A Systematic Review of the Behavioural Changes and Physiological Adjustments of Elasmobranchs and Teleost’s to Ocean Acidification with a Focus on Sharks
Abstract
:1. Introduction
2. Methods
- “CO2” OR “carbon” AND “dioxide” OR “ocean” AND “acidification”; and
- “shark” OR “sharks” OR “elasmobranch” OR “elasmobranches” OR “cartilaginous”; and
- “behaviour” OR “behavior” OR “physiology” OR “physiological” OR “metabolism” OR “metabolic” OR “vitality” OR “vital” OR “survive” OR “survival”.
- Digital object identifier (DOI).
- Paper ID (surname and year) as given in the article itself (e.g., [13]).
- Year of publication.
- Type of study (e.g., an experiment, a review).
- Number of individuals included in the study (if available).
- Information on the species included in the papers: scientific and common names (e.g., spiny chromis, Acanthochromis polyacanthus), type of species (based on taxonomic details of the study and the ecology of the organism, e.g., bony fish, elasmobranch), life stage (e.g., eggs, larvae, adults), life strategy (e.g., tropical, benthic, coastal), and species total body length.
- Geographical sources of the individuals (e.g., Lungsod ng Cebu, Philippines; Gullmar Fjord, Sweden).
- Climatic parameters (CO2, pH, temperature, salinity) for experiment and control.
- Acclimation periods (in days), if available.
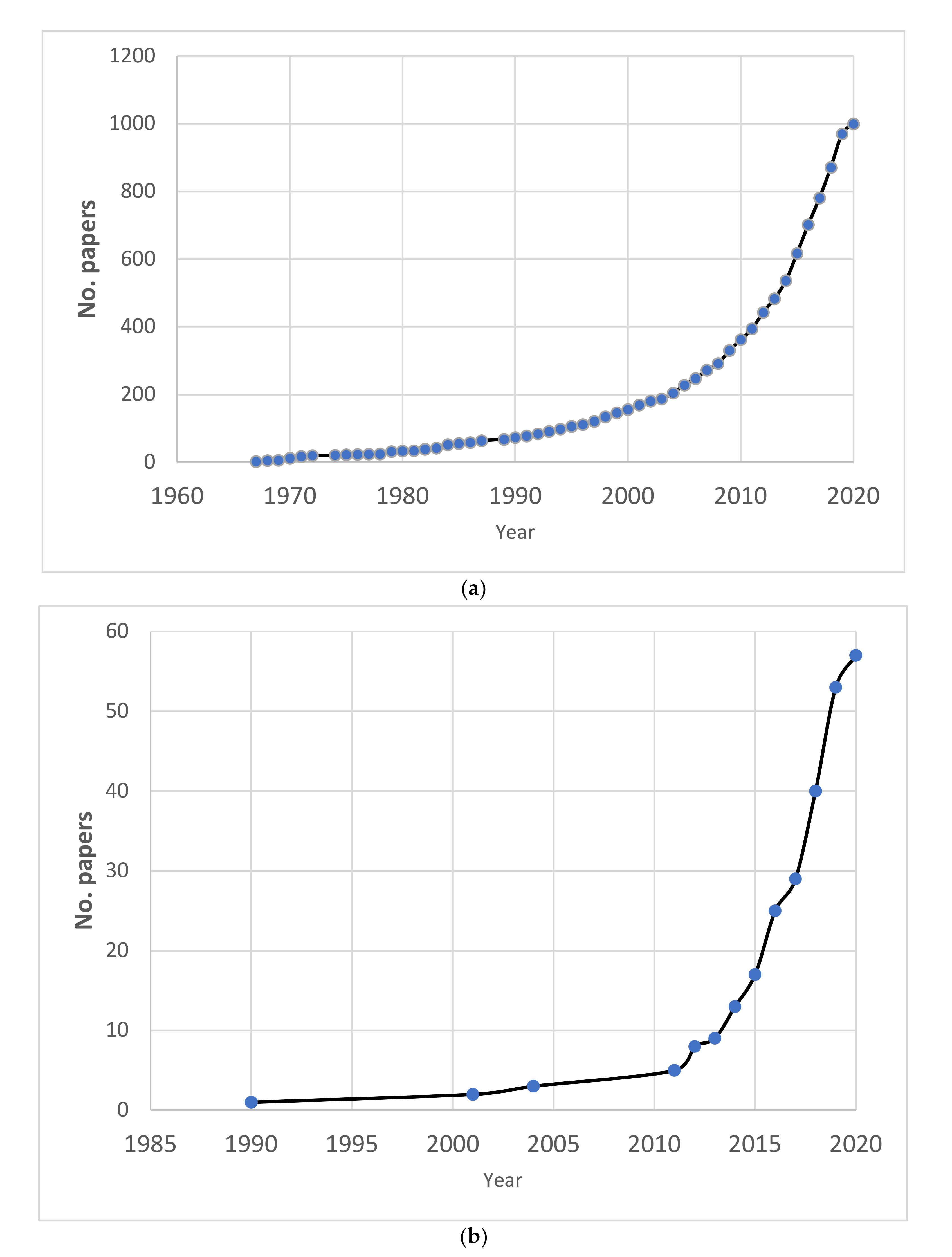
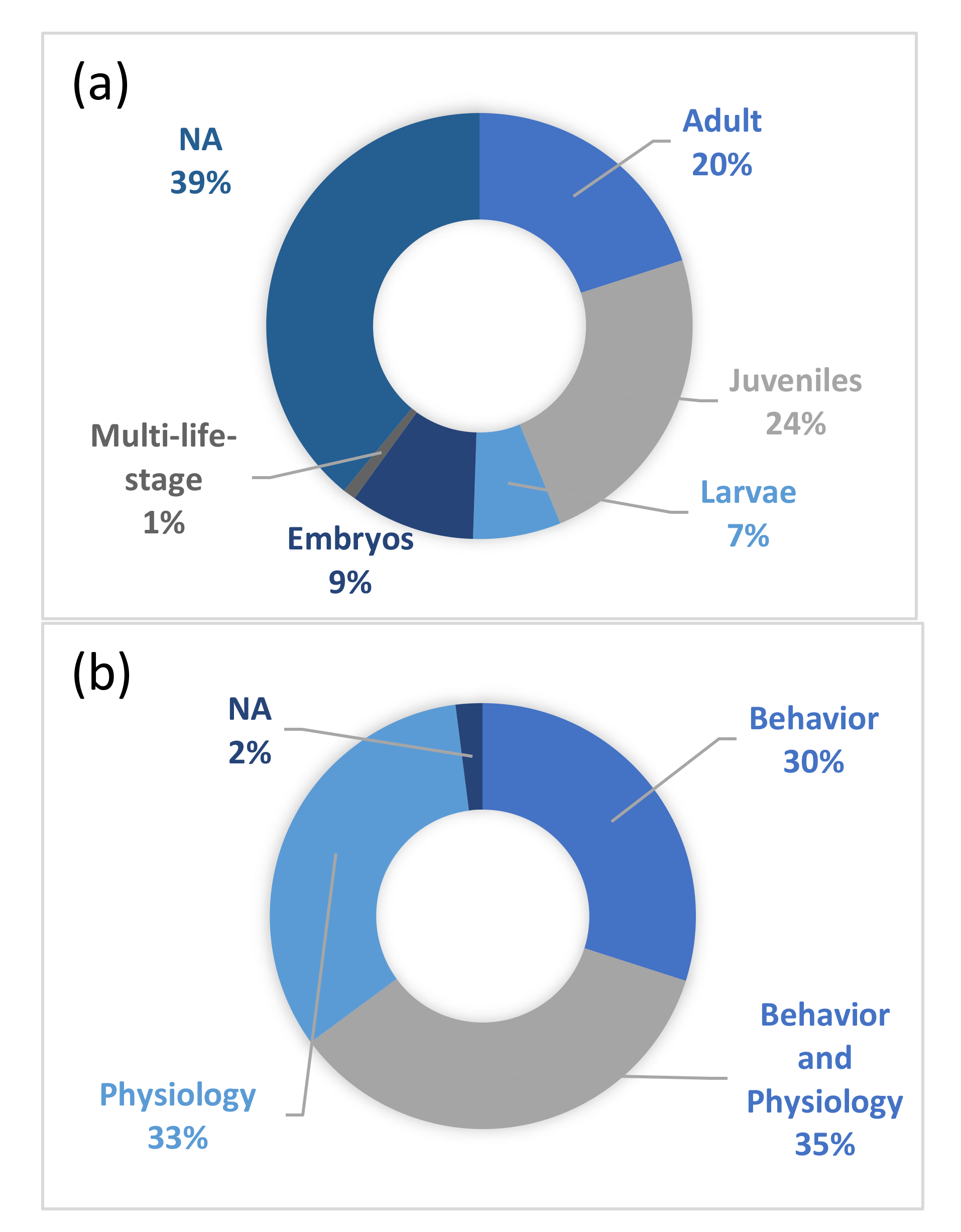
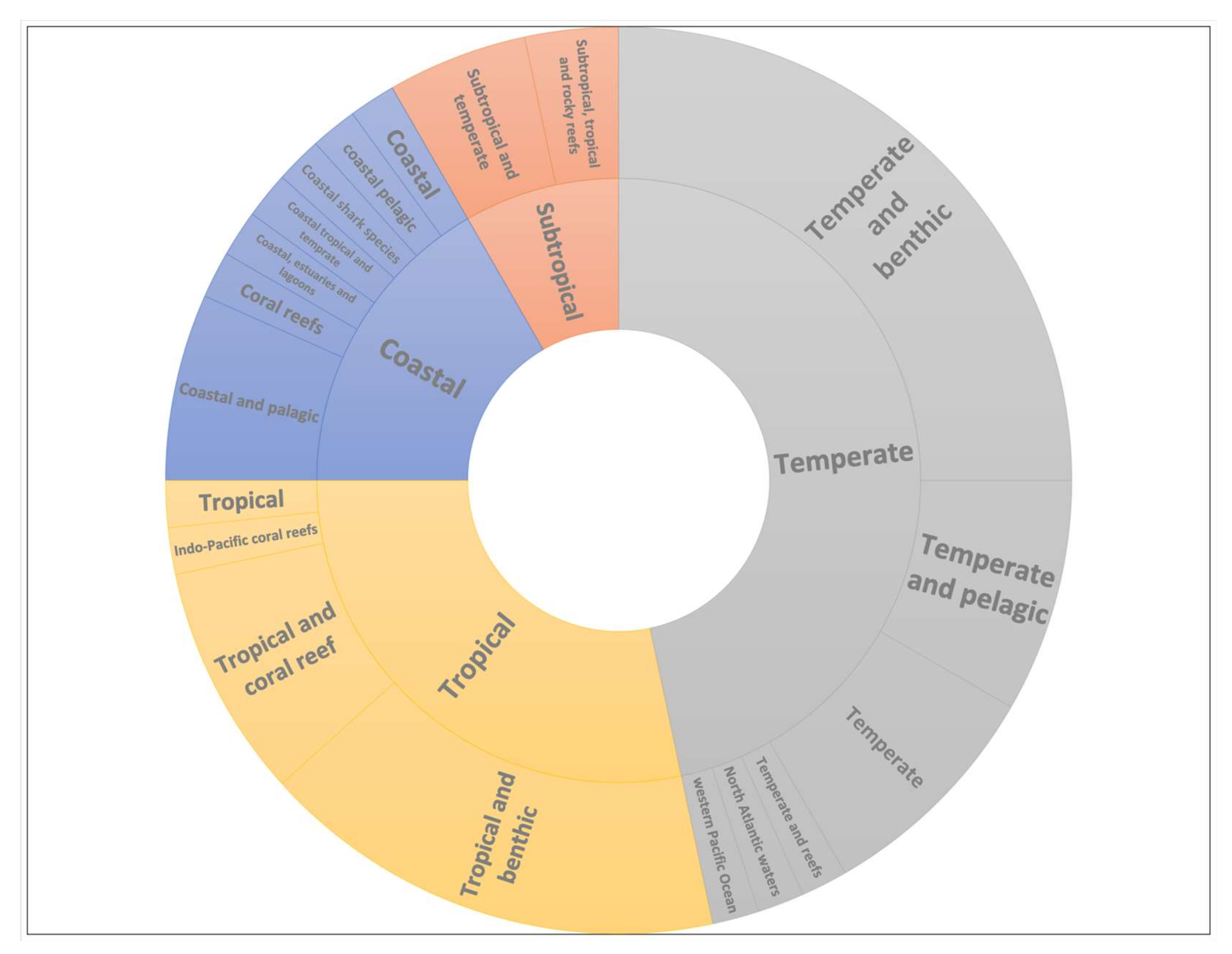
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skomal, G.; Bernal, D. Physiological responses to stress in sharks. In Sharks and Their Relatives II: Biodiversity, Adaptive Physiology, and Conservation; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 459–490. [Google Scholar] [CrossRef]
- Rosa, R.; Rummer, J.L.; Munday, P.L. Biological responses of sharks to ocean acidification. Biol. Lett. 2017, 13, 20160796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luongo, S.M.; Lowe, C.G. Seasonally acclimated metabolic Q10 of the California horn shark, Heterodontus francisci. J. Exp. Mar. Biol. Ecol. 2018, 503, 129–135. [Google Scholar] [CrossRef]
- Wheeler, C.R.; Gervais, C.R.; Johnson, M.S.; Vance, S.; Rosa, R.; Mandelman, J.W.; Rummer, J.L. Anthropogenic stressors influence reproduction and development in elasmobranch fishes. Rev. Fish Biol. Fish. 2020, 30, 373–386. [Google Scholar] [CrossRef]
- Pegado, M.R.; Santos, C.; Couto, A.; Pinto, E.; Lopes, A.R.; Diniz, M.; Rosa, R. Reduced impact of ocean acidification on growth and swimming performance of newly hatched tropical sharks (Chiloscyllium plagiosum). Mar. Freshw. Behav. Physiol. 2018, 51, 347–357. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H.; et al. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787 . [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; White, W.T. Extinction risk and conservation of the world’s sharks and rays. elife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamir, Z.Z.; Shamir, S.Z.; Becker, N.; Scheinin, A.; Tchernov, D. Evidence of the impacts of emerging shark tourism in the Mediterranean. Ocean Coast Manag. 2019, 178, 104847. [Google Scholar] [CrossRef]
- Pegado, M.R.; Santos, C.P.; Pimentel, M.; Cyrne, R.; Paulo, M.; Maulvaut, A.L.; Raffoul, D.; Diniz, M.; Bispo, R.; Rosa, R. Effects of elevated carbon dioxide on the hematological parameters of a temperate catshark. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 126–132. [Google Scholar] [CrossRef]
