Characterization of the Flavor Profile of Bigeye Tuna Slices Treated by Cold Plasma Using E-Nose and GC-IMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Cold Plasma Treatment
2.3. Sensory Ananlysis
2.4. Electronic Nose Analysis
2.5. Volatile Components Analysis by GC-IMS
2.6. Data Processing
3. Results and Discussion
3.1. Sensory Evaluation
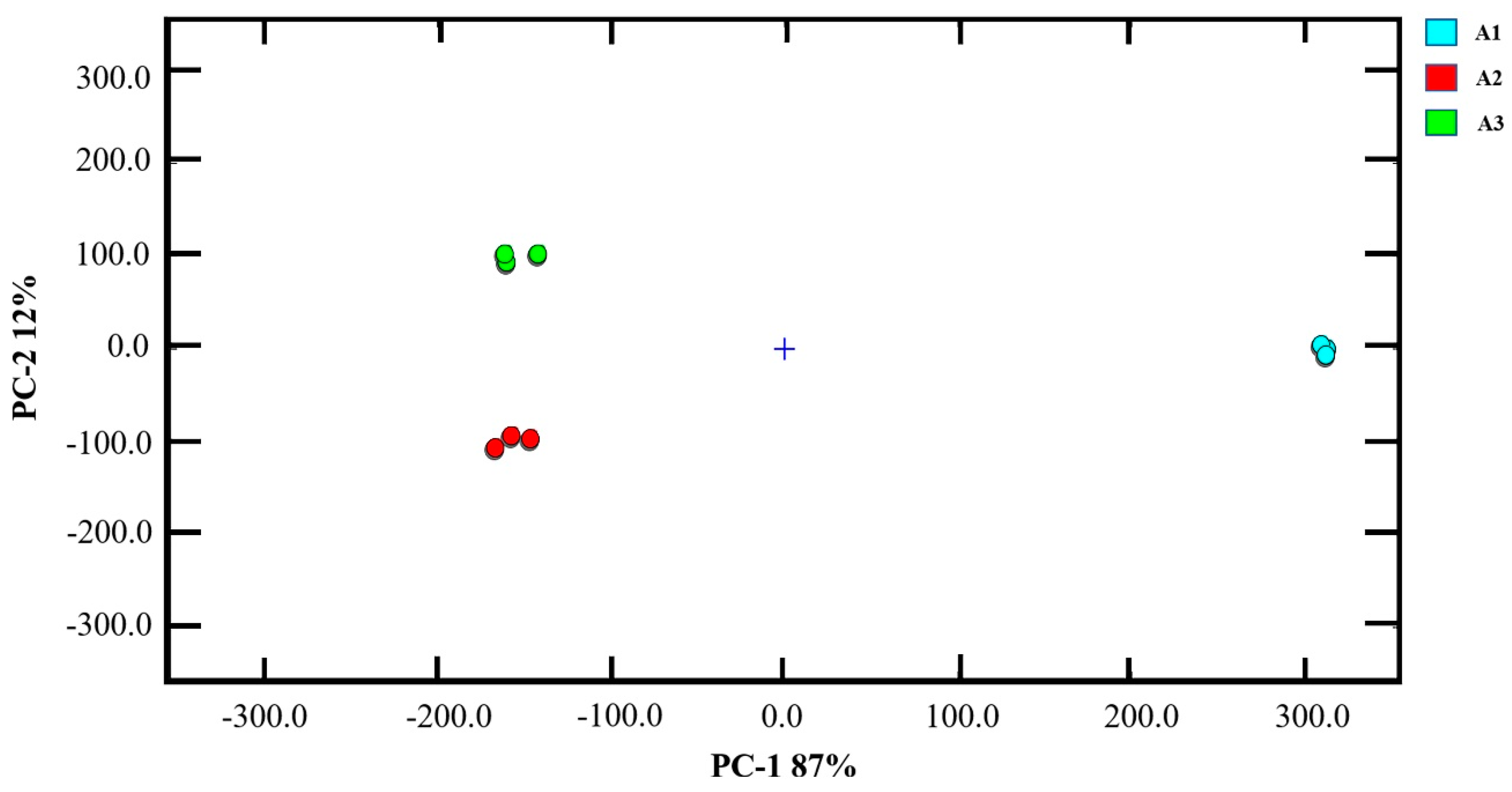
3.2. PCA Analysis of E-Nose
3.3. Data Analysis of GC-IMS
3.3.1. PCA Analysis of GC-IMS
3.3.2. Volatile Compound Identification in Tuna Sashimi of Different Treatment
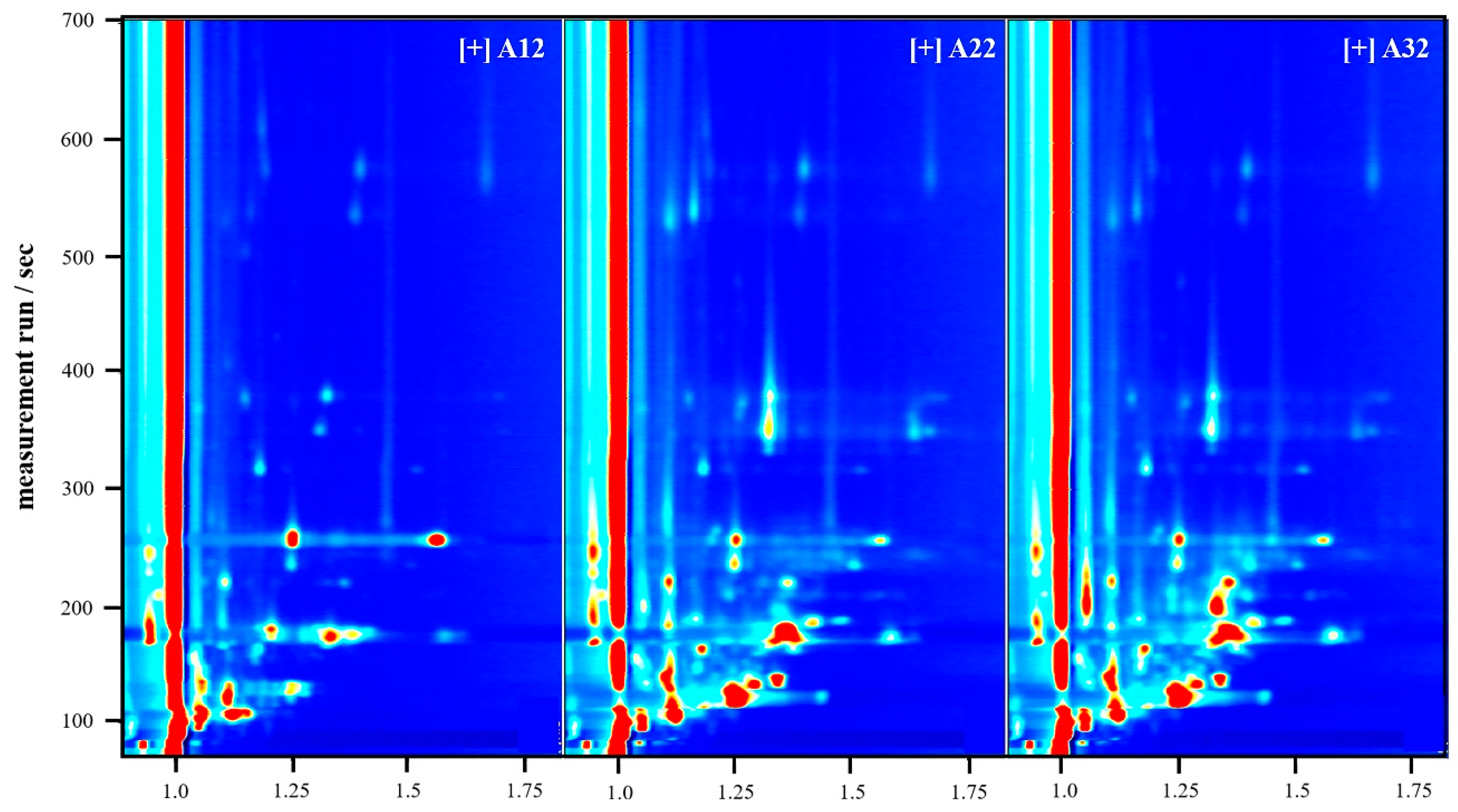
3.3.3. Comparison of Fingerprints of Volatile Compounds
3.3.4. Qualitative Analysis of Volatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, L.; Lu, J.P.; Qu, M.; Jiang, Y.H.; Li, F.L.; Guo, Y.Y.; Wang, L.Z.; Zhai, Y.X. Methodology and application of PCR-RFLP for species identification in tuna sashimi. Food Sci. Nutr. 2020, 8, 3138–3146. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Xie, J. Evaluation of water dynamics and protein changes in bigeye tuna (Thunnus obesus) during cold storage. LWT-Food Sci. Technol. 2019, 108, 289–296. [Google Scholar] [CrossRef]
- Edirisinghe, R.K.B.; Graham, A.J.; Taylor, S.J. Characterisation of the volatiles of yellowfin tuna (Thunnus albacares) during storage by solid phase microextraction and GC-MS and their relationship to fish quality parameters. Int. J. Food Sci. Technol. 2007, 42, 1139–1147. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Zhou, P.; Zhang, B.; Deng, S.G. Effect of squid pen chitooligosaccharide and epigallocatechin gallate on discoloration and shelf-life of yellowfin tuna slices during refrigerated storage. Food Chem. 2021, 351, 129296. [Google Scholar] [CrossRef] [PubMed]
- Mentana, A.; Conte, A.; Del Nobile, M.A.; Quinto, M.; Centonze, D. Investigating the effects of mild preservation technology on perishable foods by volatolomics: The case study of ready-to-cook tuna-burgers. LWT-Food Sci. Technol. 2019, 115, 108425. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, S.W.; Li, S.J.; Liu, Y. Evaluation of the preservation effect of gelatin-water soluble chitosan film incorporated with maillard peptides on bluefin tuna (Thunnus thynnus) slices packaging. LWT-Food Sci. Technol. 