGCGTT | ||
| mmp13 | Fw: | TGTCAGTGGCAGAGGTGGATGACTC | NM_001290479 |
| Rev: | CGCCACCAGGAACAGATTGTAATATTGAG | ||
| bmp2b | Fw: | GAGGAACTTAGGAGACGACGGGAACGC | NM_131360 |
| Rev: | TCTCGGGAATGAGTCCAACGGCAC | ||
| eef1a1l1 | Fw: | TTGAGAAGAAAATCGGTGGTGCTG | NM_131263 |
| Rev: | GGAACGGTGTGATTGAGGGAAATTC | ||
| actb1 | Fw: | GATGCGGAAACTGGCAAAGG | NM_131031 |
| Rev: | GAGGAGGGCAAAGTGGTAAACG | ||
| rps18 | Fw: | AACACGAACATTGATGGAAGACG | NM_173234 |
| Rev: | ATTAGCAAGGACCTGGCTGTATTT | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, R.A.; Tarasco, M.; Schatzmayr, D.; Gavaia, P. Preliminary Evaluation of Moniliformin as a Potential Threat for Teleosts. Fishes 2018, 3, 4. https://doi.org/10.3390/fishes3010004
Gonçalves RA, Tarasco M, Schatzmayr D, Gavaia P. Preliminary Evaluation of Moniliformin as a Potential Threat for Teleosts. Fishes. 2018; 3(1):4. https://doi.org/10.3390/fishes3010004
Chicago/Turabian StyleGonçalves, Rui A., Marco Tarasco, Dian Schatzmayr, and Paulo Gavaia. 2018. "Preliminary Evaluation of Moniliformin as a Potential Threat for Teleosts" Fishes 3, no. 1: 4. https://doi.org/10.3390/fishes3010004
APA StyleGonçalves, R. A., Tarasco, M., Schatzmayr, D., & Gavaia, P. (2018). Preliminary Evaluation of Moniliformin as a Potential Threat for Teleosts. Fishes, 3(1), 4. https://doi.org/10.3390/fishes3010004






