eDNA and Acoustic Tag Monitoring Reveal Congruent Overwintering Distributions of Striped Bass in a Hydrologically Complex Estuarine Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Work
2.2.1. Water Collection
2.2.2. River Depth and Flow
2.2.3. Acoustic Tag Detection
2.3. Filtration and DNA Extraction
2.4. PCR and qPCR
2.4.1. Tracer Production and Release
2.4.2. qPCR Assays
2.5. Statistical Analysis
3. Results
3.1. qPCR Assay Validation
3.2. Acoustic Tag Detection
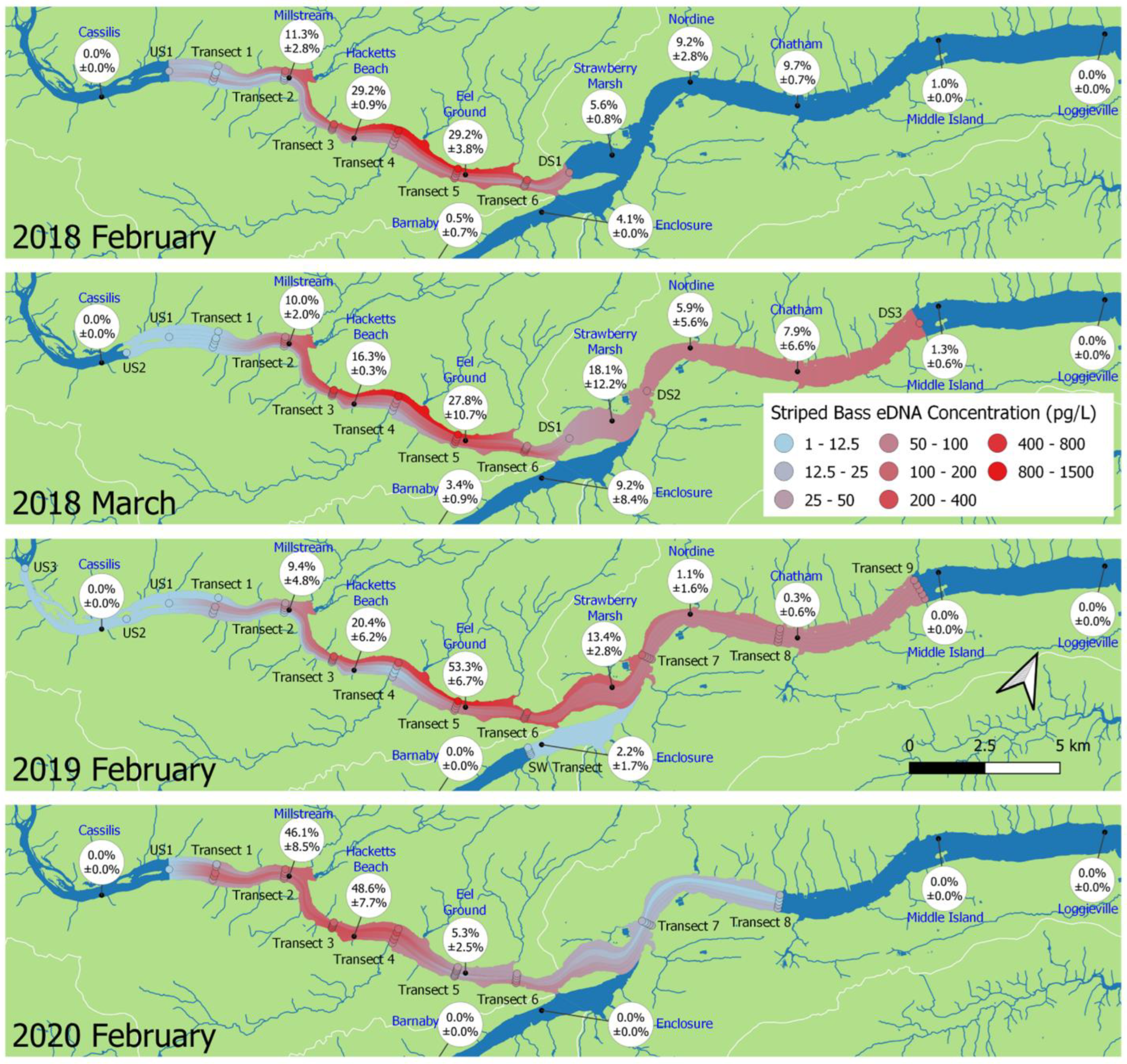
3.3. Interannual Comparisons of eDNA Distribution
3.4. Effect of Sampling Month
3.5. Effect of Sampling Depth
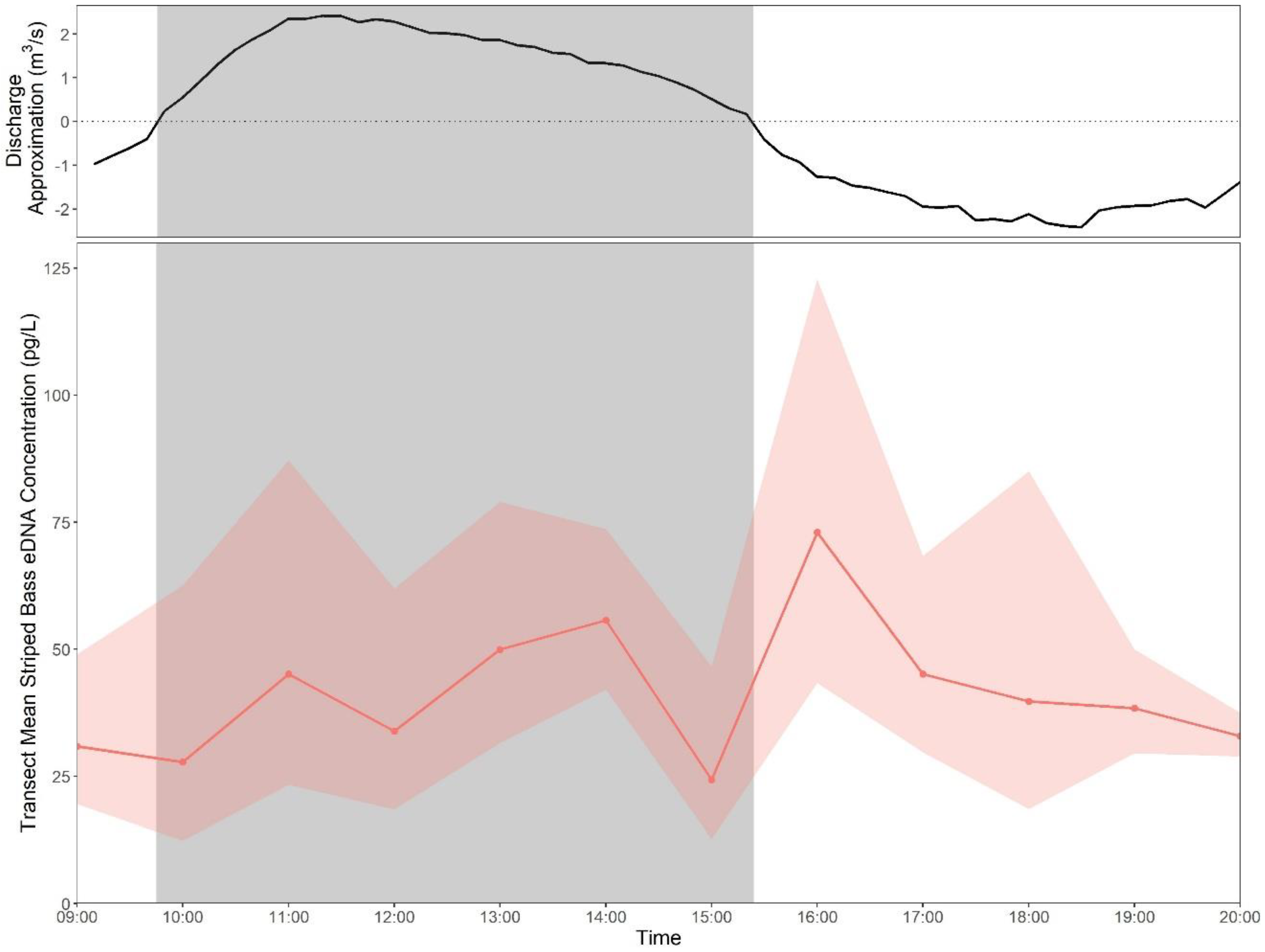
3.6. Effect of Tides
3.6.1. Tidal Time Series
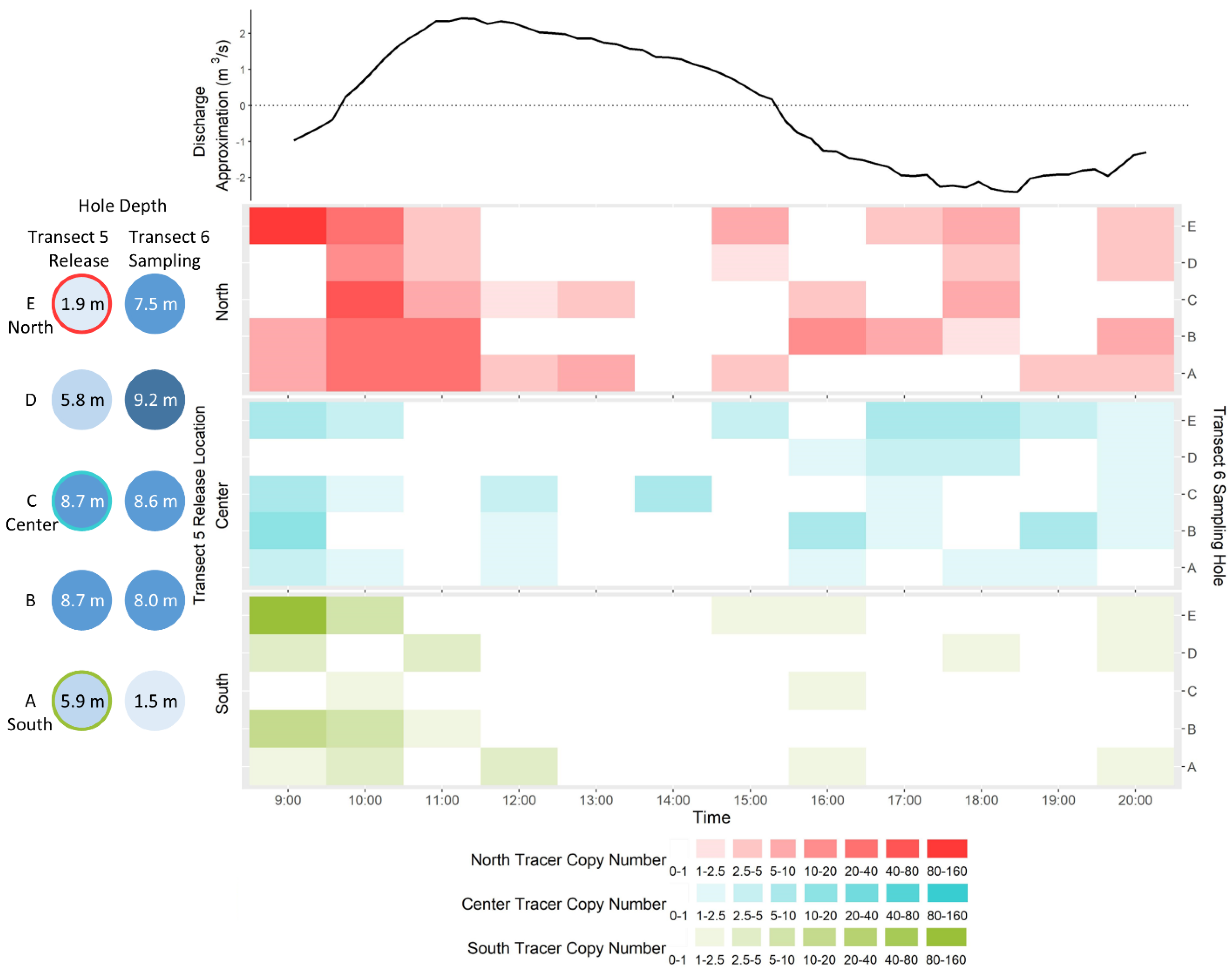
3.6.2. DNA Tracers
4. Discussion
4.1. eDNA Distribution
4.2. eDNA Recirculation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douglas, S.G.; Bradford, R.G.; Chaput, G. Assessment of Striped Bass (Morone saxatilis) in the Maritime Provinces in the Context of Species at Risk. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2003. [Google Scholar]