- Bouyoucos, I.A.; Shipley, O.N.; Jones, E.; Brooks, E.J.; Mandelman, J.W. Wound healing in an elasmobranch fish is not impaired by high-CO2 exposure. J. Fish Biol. 2020, 96, 1508–1511. [Google Scholar] [CrossRef]
- Schwieterman, G.D. The Impacts of Acute Hypoxic Exposure and Other Concomitant Stressors on the Cardiorespiratory Physiology of Coastal Elasmobranch Fishes. Ph.D. Thesis, The College of William and Mary, Williamsburg, VA, USA, 2020. [Google Scholar]
- Rummer, J.L.; Munday, P.L. Climate change and the evolution of reef fishes: Past and future. Fish. 2017, 18, 22–39. [Google Scholar] [CrossRef] [Green Version]
- Jarrold, M.D.; Welch, M.J.; McMahon, S.J.; McArley, T.; Allan, B.J.; Watson, S.A.; Parsons, D.M.; Pether, S.M.; Pope, S.; Nicol, S.; et al. Elevated CO2 affects anxiety but not a range of other behaviours in juvenile yellowtail kingfish. Mar. Environ. Res. 2020, 157, 104863. [Google Scholar] [CrossRef] [PubMed]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 2004, 305, 362–366 . [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarrold, M.D.; Munday, P.L. Elevated temperature does not substantially modify the interactive effects between elevated CO2 and diel CO2 cycles on the survival, growth, and behavior of a coral reef fish. Front. Mar. Sci. 2018, 5, 458. [Google Scholar] [CrossRef]
- Pimentel, M.S.; Faleiro, F.; Machado, J.; Pousão-Ferreira, P.; Rosa, R. Seabream Larval Physiology under Ocean Warming and Acidification. Fishes 2020, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Yool, A. Anthropogenic Ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Bopp, L.; Resplandy, L.; Orr, J.C.; Doney, S.C.; Dunne, J.P.; Gehlen, M.; Vichi, M. Multiple stressors of ocean ecosystems in the 21st century: Projections with CMIP5 models. Biogeosciences 2013, 10, 6225–6245. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, D.D.; Watson, S.A.; Rummer, J.L.; Brandl, S.J.; Simpfendorfer, C.A.; Heupel, M.R.; Munday, P.L. Foraging behaviour of the epaulette shark Hemiscyllium ocellatum is not affected by elevated CO2. ICES J. Mar. Sci. 2016, 73, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Bindoff, N.L.; Cheung, W.W.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.J.M.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing ocean, marine ecosystems, and dependent communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; pp. 477–587. [Google Scholar]
- Dixson, D.L.; Jennings, A.R.; Atema, J.; Munday, P.L. Odor tracking in sharks is reduced under future ocean acidification conditions. Glob. Change Biol. 2015, 21, 1454–1462. [Google Scholar] [CrossRef]
- Cominassi, L.; Moyano, M.; Claireaux, G.; Howald, S.; Mark, F.C.; Zambonino-Infante, J.L.; Peck, M.A. Food availability modulates the combined effects of ocean acidification and warming on fish growth. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Baumann, H.; Talmage, S.C.; Gobler, C.J. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat. Clim. Change 2012, 2, 38–41. [Google Scholar] [CrossRef]
- Dziergwa, J.; Singh, S.; Bridges, C.R.; Kerwath, S.E.; Enax, J.; Auerswald, L. Acid-base adjustments and first evidence of denticle corrosion caused by ocean acidification conditions in a demersal shark species. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Lopes, A.R.; Sampaio, E.; Santos, C.; Couto, A.; Pegado, M.R.; Diniz, M.; Munday, P.L.; Rummer, J.L.; Rosa, R. Absence of cellular damage in tropical newly hatched sharks (Chiloscyllium plagiosum) under ocean acidification conditions. Cell Stress Chaperones 2018, 23, 837–846. [Google Scholar] [CrossRef]
