Bacillus subtilis Supplementation in a High-Fat Diet Modulates the Gut Microbiota and Ameliorates Hepatic Lipid Accumulation in Grass Carp (Ctenopharyngodon idella)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Bacteria
2.2. Feed Preparation and Feeding
2.3. Growth Indices
2.4. Serum Biochemical Indices
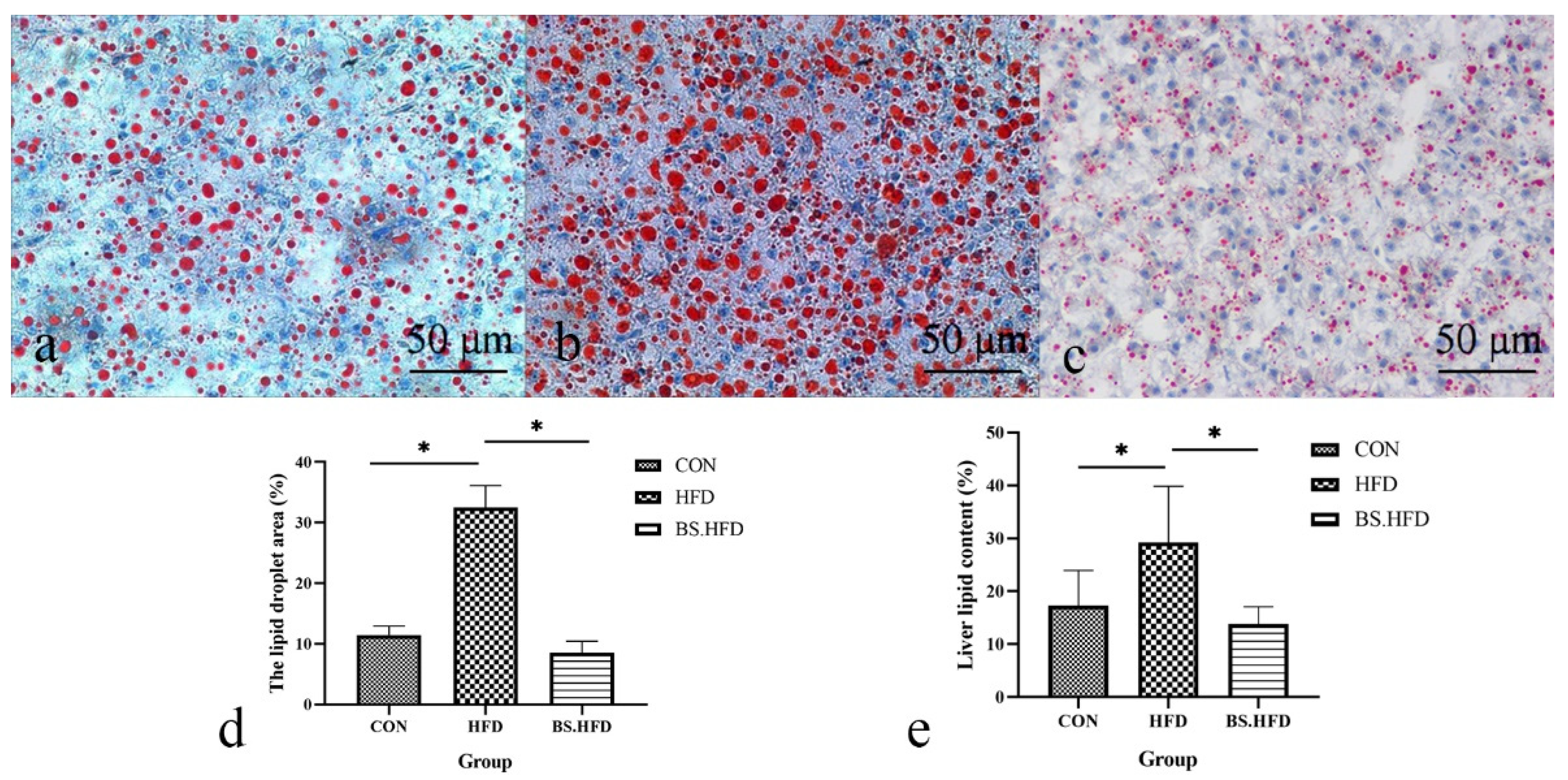
2.5. Oil Red O Staining
2.6. Total Liver Lipid
2.7. Intestinal Microbiota
2.8. Bioinformatic Analysis
2.9. Statistical Analysis
3. Result
3.1. Role of B. subtilis in the Effect of High-Fat Diet on Growth Index
3.2. Role of B. subtilis in the Effect of High-Fat Diet on Serum Biochemical Indices
3.3. Role of B. subtilis in the Effect of High-Fat Diet on Liver Lipid Content
3.4. Role of B. subtilis in the Effect of High-Fat Diet on the Intestinal Microbiota
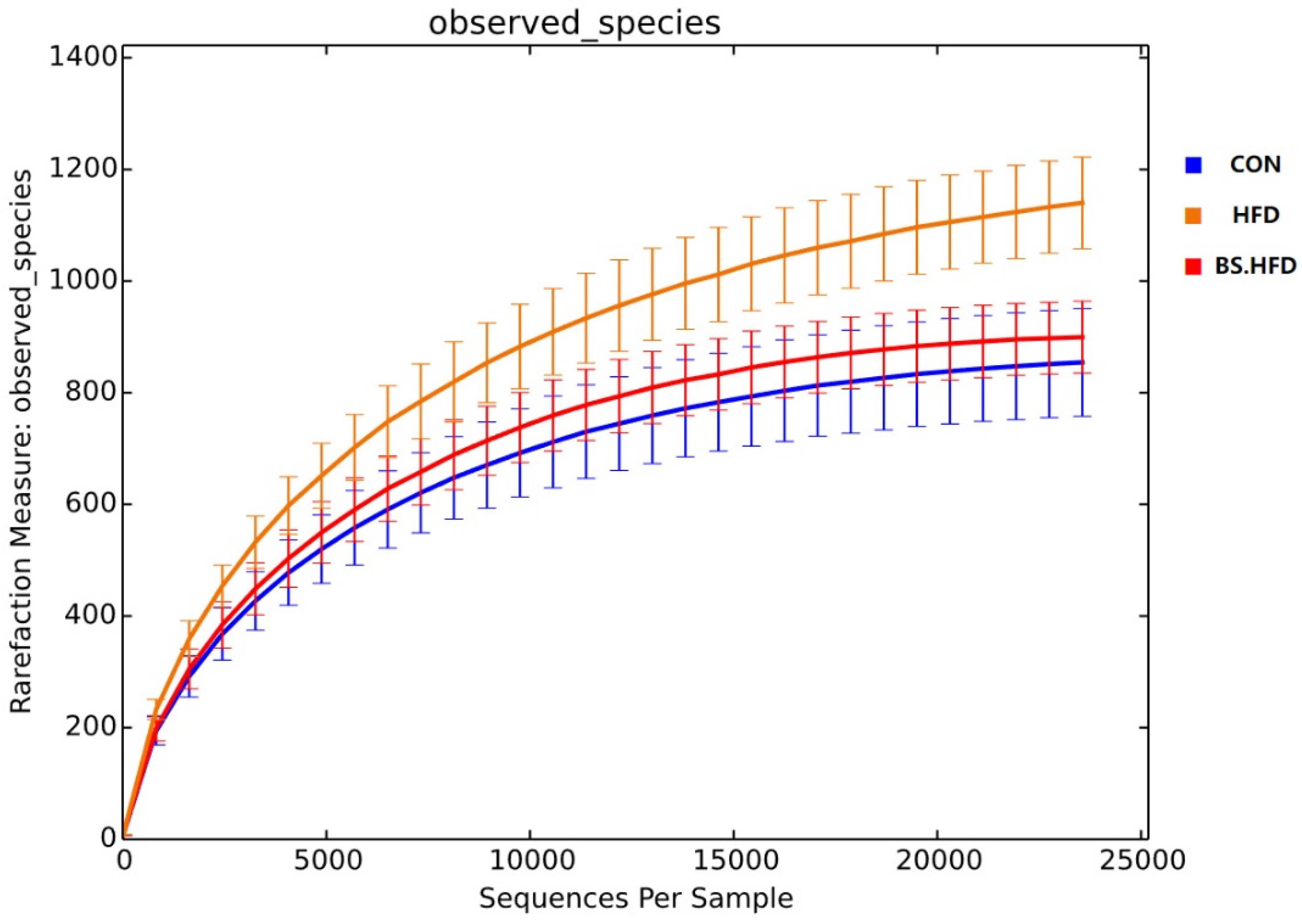
3.4.1. General Analysis of High-Throughput Sequencing
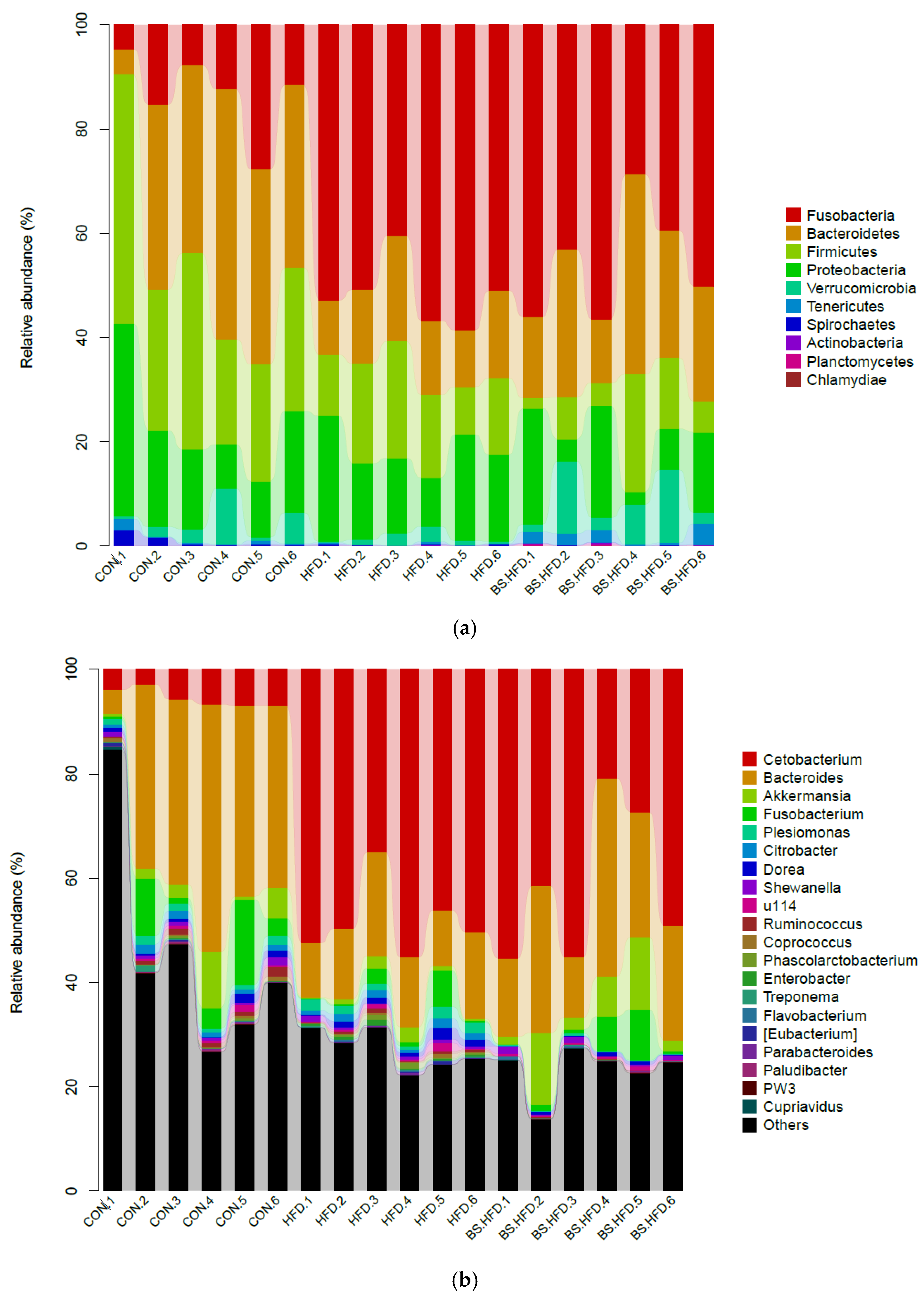
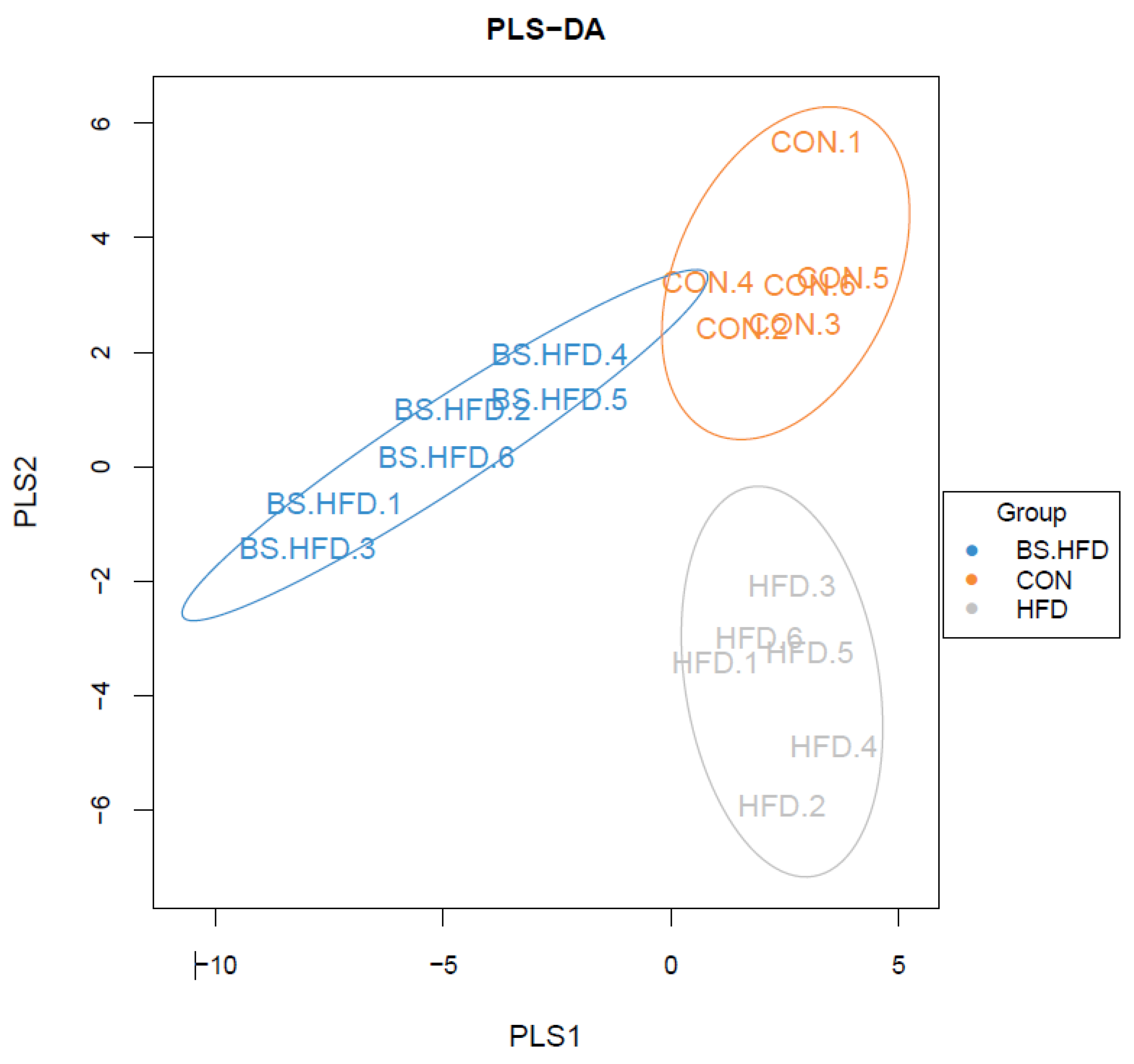
3.4.2. Comparison and Structure of the Intestinal Microbiota
3.4.3. Analysis of the Differences in the Intestinal Microbiota of the Three Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, H.M.; Wang, X.M.; Zhang, G.H. Nutritional metabolic diseases of fish. J. Hydroecol. 2004, 24, 67–69. [Google Scholar]