- Fisheries and Oceans Canada (DFO). Update of Spawner Abundance and Biological Characteristics of Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence to 2018. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2019; p. 9. [Google Scholar]
- COSEWIC. Assessment and Status Report on the Striped Bass Morone saxatilis in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2004. [Google Scholar]
- Wirgin, I.; Ong, T.-L.; Maceda, L.; Waldman, J.R.; Moore, D.; Courtenay, S. Mitochondrial DNA Variation in Striped Bass (Morone saxatilis) from Canadian Rivers. Can. J. Fish. Aquat. Sci. 1993, 50, 80–87. [Google Scholar] [CrossRef]
- Fisheries and Oceans Canada (DFO). Spawner Abundance and Biological Characteristics of Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence in 2017. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2018; p. 9. [Google Scholar]
- Andrews, S.N.; Buhariwalla, C.F.; Fleet-Pardy, B.; Dadswell, M.J.; Linnansaari, T.; Curry, R.A. Left out in the Cold: The Understudied Overwintering Ecology of Striped Bass in Canada. Environ. Biol. Fishes 2019, 102, 499–518. [Google Scholar] [CrossRef]
- Bradford, R.G.; Tremblay, E.; Chaput, G. Winter Distribution of Striped Bass (Morone saxatilis) and Associated Environmental Conditions in Kouchibouguac National Park during 1996–1997; Canadian Heritage, Parks Canada: Gatineau, QC, Canada, 1998; Volume 59. [Google Scholar]
- Rulifson, R.A.; Dadswell, M.J. Life History and Population Characteristics of Striped Bass in Atlantic Canada. Trans. Am. Fish. Soc. 1995, 124, 477–507. [Google Scholar] [CrossRef]
- Bradford, R.G.; Chaput, G. Status of Striped Bass (Morone saxatilis) in the Gulf of St. Lawrence in 1996 and Revised Estimates of Spawner Abundance for 1994 and 1995. In CSAS, Canadian Stock Assessment Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 1997; pp. 1–31. [Google Scholar]
- Buhariwalla, C.F.; Macmillan, J.L.; Gregoire, M.J.; Dadswell, M.J.; Stokesbury, M.J.W. Population Characteristics of Striped Bass Killed by Cold Shock during Winter Shutdown of a Power Plant in Nova Scotia. Northeast. Nat. 2016, 23, 163–173. [Google Scholar] [CrossRef]
- Douglas, S.G.; Chaput, G.; Caissie, D. Assessment of Status and Recovery Potential for Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence. In Science Advisory Secretariat Research Document; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2006; p. 103. [Google Scholar]
- Andrews, S.N.; Linnansaari, T.; Curry, R.A.; Leblanc, N.M.; Pavey, S.A. Winter Ecology of Striped Bass (Morone saxatilis) near Its Northern Limit of Distribution in the Saint John River, New Brunswick. Environ. Biol. Fishes 2020, 103, 1343–1358. [Google Scholar] [CrossRef]
- Bradford, R.G.; Chaput, G. The Status of Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence in 1995; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 1996; Volume 1996.