2019, 113, 108294. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xie, J.; Chen, X.J. Differences in lipid composition of Bigeye tuna (Thunnus obesus) during storage at 0 °C and 4 °C. Food Res. Int. 2021, 143, 110233. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, I.; Lopez-Caballero, M.E.; Gomez-Guillen, M.C.; Mauri, A.N.; Montero, M.P. Active nanocomposite films based on soy proteins-montmorillonite- clove essential oil for the preservation of refrigerated bluefin tuna (Thunnus thynnus) fillets. Int. J. Food Microbiol. 2018, 266, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Perez-Andres, J.M.; de Alba, M.; Harrison, S.M.; Brunton, N.P.; Cullen, P.J.; Tiwari, B.K. Effects of cold atmospheric plasma on mackerel lipid and protein oxidation during storage. LWT-Food Sci. Technol. 2020, 118, 108697. [Google Scholar] [CrossRef]
- Koddy, J.K.; Miao, W.H.; Hatab, S.; Tang, L.L.; Xu, H.Q.; Nyaisaba, B.M.; Chen, M.L.; Deng, S.G. Understanding the role of atmospheric cold plasma (ACP) in maintaining the quality of hairtail (Trichiurus lepturus). Food Chem. 2021, 343, 128418. [Google Scholar] [CrossRef]
- Barbhuiya, R.I.; Singha, P.; Singh, S.K. A comprehensive review on impact of non-thermal processing on the structural changes of food components. Food Res. Int. 2021, 149, 110647. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.Y.; Su, Y.; Liu, D.H.; Chen, S.G.; Hu, Y.Q.; Ye, X.Q.; Wang, J.; Ding, T. Application of atmospheric cold plasma-activated water (PAW) ice for preservation of shrimps (Metapenaeus ensis). Food Control 2018, 94, 307–314. [Google Scholar] [CrossRef]
- Sruthi, N.U.; Josna, K.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Khaneghah, A.M. Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: A comprehensive review. Food Chem. 2022, 368, 130809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Wei, J.P.; Yuan, Y.H.; Chen, H.; Dai, L.; Wang, X.; Yue, T.L. Bactericidal effect of cold plasma on microbiota of commercial fish balls. Innov. Food Sci. Emerg. 2019, 52, 394–405. [Google Scholar] [CrossRef]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli in liquid media inside a sealed package. J. Appl. Microbiol. 2013, 114, 778–787. [Google Scholar] [CrossRef]