- Zhang, H.T.; Wang, A.L.; Li, G.L.; Sun, C.C. Effect of nutrient on the fatty liver disease of fish. Marin. Sci. Bull. 2004, 23, 82–89. [Google Scholar]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Chen, W.X.; Yu, Z.H. The relationship between nonalcoholic fatty liver and insulin resistance with abnormal glucose metabolism. Chin. J. Hepatol. 2000, 8, 76–77. [Google Scholar]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.Q.; Zhang, S.Y.; Xu, L.L.; Wu, Z.X.; Yuan, J.F.; Chen, X.X. Comparison of the intestinal microbiota during the different growth stages of red swamp crayfish (Procambarus clarkii). Front. Microbiol. 2021, 12, 696281. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C. The gut as a potential trigger of exercise-induced inflammatory responses. Can. J. Physiol. Pharmacol. 1998, 76, 479–484. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Isolauri, E. Positive interactions with the microbiota: Probiotics. Adv. Exp. Med. Biol. 2008, 635, 57–66. [Google Scholar]
- Velayudham, A.; Dolganiuc, A.; Ellis, M.; Petrasek, J.; Kodys, K.; Mandrekar, P.; Szabo, G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology 2010, 49, 989–997. [Google Scholar]
- Verdam, F.J.; Rensen, S.S.; Driessen, A.; Greve, J.W.; Buurman, W.A. Novel evidence for chronic exposure to endotoxin in human nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2011, 45, 149–152. [Google Scholar] [CrossRef]
- Feng, L.Y.; Shen, Y.J.; Yu, S.L. Effect of enteric Bifid-triple viable capsule on no-alcoholic fatty liver disease in rats and its mechanism. Chin. J. Microecol. 2013, 25, 793–796. [Google Scholar]