- Robichaud-LeBlanc, K.A.; Courtenay, S.; Locke, A. Spawning and Early Life History of a Northern Population of Striped Bass (Morone saxatilis) in the Miramichi River Estuary, Gulf of St. Lawrence. Can. J. Zool. 1996, 74, 1645–1655. [Google Scholar] [CrossRef]
- Douglas, S.G.; Chaput, G.; Hayward, J.; Sheasgreen, J. Prespawning, Spawning, and Postspawning Behavior of Striped Bass in the Miramichi River. Trans. Am. Fish. Soc. 2009, 138, 121–134. [Google Scholar] [CrossRef]
- Fisheries and Oceans Canada (DFO). Update to 2012 on Spawner Abundance and Biological Characteristics for Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2013. [Google Scholar]
- COSEWIC. Assessment and Status Report on the Striped Bass Morone saxatilis in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2012. [Google Scholar]
- Douglas, S.G.; Chaput, G. Assessment and Status of Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence, 2006 to 2010. In CSAS, Canadian Stock Assessment Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2011. [Google Scholar]
- Fisheries and Oceans Canada (DFO). Recreation Fishery Catches, Spawner Abundance, and Biological Characteristics of Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence in 2014. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2015. [Google Scholar]
- Fisheries and Oceans Canada (DFO). Spawner Abundance and Biological Characteristics of Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence in 2016. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2017. [Google Scholar]
- Fisheries and Oceans Canada (DFO). Recreational Fishery Catches, Spawner Abundance, and Biological Characteristics of Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence in 2013. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2014. [Google Scholar]
- Fisheries and Oceans Canada (DFO). Spawner Abundance and Biological Characteristics of Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence in 2015. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2016. [Google Scholar]
- Chaput, G.; Douglas, S.G. Hierarchical Bayesian Model to Estimate the Spawning Stock of Striped Bass (Morone saxatilis) in the Northwest Miramichi River, 1994 to 2010. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2011; Volume iv, 51p. [Google Scholar]
- Fisheries and Oceans Canada (DFO). Update of Spawner Abundance and Biological Characteristics of Striped Bass (Morone saxatilis) in the Southern Gulf of St. Lawrence to 2019. In Canadian Science Advisory Secretariat; Canada Department of Fisheries and Oceans: Ottawa, ON, Canada, 2020; pp. 1–9. [Google Scholar]
- Khalsa, N.S.; Smith, J.; Jochum, K.A.; Savory, G.; López, J.A. Identifying Under-Ice Overwintering Locations of Juvenile Chinook Salmon by Using Environmental DNA. N. Am. J. Fish. Manag. 2020, 40, 762–772. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An Emerging Tool in Conservation for Monitoring Past and Present Biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Strickler, K.M.; Pilliod, D.S. Moving Environmental DNA Methods from Concept to Practice for Monitoring Aquatic Macroorganisms. Biol. Conserv. 2015, 183, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Rees, H.C.; Maddison, B.C.; Middleditch, D.J.; Patmore, J.R.M.; Gough, K.C. The Detection of Aquatic Animal Species Using Environmental DNA—A Review of eDNA as a Survey Tool in Ecology. J. Appl. Ecol. 2014, 51, 1450–1459. [Google Scholar] [CrossRef]