- Sen Gupta, A.; McNeil, B. Variability and change in the ocean. In The Future of the World’s Climate; Elsevier: Amsterdam, The Netherlands, 2012; pp. 141–165. [Google Scholar] [CrossRef]
- Doubleday, Z.A.; Nagelkerken, I.; Coutts, M.D.; Goldenberg, S.U.; Connell, S.D. A triple trophic boost: How carbon emissions indirectly change a marine food chain. Glob. Change Biol. 2019, 25, 978–984. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Kikkawa, T.; Hayashi, M.; Lee, K.S.; Kita, J. Effects of CO2 on marine fish: Larvae and adults. J. Oceanogr. 2004, 60, 731–741. [Google Scholar] [CrossRef]
- Green, L.; Jutfelt, F. Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biol. Lett. 2014, 10, 20140538. [Google Scholar] [CrossRef]
- Pinto, E.F.C. Physiological Responses of Whitespotted Bamboo Shark (Chiloscyllium Plagiosum) to High CO2 Levels. Master’s Thesis, University of Lisbon Faculty of Sciences Department of Animal Biology, Lisbon, Portugal, 2018. [Google Scholar]
- Fink, G. Stress: Definition and history. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 549–555. [Google Scholar] [CrossRef]
- Skomal, G.B.; Mandelman, J.W. The physiological response to anthropogenic stressors in marine elasmobranch fishes: A review with a focus on the secondary response. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 162, 146–155. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- McCormick, M.I.; Watson, S.A.; Simpson, S.D.; Allan, B.J. Effect of elevated CO2 and small boat noise on the kinematics of predator–prey interactions. Proc. Royal Soc. B 2018, 285, 20172650. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.H. Global Order: Values and Power in International Relations; Routledge: Oxfordshire, UK, 2018. [Google Scholar]
- Munday, P.L.; Jarrold, M.D.; Nagelkerken, I. Ecological effects of elevated CO2 on marine and freshwater fishes: From individual to community effects. In Fish Physiology; Grosell, M., Munday, P.L., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 37, pp. 323–368. [Google Scholar] [CrossRef]
- Pistevos, J.C.A. Early Life Behaviour and Sensory Ecology of Predatory Fish Under Climate Change and Ocean Acidification. Ph.D. Dissertation, School of Biological Science, University of Adelaide, Adelaide, Austraila, 2016. [Google Scholar] [CrossRef]
- Jutfelt, F.; Hedgärde, M. Atlantic cod actively avoid CO2 and predator odour, even after long-term CO2 exposure. Front. Zool. 2013, 10, 81. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- De Winter, J.C.F. Using the student’s t-test with extremely small sample sizes. Pract. Assess. Res. Eval. 2013, 18, 10. [Google Scholar]
- Simpson, S.D.; Munday, P.L.; Wittenrich, M.L.; Manassa, R.; Dixson, D.L.; Gagliano, M.; Yan, H.Y. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 2011, 7, 917–920. [Google Scholar] [CrossRef] [Green Version]
- Clements, J.C.; Hunt, H.L. Marine animal behaviour in a high CO2 Ocean. Mar. Ecol. Prog. Ser. 2015, 536, 259–279 . [Google Scholar] [CrossRef]
- Schwieterman, G.D.; Crear, D.P.; Anderson, B.N.; Lavoie, D.R.; Sulikowski, J.A.; Bushnell, P.G.; Brill, R.W. Combined effects of acute temperature change and elevated pCO2 on the metabolic rates and hypoxia tolerances of clearnose skate (Rostroraja eglanteria), summer flounder (Paralichthys dentatus), and thorny skate (Amblyraja radiata). Biology 2019, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, G.E.; Lefevre, S. Physiological challenges to fishes in a warmer and acidified future. Physiology 2016, 31, 409–417. [Google Scholar] [CrossRef]