- Zhao, H.Y.; Jin, H.L.; Yang, X.Y.; Zhang, F.R. Application of microbiotics in non-alcoholic fatty liver disease. Harbin Med. J. 2013, 33, 190–191. [Google Scholar]
- Alisi, A.; Bedogni, G.; Baviera, G.; Giorgio, V.; Porro, E.; Paris, C.; Giammaria, P.; Reali, L.; Anania, F.; Nobili, V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharm. Ther. 2014, 39, 1276–1285. [Google Scholar]
- Wang, J.; Zhao, Y.; Ruan, Y. Effects of bio-organic fertilizers produced by four Bacillus amyloliquefaciens strains on banana fusarium wilt disease. Compost. Sci. Util. 2015, 23, 185–198. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.L.; Unniappan, S.; Carnevali, O. Dietary lipid content reorganizes gut microbiota and probiotic L-rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Sci. Rep. 2017, 7, 5512. [Google Scholar] [CrossRef] [PubMed]
- Banda, I.; Lobo, C.; León-Rubio, J.M.; Tapia-Paniagua, S.; Balebona, M.C.; Moriñigo, M.A.; Moreno-Ventas, X.; Lucas, L.M.; Linares, F.; Arce, F.; et al. Influence of two closely related probiotics on juvenile Senegalese sole (Solea senegalensis, Kaup 1858) performance and protection against Photobacterium damselae subsp. Piscicida. Aquac. 2010, 306, 281–288. [Google Scholar] [CrossRef]
- Li, W.F.; Shen, T.; Chen, N.N.; Deng, B.; Fu, L.Q.; Zhou, X.X. Effect of dietary Bacillus subtilis on digestive enzyme activity and intestinal microflora in grass carp Ctenopharyngodon idella. J. Dalian Fish Univ. 2012, 27, 221–225. [Google Scholar] [CrossRef]