- Doi, H.; Uchii, K.; Takahara, T.; Matsuhashi, S.; Yamanaka, H.; Minamoto, T. Use of Droplet Digital PCR for Estimation of Fish Abundance and Biomass in Environmental DNA Surveys. PLoS ONE 2015, 10, e0122763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klymus, K.E.; Richter, C.A.; Chapman, D.C.; Paukert, C. Quantification of eDNA Shedding Rates from Invasive Bighead Carp Hypophthalmichthys nobilis and Silver Carp Hypophthalmichthys molitrix. Biol. Conserv. 2015, 183, 77–84. [Google Scholar] [CrossRef]
- Lacoursière-Roussel, A.; Rosabal, M.; Bernatchez, L. Estimating Fish Abundance and Biomass from eDNA Concentrations: Variability among Capture Methods and Environmental Conditions. Mol. Ecol. Resour. 2016, 16, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Minamoto, T.; Yamanaka, H.; Doi, H.; Kawabata, Z. Estimation of Fish Biomass Using Environmental DNA. PLoS ONE 2012, 7, e35868. [Google Scholar] [CrossRef] [Green Version]
- Lacoursière-Roussel, A.; Côté, G.; Leclerc, V.; Bernatchez, L. Quantifying Relative Fish Abundance with eDNA: A Promising Tool for Fisheries Management. J. Appl. Ecol. 2016, 53, 1148–1157. [Google Scholar] [CrossRef]
- Wilcox, T.M.; McKelvey, K.S.; Young, M.K.; Sepulveda, A.J.; Shepard, B.B.; Jane, S.F.; Whiteley, A.R.; Lowe, W.H.; Schwartz, M.K. Understanding Environmental DNA Detection Probabilities: A Case Study Using a Stream-Dwelling Char Salvelinus fontinalis. Biol. Conserv. 2016, 194, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Pilliod, D.S.; Goldberg, C.S.; Arkle, R.S.; Waits, L.P. Estimating Occupancy and Abundance of Stream Amphibians Using Environmental DNA from Filtered Water Samples. Can. J. Fish. Aquat. Sci. 2013, 70, 1123–1130. [Google Scholar] [CrossRef]
- Barnes, M.A.; Turner, C.R. The Ecology of Environmental DNA and Implications for Conservation Genetics. Conserv. Genet. 2016, 17, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Pilliod, D.S.; Goldberg, C.S.; Arkle, R.S.; Waits, L.P. Factors Influencing Detection of eDNA from a Stream-Dwelling Amphibian. Mol. Ecol. Resour. 2014, 14, 109–116. [Google Scholar] [CrossRef]
- Strickler, K.M.; Fremier, A.K.; Goldberg, C.S. Quantifying Effects of UV-B, Temperature, and pH on eDNA Degradation in Aquatic Microcosms. Biol. Conserv. 2015, 183, 85–92. [Google Scholar] [CrossRef]
- Tillotson, M.D.; Kelly, R.P.; Duda, J.J.; Hoy, M.; Kralj, J.; Quinn, T.P. Concentrations of Environmental DNA (eDNA) Reflect Spawning Salmon Abundance at Fine Spatial and Temporal Scales. Biol. Conserv. 2018, 220, 1–11. [Google Scholar] [CrossRef]
- Yates, M.C.; Fraser, D.J.; Derry, A.M. Meta-analysis Supports Further Refinement of eDNA for Monitoring Aquatic Species-specific Abundance in Nature. Environ. DNA 2019, 1, 5–13. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada (ECCC). Daily Discharge Graph for Northwest Miramichi River at Trout Brook (01BQ001) [NB]. Historical Hydrometric Data. 2022. Available online: https://wateroffice.ec.gc.ca/report/historical_e.html?stn=01BQ001 (accessed on 5 May 2022).
- Vilks, G.; Krauel, D.P. Environmental Geology of the Miramichi Estuary: Physical Oceanography; Geological Survey of Canada: Ottawa, ON, Canada, 1982; Volume 81, pp. 1–53.