- Pistevos, J.C.; Nagelkerken, I.; Rossi, T.; Olmos, M.; Connell, S.D. Ocean acidification and global warming impair shark hunting behaviour and growth. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tierney, K.B.; Baldwin, D.H.; Hara, T.J.; Ross, P.S.; Scholz, N.L.; Kennedy, C.J. Olfactory toxicity in fishes. Aquat. Toxicol. 2010, 96, 2–26. [Google Scholar] [CrossRef]
- Munday, P.L.; Welch, M.J.; Allan, B.J.; Watson, S.A.; McMahon, S.J.; McCormick, M.I. Effects of elevated CO2 on predator avoidance behaviour by reef fishes is not altered by experimental test water. Peer J. 2016b, 4, e2501. [Google Scholar] [CrossRef] [Green Version]
- Devine, B.M.; Munday, P.L.; Jones, G.P. Homing ability of adult cardinalfish is affected by elevated carbon dioxide. Oecologia 2012a, 168, 269–276. [Google Scholar] [CrossRef]
- Chapuis, L.; Collin, S.P.; Yopak, K.E.; McCauley, R.D.; Kempster, R.M.; Ryan, L.A.; Schmidt, C.; Kerr, C.C.; Gennari, E.; Egeberg, C.A.; et al. The effect of underwater sounds on shark behaviour. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ashur, M.M.; Johnston, N.K.; Dixson, D.L. Impacts of ocean acidification on sensory function in marine organisms. Integr. Comp. Biol. 2017, 57, 63–80. [Google Scholar] [CrossRef] [Green Version]
- Pistevos, J.C.; Nagelkerken, I.; Rossi, T.; Connell, S.D. Antagonistic effects of ocean acidification and warming on hunting sharks. Oikos 2017, 126, 241–247. [Google Scholar] [CrossRef]
- Gobler, C.J.; Baumann, H. Hypoxia and acidification in ocean ecosystems: Coupled dynamics and effects on marine life. Biol. Lett. 2016, 12, 20150976. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.P.; Sampaio, E.; Pereira, B.P.; Pegado, M.R.; Borges, F.O.; Wheeler, C.R.; Bouyoucos, I.A.; Rummer, J.L.; Santos, C.F.; Rosa, R. Elasmobranch Responses to Experimental Warming, Acidification, and Oxygen Loss—A Meta-Analysis. Front. Mar. Sci. 2021, 8, 1380. [Google Scholar] [CrossRef]
- Rosa, R.; Pimentel, M.; Galan, J.G.; Baptista, M.; Lopes, V.M.; Couto, A.; Guerreiro, M.; Sampaio, E.; Castro, J.; Santos, C.; et al. Deficit in digestive capabilities of bamboo shark early stages under climate change. Mar. Biol. 2016b, 163, 60. [Google Scholar] [CrossRef]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Hayashi, M.; Kikkawa, T. Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 2008, 373, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Kita, J.; Ishimatsu, A. Acid-base responses to lethal aquatic hypercapnia in three marine fishes. Mar. Biol. 2004, 144, 153–160. [Google Scholar] [CrossRef]
- Hannan, K.D.; Rummer, J.L. Aquatic acidification: A mechanism underpinning maintained oxygen transport and performance in fish experiencing elevated carbon dioxide conditions. J. Exp. Biol. 2018, 221, jeb154559. [Google Scholar] [CrossRef] [Green Version]
- Kormanik, G.A.; Evans, D.H. The acid-base status of prenatal pups of the dogfish, Squalus acanthias, in the uterine environment. J. Exp. Biol. 1986, 125, 173–179. [Google Scholar] [CrossRef]
- Munday, P.L. Transgenerational acclimation of fishes to climate change and ocean acidification. F1000Prime Rep. 2014, 6, 99. [Google Scholar] [CrossRef] [Green Version]