- Luo, Y.E.; Zhao, H.; Guo, D.Y.; Wang, H.; Chen, X.X. Effect of Bacillus subtilis on the hepatic lipid metabolism of Ctenopharyngodon idella. Acta Hydrobiol. Sin. 2020, 44, 485–493. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Btotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randazzo, B.; Zarantoniello, M.; Gioacchini, G.; Cardinaletti, G.; Belloni, A.; Giorgini, E.; Faccenda, F.; Cerri, R.; Tibaldi, E.; Olivotto, I. Physiological response of rainbow trout (Oncorhynchus mykiss) to graded levels of Hermetia illucens or poultry by-product meals as single or combined substitute ingredients to dietary plant proteins. Aquaculture 2021, 538, 736550. [Google Scholar] [CrossRef]
- Randazzo, B.; Zarantoniello, M.; Cardinaletti, G.; Cerri, R.; Giorgini, E.; Belloni, A.; Conto, M.; Tibaldi, E.; Olivotto, I. Hermetia illucens and poultry by-product meals as alternatives to plant protein sources in gilthead seabream (Sparus aurata) diet: A multidisciplinary study on fish gut status. Animals 2021, 11, 677. [Google Scholar] [CrossRef]
- Hua, K.; Bureau, D.P. Development of a model to estimate digestible lipid content of salmonid fish feeds. Aquaculture 2009, 286, 271–276. [Google Scholar] [CrossRef]
- Diaz, J.P.; Guyot, E.; Vigier, S.; Connes, R. First events in lipid absorption during post-embryonic development of the anterior intestine in gilt-head sea bream. J. Fish Biol. 1997, 51, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.A.; Garcia, M.I.; Riera, H.N.; Vélez, E.J.