- Nyaisaba, B.M.; Miao, W.H.; Hatab, S.; Siloam, A.; Chen, M.L.; Deng, S.G. Effects of cold atmospheric plasma on squid proteases and gel properties of protein concentrate from squid (Argentinus ilex) mantle. Food Chem. 2019, 291, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, W.; Wang, Z.H.; Wang, J.; Huo, Y.J. Rapid detection of Aspergillus spp. infection levels on milled rice by headspace-gas chromatography ion-mobility spectrometry (HS-GC-IMS) and E-nose. LWT-Food Sci. Technol. 2020, 132, 109758. [Google Scholar] [CrossRef]
- Wang, S.Q.; Chen, H.T.; Sun, B.G. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Li, M.Q.; Du, H.T.; Lin, S.Y. Flavor Changes of Tricholoma matsutake Singer under Different Processing Conditions by Using HS-GC-IMS. Foods 2021, 10, 531. [Google Scholar] [CrossRef]
- Lv, W.S.; Lin, T.; Ren, Z.Y.; Jiang, Y.Q.; Zhang, J.; Bi, F.J.; Gu, L.H.; Hou, H.C.; He, J.N. Rapid discrimination of Citrus reticulata ‘Chachi’ by headspace-gas chromatography-ion mobility spectrometry fingerprints combined with principal component analysis. Food Res. Int. 2020, 131, 108985. [Google Scholar] [CrossRef]
- Liu, D.Y.; Bai, L.; Feng, X.; Chen, Y.P.; Zhang, D.N.; Yao, W.S.; Zhang, H.; Chen, G.L.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Y.Q.; Wang, H.B.; Xi, B.; He, X.N.; Yang, X.L.; Li, W.H. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, X.Y.; Ma, Y.; Wang, Y.B.; Wang, D. Fingerprints and changes analysis of volatile compounds in fresh-cut yam during yellowing process by using HS-GC-IMS. Food Chem. 2022, 369, 130939. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Martin-Gomez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace—Gas chromatography-ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Misra, N.N.; Cullen, P.J. Kinetics of tomato peroxidase inactivation by atmospheric pressure cold plasma based on dielectric barrier discharge. Innov. Food Sci. Emerg. 