- Rummer, J.L.; Bouyoucos, I.A.; Mourier, J.; Nakamura, N.; Planes, S. Responses of a coral reef shark acutely exposed to ocean acidification conditions. Coral Reefs 2020, 39, 1215–1220. [Google Scholar] [CrossRef]
- Gattuso, J.P.; Magnan, A.; Billé, R.; Cheung, W.W.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, aac4722. [Google Scholar] [CrossRef]
- Derraik, J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bul. 2002, 44, 842–852 . [Google Scholar] [CrossRef]
- Nagelkerken, I.; Munday, P.L. Animal behaviour shapes the ecological effects of ocean acidification and warming: Moving from individual to community-level responses. Glob. Change Biol. 2016, 22, 974–989. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.; Chan, K.M.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366, aax3100. [Google Scholar] [CrossRef] [Green Version]
- Bruder, A.; Frainer, A.; Rota, T.; Primicerio, R. The importance of ecological networks in multiple-stressor research and management. Front. Environ. Sci. 2019, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Worm, B.; Davis, B.; Kettemer, L.; Ward-Paige, C.A.; Chapman, D.; Heithaus, M.R.; Kessel, S.T.; Gruber, S.H. Global catches, exploitation rates, and rebuilding options for sharks. Mar. Policy 2013, 40, 194–204. [Google Scholar] [CrossRef]
- Zemah-Shamir, Z.; Zemah-Shamir, S.; Tchernov, D.; Scheinin, A.; Becker, N. (Shark aggregation and tourism: Opportunities and challenges of an emerging phenomenon. Int. J. Sust. Dev. World 2019, 26, 406–414. [Google Scholar] [CrossRef]
- Leis, J.M. Paradigm lost: Ocean acidification will overturn the concept of larval-fish biophysical dispersal. Front. Mar. Sci. 2018, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Di Santo, V. Ocean acidification exacerbates the impacts of global warming on embryonic little skate, Leucoraja erinacea (Mitchill). J. Exp. Mar. Biol. Ecol. 2015, 463, 72–78. [Google Scholar] [CrossRef]
- Burleson, M.L. Oxygen availability: Sensory systems. In Biochemistry and Molecular Biology of Fishes, Environmental and Ecological Biochemistry; Hochachka, P.W., Mommsen., T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 5, pp. 1–18. [Google Scholar] [CrossRef]
- Rosa, R.; Baptista, M.; Lopes, V.M.; Pegado, M.R.; Ricardo Paula, J.; Trübenbach, K.; Leal, M.C.; Calado, R.; Repolho, T. Early-life exposure to climate change impairs tropical shark survival. Proc. R. Soc. B 2014, 281, 20141738. [Google Scholar] [CrossRef] [Green Version]
- Rosa, R.; Paula, J.R.; Sampaio, E.; Pimentel, M.; Lopes, A.R.; Baptista, M.; Guerreiro, M.; Santos, C.; Campos, D.; Almeida-Val, V.M.; et al. Neuro-oxidative damage and aerobic potential loss of sharks under elevated CO2 and warming. Mar. Biol. 2016a, 163, 119. [Google Scholar] [CrossRef]
- Larsen, E.H.; Deaton, L.E.; Onken, H.; O’Donnell, M.; Grosell, M.; Dantzler, W.H.; Weihrauch, D. Osmoregulation and excretion. Compr. Physiol. 2014, 4, 405–573. [Google Scholar] [CrossRef]
- Heithaus, M.R.; Delius, B.K.; Wirsing, A.J.; Dunphy-Daly, M.M. Physical factors influencing the distribution of a top predator in a subtropical oligotrophic estuary. Limnol. Oceanogr. 2009, 54, 472–482. [Google Scholar] [CrossRef]
- Wise, G.; Mulvey, J.M.; Renshaw, G.M.C. Hypoxia tolerance in the epaulette shark (Hemiscyllium ocellatum). J. Exp. Zool. 1998, 281, 1–5. [Google Scholar] [CrossRef]
- Baumann, H. Experimental assessments of marine species sensitivities to ocean acidification and co-stressors: How far have we come? Can. J. Zool. 2019, 97, 399–408. [Google Scholar] [CrossRef]