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of different dietary vegetable oils on growth and intestinal performance, lipid metabolism and flesh quality in gilthead sea bream. Aquaculture 2019, 519, 734881. [Google Scholar] [CrossRef]
- Zhang, C.N.; Wang, A.M.; Liu, W.B.; Yang, W.P.; Yu, Y.B.; Lv, L.L.; Huang, J.T.; Qi, Z.T. Effect of dietary lipid levels on fat deposition, lipid metabolize enzyme and antiocidantic activities of Chelon haematocheilus. J. Fish Sci. Chin. 2013, 20, 108–115. [Google Scholar] [CrossRef]
- Xu, J.H.; Qin, J.; Yan, B.L.; Zhu, M.; Luo, G. Effects of dietary lipid levels on growth performance, feed utilization and fatty acid composition of juvenile Japanese seabass (Lateolabrax japonicus) reared in seawater. Aquacult. Int. 2011, 19, 79–89. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Xu, Z.C.; Wang, C.A.; Zhao, Z.G.; Luo, L. Effect of dietary lipid levels on liver free fatty acids, serum biochemical parameters and liver histological structure in mirror common carp at different temperatures. J. Northeast. Agric. Univ. 2012, 43, 118–126. [Google Scholar] [CrossRef]
- Miao, C.H. Pharmacology study of Penthorum chinense pursh extract in grass carp fatty liver disease. Sichuan Agric. Univ. 2012. Available online: https://scholar.google.com.hk/scholar?hl=zh-CN&as_sdt=0%2C5&q=Supplementation+with+probiotics+modifies+gut+flora+and+attenuates+liver+fat+accumulation+in+rat+nonalcoholic+fatty+liver+disease+model&btnG= (accessed on 4 April 2022).