- Feng, W.; Bulté, G.; Lougheed, S.C. Environmental DNA Surveys Help to Identify Winter Hibernacula of a Temperate Freshwater Turtle. Environ. DNA 2020, 2, 200–209. [Google Scholar] [CrossRef]
- Minamoto, T.; Hayami, K.; Sakata, M.K.; Imamura, A. Real-Time Polymerase Chain Reaction Assays for Environmental DNA Detection of Three Salmonid Fish in Hokkaido, Japan: Application to Winter Surveys. Ecol. Res. 2019, 34, 237–242. [Google Scholar] [CrossRef]
- Kelly, R.P.; Gallego, R.; Jacobs-Palme, E. The Effect of Tides on Nearshore Environmental DNA. PeerJ 2018, 6, e4521. [Google Scholar] [CrossRef] [Green Version]
- Lafleur, C.; Pettigrew, B.; Booth, D.A.; Chadwick, M. Chapter 4 Seasonal and Short-Term Variations in the Estuarine Structure of the Miramichi. Can. Spec. Publ. Fish. Aquat. Sci. 1995, 123, 45–72. [Google Scholar]
- Schwentner, M.; Zahiri, R.; Yamamoto, S.; Husemann, M.; Kullmann, B.; Thiel, R. eDNA as a Tool for Non-Invasive Monitoring of the Fauna of a Turbid, Well-Mixed System, the Elbe Estuary in Germany. PLoS ONE 2021, 16, e0250452. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Garcia-Vedrenne, A.E.; McLaughlin, J.P.; Childress, J.N.; Morse, M.F.; Jerde, C.L. At Palmyra Atoll, the Fish-Community Environmental DNA Signal Changes across Habitats but Not with Tides. J. Fish Biol. 2021, 98, 415–425. [Google Scholar] [CrossRef]
- Ely, T.; Barber, P.H.; Man, L.; Gold, Z. Short-Lived Detection of an Introduced Vertebrate eDNA Signal in a Nearshore Rocky Reef Environment. PLoS ONE 2021, 16, e0245314. [Google Scholar] [CrossRef]
- Merkes, C.; Klymus, K.; Allison, M.; Goldberg, C.; Helbing, C.; Hunter, M.; Jackson, C.; Lance, R.; Mangan, A.; Monroe, E.; et al. Generic qPCR Limit of Detection (LOD)/Limit of Quantification (LOQ) Calculator. R Script 2019. [Google Scholar] [CrossRef]
- Klymus, K.E.; Merkes, C.M.; Allison, M.J.; Goldberg, C.S.; Helbing, C.C.; Hunter, M.E.; Jackson, C.A.; Lance, R.F.; Mangan, A.M.; Monroe, E.M.; et al. Reporting the Limits of Detection and Quantification for Environmental DNA Assays. Environ. DNA 2019, 2, 271–282. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 14 May 2021).
- Fox, J.; Sanford, W. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Length, R.V. Least-Squares Means: The R Package Lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Gplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Auguie, B. GridExtra: Miscellaneous Functions for “Grid” Graphics. R Package Version 2.3. 2017. Available online: https://CRAN.R-project.org/package=gridExtra (accessed on 14 May 2021).
- QGIS Geographic Information System. QGIS Association, 2021. Available online: http://www.qgis.org (accessed on 13 December 2021).
- Service New Brunswick, New Brunswick Hydrographic Network (NBHN). GeoNB. Available online: http://www.snb.ca/geonb1/e/DC/NBHN.asp (accessed on 20 September 2018).
- Curtis, A.N.; Larson, E.R.; Davis, M.A. Field Storage of Water Samples Affects Measured Environmental DNA Concentration and Detection. Limnology 2021, 22, 1–4. [Google Scholar] [CrossRef]
- Eichmiller, J.J.; Best, S.E.; Sorensen, P.W. Effects of Temperature and Trophic State on Degradation of Environmental DNA in Lake Water. Environ. Sci. Technol. 2016, 50, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Pont, D.; Rocle, M.; Valentini, A.; Civade, R.; Jean, P.; Maire, A.; Roset, N.; Schabuss, M.; Zornig, H.; Dejean, T. Environmental DNA Reveals Quantitative Patterns of Fish Biodiversity in Large Rivers despite Its Downstream Transportation. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Deiner, K.; Altermatt, F. Transport Distance of Invertebrate Environmental DNA in a Natural River. PLoS ONE 2014, 9, e88786. [Google Scholar] [CrossRef] [Green Version]
- Villacorta-Rath, C.; Hoskin, C.J.; Strugnell, J.M.; Burrows, D. Long Distance (>20 km) Downstream Detection of Endangered Stream Frogs Suggests an Important Role for eDNA in Surveying for Remnant Amphibian Populations. PeerJ 2021, 9, e12013. [Google Scholar] [CrossRef]
- Douglas, S. Personal Communication; DFO: Moncton, NB, Canada, 2022.