- Lefevre, S. Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv. Physiol. 2016, 4, cow009. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, D.D.; Rummer, J.L.; Morash, A.J.; Watson, S.A.; Simpfendorfer, C.A.; Heupel, M.R.; Munday, P.L. A product of its environment: The epaulette shark (Hemiscyllium ocellatum) exhibits physiological tolerance to elevated environmental CO2. Conserv. Physiol. 2014, 2, cou047. [Google Scholar] [CrossRef] [Green Version]
- Fry, F.E.J. The effect of environmental factors on the physiology of fish. In Fish Physiology; Hoar, W.S., Randall, D.J., Eds.; Academic Press: New York, NY, USA, 1971; Volume 6, pp. 1–98. [Google Scholar] [CrossRef]
- Bernal, D.; Reid, J.P.; Roessig, J.M.; Matsumoto, S.; Sepulveda, C.A.; Cech, J.J.; Graham, J.B. Temperature effects on the blood oxygen affinity in sharks. Fish Physiol. Biochem. 2018, 44, 949–967. [Google Scholar] [CrossRef]
- Casper, B.M.; Mann, D.A. Dipole hearing measurements in elasmobranch fishes. J. Exp. Biol. 2007, 210, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Chin, A.; Kyne, P.M.; Walker, T.I.; McAuley, R.B. An integrated risk assessment for climate change: Analysing the vulnerability of sharks and rays on Australia’s Great Barrier Reef. Glob. Change Biol. 2010, 16, 1936–1953. [Google Scholar] [CrossRef]
- Clark, T.D.; Raby, G.D.; Roche, D.G.; Binning, S.A.; Speers-Roesch, B.; Jutfelt, F.; Sundin, J. Ocean acidification does not impair the behaviour of coral reef fishes. Nature 2020, 577, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Crespel, A.; Katja, A.; Pernelle, L.; Patrick, Q.; Nicolas, L.B.; José-Luis, Z.I.; Denis, C.; Guy, C. Long-term effects of ocean acidification upon energetics and oxygen transport in the European sea bass (Dicentrarchus labrax, Linnaeus). Mar. Biol. 2019, 166, 1–12. [Google Scholar] [CrossRef]
- Devine, B.M.; Munday, P.L.; Jones, G.P. Rising CO2 concentrations affect settlement behaviour of larval damselfishes. Coral Reefs 2012, 31, 229–238. [Google Scholar] [CrossRef]
- Di Santo, V. Intraspecific variation in physiological performance of a benthic elasmobranch challenged by ocean acidification and warming. J. Exp. Biol. 2016, 219, 1725–1733. [Google Scholar] [CrossRef] [Green Version]
- Di Santo, V. Ocean acidification and warming affect skeletal mineralization in a marine fish. Proc. R. Soc. B 2019, 286, 20182187. [Google Scholar] [CrossRef] [Green Version]
- Draper, A.M.; Weissburg, M.J. Impacts of global warming and elevated CO2 on sensory behavior in predator-prey interactions: A review and synthesis. Front. Ecol. Evol. 2019, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Esbaugh, A.J. Physiological implications of ocean acidification for marine fish: Emerging patterns and new insights. J. Comp. Physiol. B 2018, 188, 1–13. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef]
- Gilmour, K.M. New insights into the many functions of carbonic anhydrase in fish gills. Respir. Physiol. Neurobiol. 2012, 184, 223–230. [Google Scholar] [CrossRef]
- Graham, C.T.; Harrod, C. Implications of climate change for the fishes of the British Isles. J. Fish Biol. 2009, 74, 1143–1205. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.S.; Turner, J.D.; Wood, C.M. Control of ventilation in the hypercapnic skate Raja ocellata: I. Blood and extradural fluid. Respir. Physiol. 1990, 80, 259–277. [Google Scholar] [CrossRef]