- Lee, H.Y.; Park, J.H.; Seok, S.H.; Baek, M.W.; Kim, D.J.; Lee, K.E.; Paek, K.S.; Lee, Y.; Park, J.H. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim. Biophys. Acta. 2006, 1761, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jing, H.; Li, Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J. Hepatol. 2008, 49, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.Y.; Wan, Y.P.; Fang, Q.Y.; Lu, W.; Cai, W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J. Clin. Biochem. Nutr. 2012, 50, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia-Paniagua, S.T.; Diaz-Rosales, P.; de la Banda, I.G.; Lobo, C.; Clavijo, E.; Balebona, M.C.; Moriñigo, M.A. Modulation of certain liver fatty acids in Solea senegalensis is influenced by the dietary administration of probiotic microorganisms. Aquaculture 2014, 424–425, 234–238. [Google Scholar] [CrossRef]
- Falcinelli, S.; Picchietti, S.; Rodiles, A.; Cossignani, L.; Merrifield, D.; Taddei, A.R.; Maradonna, F.; Olivotto, I.; Gioacchini, G.; Carnevali, O. Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci. Rep. 2015, 5, 9336. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Luo, Y.E.; Zhang, Y.G.; Chen, X.X.; Wang, H.; Wu, Z.X. Effects of Bacillus subtilis on hepatic lipid metabolism and oxidative stress response in grass carp (Ctenopharyngodon idella) fed a high-fat diet. MLST 2020, 2, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.Q.; Chen, J.M.; Guo, J.L.; Pan, Q.; Sun, L.H.; Ye, J.Y. Effect of adding Bacillus subtilis to diets on growth performance, digestive enzymes activity and body composition of fingerling black carp (Mylopharyngodon piceus). Acta Hydrobiol. Sin. 2013, 37, 48–53. [Google Scholar]
- Karimzadeh, S.; Amirkolaie, A.K.; Miandehy, S.P. The effects of different levels of beta plus on growth performance, microbial flora and blood parameters of Caspian trout, Salmo caspius (Kessler, 1877). IJAB 2014, 2, 292–298. [Google Scholar]
- Wu, Z.X.; Feng, X.; Xie, L.L.; Peng, X.Y.; Yuan, J.; Chen, X.X. Effect of probiotic Bacillus subtilis Ch9 for grass carp, Ctenopharyngodon idella (Valenciennes, 1844), on growth performance, digestive enzyme activities and intestinal microflora. J. Appl. Ichthyol. 2012, 28, 721–727. [Google Scholar] [CrossRef]
- Cheng, W.; Chiu, C.S.; Guu, Y.K.; Tsai, S.T.; Liu, C.H. Expression of recombinant phytase of Bacillus subtilis E20 in Escherichia coli HMS 174 and improving the growth performance of white shrimp, Litopenaeus vannamei, juveniles by using phytase-pretreated soybean meal-containing diet. Aquacul. Nutr. 2013, 19, 117–127. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, K.; Chu, T.W.; Meng, W.T. Dietary supplementation of probiotic, Bacillus subtilis E20, enhances the growth performance and disease resistance against Vibrio alginolyticus in parrot fish (Oplegnathus fasciatus). Aquacul. Int. 2018, 26, 63–74. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Soltan, M.A.; Jarmo, O.S.; Abdo, H.S. Combined effects of dietary malic acid and Bacillus subtilis on growth, gut microbiota and blood parameters of Nile tilapia (Oreochromis niloticus). Aquacul. Nutr. 2018, 24, 83–93. [Google Scholar] [CrossRef]