2013, 19, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Wen, R.X.; Kong, B.H.; Yin, X.Y.; Zhang, H.; Chen, Q. Characterisation of flavour profile of beef jerky inoculated with different autochthonous lactic acid bacteria using electronic nose and gas chromatography-ion mobility spectrometry. Meat Sci. 2022, 183, 108658. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, Y.C.; Gu, S.Q.; Zhu, S.C.; Zhou, X.X.; Ding, Y.T. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Res. Int. 2020, 137, 109339. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.Z.; Chen, J.Y.; Chen, D.Z.; Deng, S.G.; Xu, B. Effect of cold plasma on maintaining the quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. J. Sci. Food Agric. 2019, 99, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chutia, H.; Mahanta, C.L.; Ojah, N.; Choudhury, A.J. Fuzzy logic approach for optimization of blended beverage of cold plasma treated TCW and orange juice. J. Food Meas. Charact. 2020, 14, 1926–1938. [Google Scholar] [CrossRef]
- Chen, X.A.; Chen, H.Q.; Xiao, J.; Liu, J.Y.; Tang, N.; Zhou, A.M. Variations of volatile flavour compounds in finger citron (Citrus medico L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef] [PubMed]
- Guney, S.; Atasoy, A. Study of fish species discrimination via electronic nose. Comput. Electron. Agric. 2015, 119, 83–91. [Google Scholar] [CrossRef]
- Wu, Z.B.; Chen, L.Z.; Wu, L.M.; Xue, X.F.; Zhao, J.; Li, Y.; Ye, Z.H.; Lin, G.H. Classification of Chinese Honeys According to Their Floral Origins Using Elemental and Stable Isotopic Compositions. J. Agric. Food Chem. 2015, 63, 5388–5394. [Google Scholar] [CrossRef]
- Li, M.Q.; Yang, R.W.; Zhang, H.; Wang, S.L.; Chen, D.; Lin, S.Y. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Ma, X.T.; Dai, Z.Y. Comparison of nonvolatile and volatile compounds in raw, cooked, and canned yellowfin tuna (Thunnus albacores). J. Food Process. Preserv. 2019, 43, 14111. [Google Scholar] [CrossRef]
- Lantsuzskaya, E.V.; Krisilov, A.V.; Levina, A.M. Structure of the cluster ions of ketones in the gas phase according to ion mobility spectrometry and ab initio calculations. Russ. J. Phys. Chem. A 2015, 89, 1838–1842. [Google Scholar] [CrossRef]