- Hamilton, T.J.; Holcombe, A.; Tresguerres, M. CO2-induced Ocean acidification increases anxiety in rockfish via alteration of GABAA receptor functioning. Proc. R. Soc. B 2014, 281, 20132509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, N.S.; Collin, S.P. Sharks senses and shark repellents. Integr. Zool. 2015, 10, 38–64. [Google Scholar] [CrossRef]
- Heuer, R.M.; Grosell, M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1061–R1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiahuan, R.; Wenhao, S.; Xiaofan, G.; Wei, S.; Shanjie, Z.; Maolong, H.; Haifeng, W.; Guangxu, L. Ocean acidification impairs foraging behavior by interfering with olfactory neural signal transduction in black sea bream, Acanthopagrus schlegelii. Front. Physiol. 2018, 9, 1592. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.S.; Kraver, D.W.; Renshaw, G.M.; Rummer, J.L. Will ocean acidification affect the early ontogeny of a tropical oviparous elasmobranch (Hemiscyllium ocellatum)? Conserv. Physiol. 2016, 4, cow003. [Google Scholar] [CrossRef] [Green Version]
- Jutfelt, F.; Hedgärde, M. Juvenile Atlantic cod behavior appears robust to near-future CO2 levels. Front. Zool. 2015, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Kimber, J.A.; Sims, D.W.; Bellamy, P.H.; Gill, A.B. The ability of a benthic elasmobranch to discriminate between biological and artificial electric fields. Mar. Biol. 2011, 158, 1–8. [Google Scholar] [CrossRef]
- Kwan, G.T.; Hamilton, T.J.; Tresguerres, M. CO2-induced Ocean acidification does not affect individual or group behaviour in a temperate damselfish. R. Soc. Open Sci. 2017, 4, 170283. [Google Scholar] [CrossRef] [Green Version]
- Malvezzi, A.J.; Murray, C.S.; Feldheim, K.A.; DiBattista, J.D.; Garant, D.; Gobler, C.J.; Chapman, D.D.; Baumann, H. A quantitative genetic approach to assess the evolutionary potential of a coastal marine fish to ocean acidification. Evol. Appl. 2015, 8, 352–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazurais, D.; Servili, A.; Le Bayon, N.; Gislard, S.; Madec, L.; Zambonino-Infante, J.L. Long-term exposure to near-future ocean acidification does not affect the expression of neurogenesis-and synaptic transmission-related genes in the olfactory bulb of European sea bass (Dicentrarchus labrax). J. Comp. Physiol. B 2020, 190, 161–167. [Google Scholar] [CrossRef] [PubMed]
- McKendry, J.E.; Milsom, W.K.; Perry, S.F. Branchial CO2 receptors and cardiorespiratory adjustments during hypercarbia in Pacific spiny dogfish (Squalus acanthias). J. Exp. Biol. 2001, 204, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; Gagliano, M.; Donelson, J.M.; Dixson, D.L.; Thorrold, S.R. Ocean acidification does not affect the early life history development of a tropical marine fish. Mar. Ecol. Prog. Ser. 2011, 423, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Munday, P.L.; Watson, S.A.; Parsons, D.M.; King, A.; Barr, N.G.; Mcleod, I.M.; Allan, B.J.; Pether, S.M. Effects of elevated CO2. on early life history development of the yellowtail kingfish, Seriola lalandi, a large pelagic fish. ICES J. Mar. Sci. 2016, 73, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Paula, J.R.; Baptista, M.; Carvalho, F.; Repolho, T.; Bshary, R.; Rosa, R. The past, present and future of cleaner fish cognitive performance as a function of CO2 levels. Biol. Lett. 2019, 15, 20190618. [Google Scholar] [CrossRef] [Green Version]
- Pistevos, J.C.; Nagelkerken, I.; Rossi, T.; Connell, S.D. Ocean acidification alters temperature and salinity preferences in larval fish. Oecologia 2017, 183, 545–553. [Google Scholar] [CrossRef]