- Lei, K.; Li, Y.L.; Wang, Y.; Wen, J.; Wu, H.Z.; Yu, D.Y.; Li, W.F. Effect of dietary supplementation of Bacillus subtilis B10 on biochemical and molecular parameters in the serum and liver of high-fat diet-induced obese mice. J. Zhejiang Univ.-Sci. B. 2015, 16, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.J.; Wei, F.; Yao, G.; Du, H.; Wang, M.; Liu, Z.; Li, Q.; An, L.; Tian, J.; Li, M.; et al. Drinking warm water improves growth performance and optimizes the gut microbiota in early postweaning rabbits during winter. Animals 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.; Stephens, W.Z.; Adam, R.B.; Burns, A.R.; Stagaman, K.; David, L.A.; Bohannan, B.J.M.; Guillemin, K.; Rawls, J.F. Ontogenetic differences in dietary fat influence microbiota assembly in the zebrafish gut. mBio 2015, 6, e00687-15. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.Y.; Li, J.J.; Yu, Y.H.; Wang, J.; He, Z.; Van Nostrand, J.D.; Kempher, M.L.; Wu, L.; Wang, Y.; Liao, L.; et al. Environmental filtering decreases with fish development for the assembly of gut microbiota. Environ. Microbiol. 2016, 18, 4739–4754. [Google Scholar] [CrossRef]
- Stephens, W.Z.; Adam, R.B.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J.M. The composition of the zebrafish intestinal microbial community varies across development. ISME J. 2016, 10, 644–654. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]

| (a) | |||
| Group | Fat Content of Feed | B. subtilisContent of Feed | |
| CON | 4% | 0 | |
| HFD | 8% | 0 | |
| BS.HFD | 8% | 107 CFU/g | |
| (b) | |||
| Ingredient | Content (g/kg) | Composition | Content (% DM) |
| Fish meal | 80 | Basal diet | |
| Soybean meal | 240 | Crude protein | 29.83 |
| Rapeseed meal | 340 | Crude lipid | 4.39 |
| Wheat Flour | 250 | Moisture | 10.16 |
| Soybean oil | 6 | Ash | 8.32 |
| Ca(H2PO4)2 | 20 | ||
| Vitamin premix1 | 1 | High-fat diet | |
| Mineral premix2 | 3 | Crude protein | 29.74 |
| NaCl | 2 | Crude lipid | 8.45 |
| Choline chloride | 2 | Moisture | 10.13 |
| Cellulose | 56 | Ash | 8.38 |
| High-fat + B. subtilis diet | |||
| Crude protein | 29.66 | ||
| Crude lipid | 8.42 | ||
| Moisture | 10.24 | ||
| Total | 1000 | Ash | 8.42 |
| Growth Index | Group | ||
|---|---|---|---|
| CON | HFD | BS.HFD | |
| Initial weight | 60.58 ± 5.52 | 60.58 ± 5.52 | 60.58 ± 5.52 |
| FBW | 94.16 ± 3.96 a | 119.9 ± 6.86 b | 100.31 ± 5.31 a |
| WGR | 55.4 ± 3.35 a | 97.9 ± 8.73 c | 65.6 ± 4.58 b |
| SGR | 0.79 ± 0.05 a | 1.22 ± 0.09 b | 0.9 ± 0.04 a |
| CF | 1.98 ± 0.04 | 2.03 ± 0.05 | 1.91 ± 0.03 |
| HSI | 3.30 ± 0.14 b | 3.39 ± 0.026 b | 2.57 ± 0.13 a |
| VSI | 12.81 ± 0.60 b | 13.26 ± 1.05 b | 10.74 ± 1.17 a |
| Serum Biochemical Indices | Group | ||
|---|---|---|---|
| CON | HFD | BS.HFD | |
| Cholesterol (CHO) | 10.64 ± 0.56 | 9.8 ± 0.45 | 9.57 ± 0.15 |
| Albumin (ALB) | 19.98 ± 1.38 | 21.3 ± 0.5 | 20.01 ± 0.83 |
| Total Protein (TP) | 32.85 ± 0.19 a | 37.11 ± 1.06 b | 31.62 ± 0.75 a |
| Triglyceride (TG) | 9.02 ± 0.12 b | 7.99 ± 0.29 a | 7.27 ± 0.05 a |
| Aspartate aminotransferase (AST) | 82.98 ± 7.2 | 97.74 ± 6.49 | 92.87 ± 6.94 |