- Ma, W.B.; Zhao, L.L.; Zhao, W.H.; Xie, Y.L. (E)-2-Hexenal, as a Potential Natural Antifungal Compound, Inhibits Aspergillus flavus Spore Germination by Disrupting Mitochondria! Energy Metabolism. J. Agric. Food Chem. 2019, 67, 1138–1145. [Google Scholar] [CrossRef]
- Mansur, M.A.; Bhadra, A.; Takamura, H.; Matoba, T. Volatile flavor compounds of some sea fish and prawn species. Fish. Sci. 2003, 69, 864–866. [Google Scholar] [CrossRef] [Green Version]



| Sensors | Performance Description |
|---|---|
| W1C | Sensitive to aromatic compounds |
| W3C | Sensitive to ammonia and aromatic compounds |
| W5C | Sensitive to short chain alkanes and aromatic compounds |
| W1W | Sensitive to sulfides, pyrazine and many terpenes |
| W2W | Sensitive to organic sulfides and aromatic compounds |
| W5S | Sensitive to nitrogen oxides |
| W6S | Sensitive to hydrides |
| W1S | Sensitive to methyl |
| W2S | Sensitive to alcohols, aldehydes, and ketones |
| W3S | Sensitive to long chain alkanes |
| Gas Phase-Ion Mobility Spectrometry Unit | |
|---|---|
| Analysis time | 30 min |
| Column type | MXT-5, 15 m ID: 0.53 mm |
| Column temperature | 60 °C |
| Carrier gas/drift gas | N2 |
| IMS temperature | 45 °C |
| Automatic headspace sampling unit | |
| Injection volume | 500 µL |
| Incubation time | 20 min |
| Incubation temperature | 40 °C |
| Syringe temperature | 85 °C |
| Incubation speed | 500 rpm |
| Volatiles | NO. | Compounds | Molecule Formula | MW | RI | RT | DT | A1 | A2 | A3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | 1 | Octanal | C8H16O | 128.2 | 1008.5 | 574.9 | 1.40 | 242.30 ± 9.37 a | 292.57 ± 9.66 b | 262.32 ± 9.99 a |
| 2 | n-Nonanal | C9H18O | 142.2 | 1105.9 | 769.1 | 1.47 | 98.43 ± 6.63 a | 92.84 ± 15.21 a | 115.20 ± 13.69 a | |
| 3 | Heptanal | C7H14O | 114.2 | 898.6 | 379.3 | 1.33 | 381.89 ± 9.34 a | 853.53 ± 17.90 c | 668.30 ± 33.38 b | |
| 4 | (E)-2-Hexenal (M) | C6H10O | 98.1 | 848.1 | 319.6 | 1.18 | 478.76 ± 10.22 a | 526.12 ± 54.10 ab | 612.68 ± 28.24 b | |
| 5 | (E)-2-Hexenal (D) | C6H10O | 98.1 | 846.1 | 317.4 | 1.52 | 44.69 ± 1.90 a | 62.09 ± 11.52 ab | 82.38 ± 9.54 b | |