- Raby, G.D.; Sundin, J.; Jutfelt, F.; Cooke, S.J.; Clark, T.D. Exposure to elevated carbon dioxide does not impair short-term swimming behaviour or shelter-seeking in a predatory coral-reef fish. J. Fish Biol. 2018, 93, 138–142. [Google Scholar] [CrossRef]
- Sswat, M.; Stiasny, M.H.; Jutfelt, F.; Riebesell, U.; Clemmesen, C. Growth performance and survival of larval Atlantic herring, under the combined effects of elevated temperatures and CO2. PLoS ONE. 2018, 13, e0191947. [Google Scholar] [CrossRef] [Green Version]
- Sundin, J.; Amcoff, M.; Mateos-González, F.; Raby, G.D.; Clark, T.D. Long-term acclimation to near-future ocean acidification has negligible effects on energetic attributes in a juvenile coral reef fish. Oecologia 2019, 190, 689–702. [Google Scholar] [CrossRef]
- Sundin, J.; Amcoff, M.; Mateos-González, F.; Raby, G.D.; Jutfelt, F.; Clark, T.D. Long-term exposure to elevated carbon dioxide does not alter activity levels of a coral reef fish in response to predator chemical cues. Behav. Ecol. Sociobiol. 2017, 71, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundin, J.; Jutfelt, F. 9–28 d of exposure to elevated p CO2 reduces avoidance of predator odour but had no effect on behavioural lateralization or swimming activity in a temperate wrasse (Ctenolabrus rupestris). ICES J. Mar. Sci. 2016, 73, 620–632. [Google Scholar] [CrossRef] [Green Version]
- Sundin, J.; Jutfelt, F. Effects of elevated carbon dioxide on male and female behavioural lateralization in a temperate goby. R. Soc. Open Sci. 2018, 5, 171550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresguerres, M.; Hamilton, T.J. Acid-base physiology, neurobiology and behaviour in relation to CO2-induced Ocean acidification. J. Exp. Biol. 2017, 220, 2136–2148. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.R.; Dittman, A.H.; McElhany, P.; Busch, D.S.; Maher, M.T.; Bammler, T.K.; MacDonald, J.W.; Gallagher, E.P. Elevated CO2 impairs olfactory-mediated neural and behavioral responses and gene expression in ocean-phase coho salmon (Oncorhynchus kisutch). Glob. Chang. Biol. 2019, 25, 963–977. [Google Scholar] [CrossRef]

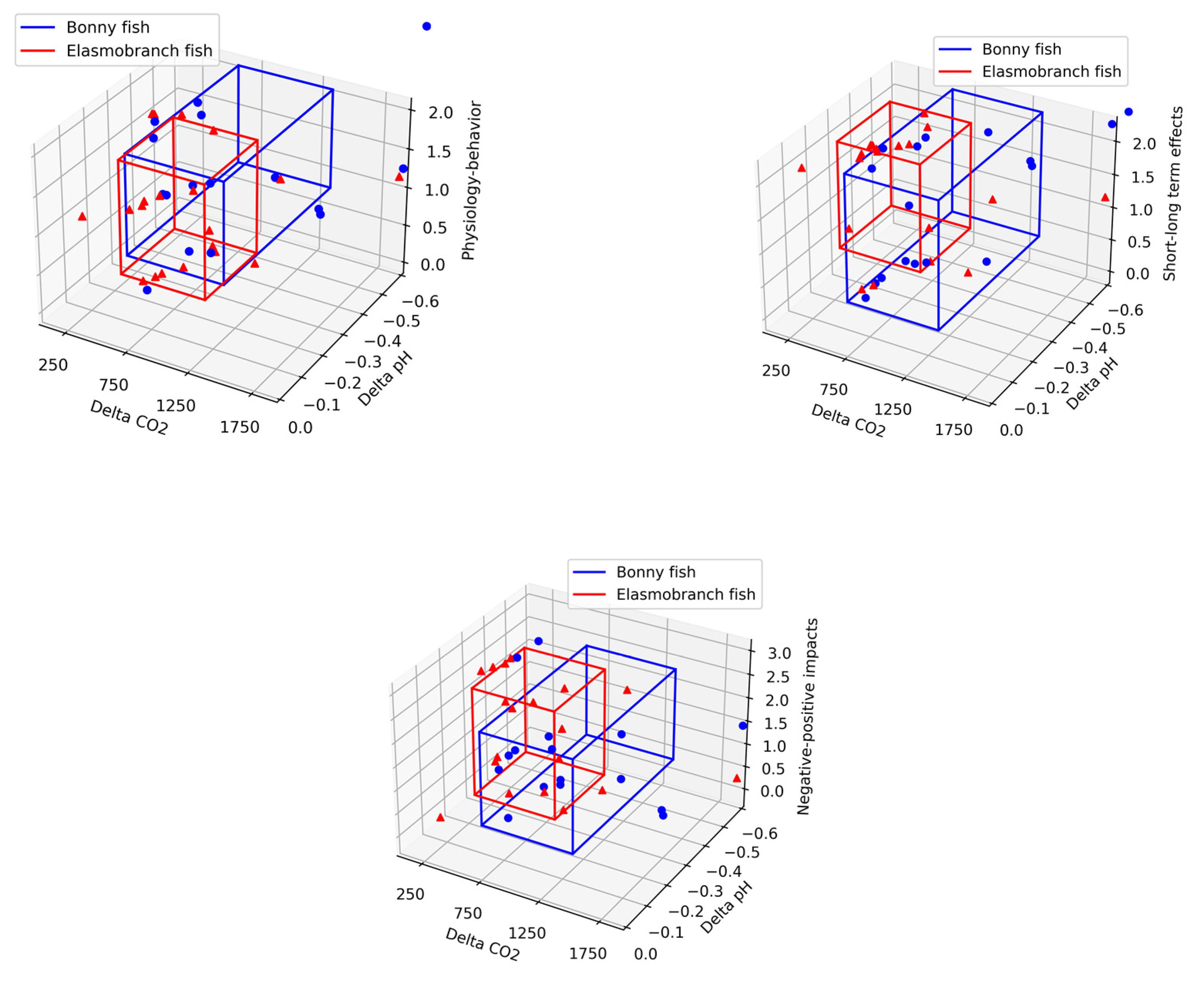



| Fitness | Behavior Avoidance | Mortality | |
|---|---|---|---|
| N | 48 | 29 | 14 |
| p value | 0.0413 ** | 0.133 | 0.225 |
| Estimated effect on the variables | 0.011 | 0.011 | 0.007 |
| Akaike information criterion (AIC) | 40.141 | 26.567 | 17.569 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemah-Shamir, Z.; Zemah-Shamir, S.; Scheinin, A.; Tchernov, D.; Lazebnik, T.; Gal, G. A Systematic Review of the Behavioural Changes and Physiological Adjustments of Elasmobranchs and Teleost’s to Ocean Acidification with a Focus on Sharks. Fishes 2022, 7, 56. https://doi.org/10.3390/fishes7020056
Zemah-Shamir Z, Zemah-Shamir S, Scheinin A, Tchernov D, Lazebnik T, Gal G. A Systematic Review of the Behavioural Changes and Physiological Adjustments of Elasmobranchs and Teleost’s to Ocean Acidification with a Focus on Sharks. Fishes. 2022; 7(2):56. https://doi.org/10.3390/fishes7020056
Chicago/Turabian StyleZemah-Shamir, Ziv, Shiri Zemah-Shamir, Aviad Scheinin, Dan Tchernov, Teddy Lazebnik, and Gideon Gal. 2022. "A Systematic Review of the Behavioural Changes and Physiological Adjustments of Elasmobranchs and Teleost’s to Ocean Acidification with a Focus on Sharks" Fishes 7, no. 2: 56. https://doi.org/10.3390/fishes7020056