| Alanine aminotransferase (ALT) | 10.45 ± 0.6 a | 16.88 ± 1.55 b | 11.29 ± 0.42 a |
| High-density lipoprotein (HDL) | 2.12 ± 0.02 a | 2.59 ± 0.08 b | 2.69 ± 0.15 b |
| Low-density lipoprotein (LDL) | 3.72 ± 0.08 a,b | 3.86 ± 0 b | 3.58 ± 0.12 a,b |
| Alpha Diversity Index | Group | ||
|---|---|---|---|
| CON | HFD | BS.HFD | |
| Simpson | 0.89 ± 0.02 | 0.95 ± 0.01 | 0.9 ± 0.02 |
| Chao1 | 883.35 ± 50.61 a | 1278.38 ± 52.91 b | 904.18 ± 28.48 a |
| ACE | 893.77 ± 50.42 a | 1285.04 ± 48.21 b | 913.47 ± 29.6 a |
| Shannon | 5.68 ± 0.33 | 6.57 ± 0.16 | 5.8 ± 0.27 |
| (a) | |||||
| CON | HFD | BS.HFD | |||
| Phylum | Percent (%) | Phylum | Percent (%) | Phylum | Percent (%) |
| Bacteroidetes | 32.77% | Fusobacteria | 51.76% | Fusobacteria | 45.66% |
| Firmicutes | 30.45% | Proteobacteria | 16.61% | Bacteroidetes | 23.48% |
| Proteobacteria | 18.28% | Firmicutes | 15.48% | Proteobacteria | 12.32% |
| Fusobacteria | 13.23% | Bacteroidetes | 14.46% | Firmicutes | 9.41% |
| Verrucomicrobia | 3.72% | Verrucomicrobia | 1.28% | Verrucomicrobia | 6.89% |
| Tenericutes | 1.95% | ||||
| (b) | |||||
| CON | HFD | BS.HFD | |||
| Genus | Percent (%) | Genus | Percent (%) | Genus | Percent (%) |
| Bacteroides | 32.33% | Cetobacterium | 48.14% | Cetobacterium | 41.57% |
| Unclassified_ Erysipelotrichaceae | 23.65% | Bacteroides | 14.04% | Bacteroides | 23.09% |
| Unclassified_ Enterobacteriaceae | 13.04% | Unclassified_ Enterobacteriaceae | 11.34% | Unclassified_ Enterobacteriaceae | 9.45% |
| Fusobacterium | 6.02% | Unclassified_ Erysipelotrichaceae | 8.78% | Akkermansia | 6.89% |
| Cetobacterium | 5.54% | Unclassified_ Ruminococcaceae | 3.04% | Unclassified_ Erysipelotrichaceae | 6.23% |
| Akkermansia | 3.72% | Fusobacterium | 1.89% | Fusobacterium | 3.11% |
| Unclassified_ Lachnospiraceae | 2.10% | Plesiomonas | 1.70% | Unclassified_ CK-1C4-19 | 1.93% |
| Plesiomonas | 1.24% | Akkermansia | 1.28% | Unclassified_ Peptostreptococcaceae | 1.11% |
| Unclassified_ Aeromonadaceae | 1.21% | Citrobacter | 1.22% | Unclassified_ Aeromonadaceae | 1.07% |
| Unclassified_ Ruminococcaceae | 1.15% | Unclassified_ Fusobacteriaceae | 1.17% | ||
| Citrobacter | 1.11% | Dorea | 1.09% | ||
| Unclassified_ Fusobacteriaceae | 1.11% | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Xie, M.; Xiao, H.; Xu, L.; Zhang, S.; Chen, X.; Wu, Z. Bacillus subtilis Supplementation in a High-Fat Diet Modulates the Gut Microbiota and Ameliorates Hepatic Lipid Accumulation in Grass Carp (Ctenopharyngodon idella). Fishes 2022, 7, 94. https://doi.org/10.3390/fishes7030094
Guo D, Xie M, Xiao H, Xu L, Zhang S, Chen X, Wu Z. Bacillus subtilis Supplementation in a High-Fat Diet Modulates the Gut Microbiota and Ameliorates Hepatic Lipid Accumulation in Grass Carp (Ctenopharyngodon idella). Fishes. 2022; 7(3):94. https://doi.org/10.3390/fishes7030094
Chicago/Turabian StyleGuo, Daoyuan, Mengqi Xie, Hang Xiao, Lili Xu, Shiyu Zhang, Xiaoxuan Chen, and Zhixin Wu. 2022. "Bacillus subtilis Supplementation in a High-Fat Diet Modulates the Gut Microbiota and Ameliorates Hepatic Lipid Accumulation in Grass Carp (Ctenopharyngodon idella)" Fishes 7, no. 3: 94. https://doi.org/10.3390/fishes7030094