| 6 | Hexanal (M) | C6H12O | 100.2 | 790.7 | 256.5 | 1.26 | 2185.48 ± 10.93 b | 1341.92 ± 30.33 a | 1366.16 ± 33.54 a | |
| 7 | Hexanal (D) | C6H12O | 100.2 | 791.2 | 257.0 | 1.57 | 2418.50 ± 38.18 b | 710.15 ± 52.44 a | 770.79 ± 37.70 a | |
| 8 | Butanal | C4H8O | 72.1 | 593.9 | 133.2 | 1.29 | 173.40 ± 2.08 a | 1090.53 ± 2.44 c | 1021.82 ± 36.86 b | |
| 9 | Acetone | C3H6O | 58.1 | 526.8 | 104.4 | 1.12 | 2109.28 ± 7.14 a | 3469.11 ± 24.91 c | 2940.57 ± 97.37 b | |
| Alcohols | 1 | 1-Octen-3-Ol | C8H16O | 128.2 | 989.1 | 538.0 | 1.17 | 175.64 ± 2.38 a | 588.33 ± 19.68 c | 373.22 ± 13.03 b |
| 2 | 1-Heptanol | C7H16O | 116.2 | 987.9 | 535.9 | 1.39 | 229.51 ± 9.39 b | 213.90 ± 20.29 b | 161.50 ± 9.10 a | |
| 3 | 1-Hexanol (M) | C6H14O | 102.2 | 878.3 | 352.8 | 1.32 | 341.37 ± 10.93 a | 1950.79 ± 2.79 c | 1377.23 ± 83.74 b | |
| 4 | 1-Hexanol (D) | C6H14O | 102.2 | 875.4 | 349.6 | 1.64 | 55.05 ± 5.04 a | 363.08 ± 3.30 c | 205.43 ± 19.24 b | |
| 5 | 1-Pentanol (M) | C5H12O | 88.1 | 769.1 | 237.4 | 1.25 | 376.08 ± 9.65 a | 950.04 ± 8.02 c | 741.16 ± 12.24 b | |
| 6 | 1-Pentanol (D) | C5H12O | 88.1 | 767.1 | 235.9 | 1.52 | 38.37 ± 0.57 a | 226.75 ± 4.15 c | 173.86 ± 7.76 b | |
| 7 | (E)-2-Pentenal (M) | C5H8O | 84.1 | 748.7 | 221.2 | 1.11 | 625.63 ± 24.94 a | 1092.38 ± 55.14 c | 922.54 ± 38.29 b | |
| 8 | (E)-2-Pentenal (D) | C5H8O | 84.1 | 747.3 | 220.2 | 1.37 | 153.78 ± 17.48 a | 643.34 ± 102.61 b | 1175.35 ± 30.79 c | |
| 9 | 1-Butanol | C4H10O | 74.1 | 663.2 | 162.9 | 1.18 | 392.71 ± 3.27 a | 746.14 ± 16.35 b | 872.89 ± 12.33 c | |
| 10 | 1-Propanol | C3H8O | 60.1 | 559.0 | 118.2 | 1.25 | 202.33 ± 3.99 a | 4201.47 ± 68.91 b | 4323.25 ± 88.08 b | |
| 11 | 2-Methyl-1-Propanol | C4H10O | 74.1 | 630.0 | 148.6 | 1.17 | 58.16 ± 7.29 a | 146.24 ± 12.57 b | 321.42 ± 21.65 c | |
| 12 | Ethanol | C2H6O | 46.1 | 503.8 | 94.6 | 1.05 | 654.27 ± 31.83 a | 1059.45 ± 33.20 b | 1080.72 ± 23.32 b | |
| Ketones | 1 | 2-Heptanone | C7H14O | 114.2 | 896.1 | 374.9 | 1.27 | 95.28 ± 5.16 a | 192.99 ± 7.40 b | 197.72 ± 3.29 b |
| 2 | 3-Pentanone (M) | C5H10O | 86.1 | 698.1 | 181.2 | 1.11 | 301.77 ± 7.06 a | 718.66 ± 33.71 c | 567.94 ± 62.88 b | |
| 3 | 3-Pentanone (D) | C5H10O | 86.1 | 700.4 | 183.1 | 1.36 | 1387.22 ± 11.34 a | 6597.84 ± 198.96 c | 4507.08 ± 91.28 b | |
| 4 | Propanal | C3H6O | 58.1 | 534.2 | 107.6 | 1.15 | 695.84 ± 24.76 b | 236.59 ± 14.24 a | 222.34 ± 2.95 a | |
| 5 | Ethyl Acetate (M) | C4H8O2 | 88.1 | 609.4 | 139.8 | 1.11 | 343.31 ± 21.21 a | 1669.06 ± 10.68 b | 1680.67 ± 17.23 b | |
| 6 | Ethyl Acetate (D) | C4H8O2 | 88.1 | 604.1 | 137.5 | 1.35 | 65.06 ± 9.10 a | 2034.90 ± 86.56 c | 1571.02 ± 77.22 b | |
| 7 | 2-Butanone | C4H8O | 72.1 | 582.8 | 128.4 | 1.25 | 1150.52 ± 14.91 a | 3720.99 ± 44.79 b | 3876.60 ± 34.83 c | |
| Esters | 1 | Ethyl Butanoate | C6H12O2 | 116.2 | 791.9 | 257.8 | 1.20 | 69.93 ± 4.93 a | 96.76 ± 1.55 c | 86.19 ± 4.67 b |
| 2 | Propyl Acetate (M) | C5H10O2 | 102.1 | 707.0 | 188.3 | 1.16 | 94.74 ± 2.61 a | 345.18 ± 32.58 b | 341.80 ± 18.35 b | |
| 3 | Propyl Acetate (D) | C5H10O2 | 102.1 | 706.7 | 188.1 | 1.48 | 45.87 ± 4.33 a | 280.11 ± 41.26 b | 339.25 ± 19.17 b | |
| 4 | Acetoin (M) | C4H8O2 | 88.1 | 721.5 | 199.7 | 1.05 | 690.26 ± 11.80 a | 1133.95 ± 191.34 b | 3322.60 ± 74.83 c | |
| 5 | Acetoin (D) | C4H8O2 | 88.1 | 723.8 | 201.6 | 1.33 | 91.65 ± 3.73 a | 511.60 ± 153.36 b | 2553.62 ± 100.37 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, W.; Benjakul, S.; Sanmartin, C.; Guidi, A.; Ying, X.; Ma, L.; Weng, X.; Yu, J.; Deng, S. Characterization of the Flavor Profile of Bigeye Tuna Slices Treated by Cold Plasma Using E-Nose and GC-IMS. Fishes 2022, 7, 13. https://doi.org/10.3390/fishes7010013
Pan W, Benjakul S, Sanmartin C, Guidi A, Ying X, Ma L, Weng X, Yu J, Deng S. Characterization of the Flavor Profile of Bigeye Tuna Slices Treated by Cold Plasma Using E-Nose and GC-IMS. Fishes. 2022; 7(1):13. https://doi.org/10.3390/fishes7010013
Chicago/Turabian StylePan, Weicong, Soottawat Benjakul, Chiara Sanmartin, Alessandra Guidi, Xiaoguo Ying, Lukai Ma, Xudong Weng, Jin Yu, and Shanggui Deng. 2022. "Characterization of the Flavor Profile of Bigeye Tuna Slices Treated by Cold Plasma Using E-Nose and GC-IMS" Fishes 7, no. 1: 13. https://doi.org/10.3390/fishes7010013