- Environment and Climate Change Canada (ECCC). Daily Data Report for Miramichi RCS New Brunswick. Historical Weather and Climate Data. 2022. Available online: https://climate.weather.gc.ca/climate_data/daily_data_e.html (accessed on 5 May 2022).
- Hurst, T.P.; Conover, D.O. Activity-Related Constraints on Overwintering Young-of-the-Year Striped Bass (Morone saxatilis). Can. J. Zool. 2001, 79, 129–136. [Google Scholar] [CrossRef]



| Amplicon | Tracer Length (bp) | Quantity Released (Copies × 1012) | Target Length (bp) | Primers and Probes |
|---|---|---|---|---|
| Striped Bass COI | F: 5′-TGCTTCCCCCATCCTTCCTC-3′ | |||
| - | - | 91 | R: 5′-TGCTTGCAAGAGGGGGGTAA-3′ | |
| Probe: 5′ FAM- TCTAGCTTCTTCAGGTGTC-MGB | ||||
| Tracer Production | - | - | See Tracer Lengths | F: 5′-GAGCGGATACATATTTGAATGTATTTAGA-3′ |
| R: 5′-TGATAATCTCATGACCAAAATCCCTT-3′ | ||||
| North Tracer Detection | F: 5′-CCACTAGCCGGCAACCTAGC-3′ | |||
| 2032 | 96.78 | 170 | R: 5′-GATCATACGAACAGAGGTGTTTGG-3′ | |
| Probe: 5′ FAM-CCTCAGTAGACCTAACTAT-MGB | ||||
| Center Tracer Detection | F: 5′-AGGTCTCGTAGGATCATCTCTTGGAT-3′ | |||
| 2311 | 53.82 | 161 | R: 5′-ATGTAATGTTCGGCACGGGTAGTT-3′ | |
| Probe: 5′ FAM-CCGGAGTAATGATTGAT-MGB | ||||
| South Tracer Detection | F: 5′-GGATTCGGGACGACCCG-3′ | |||
| 2192 | 84.65 | 149 | R: 5′-CAATCAATTTCCGAATCCTCCACT-3′ | |
| Probe: 5′ FAM-CGCCGGTAGCTCT-MGB |
| Assay | Equation | Adjusted R2 | Efficiency | LOD | LOQ |
|---|---|---|---|---|---|
| Striped Bass | eDNA Conc. = 10(Ct − 32.39)/−3.99 | 0.9931 | 78.0% | 12.4 pg/L | 46.0 pg/L |
| North Tracer | DNA Copies = 10(Ct − 43.20)/−3.90 | 0.9990 | 80.3% | 28.1 Copies | 80.0 Copies |
| Center Tracer | DNA Copies = 10(Ct − 41.46)/−3.74 | 0.9956 | 85.2% | 52.5 Copies | 52.5 Copies |
| South Tracer | DNA Copies = 10(Ct − 38.58)/−3.63 | 0.9987 | 88.7% | 48.2 Copies | 232 Copies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, M.; Brodeur, N.; LeBlanc, F.; Douglas, S.; Chamberland, P.; Guyondet, T.; Steeves, R.; Gagné, N. eDNA and Acoustic Tag Monitoring Reveal Congruent Overwintering Distributions of Striped Bass in a Hydrologically Complex Estuarine Environment. Fishes 2022, 7, 183. https://doi.org/10.3390/fishes7040183
Harris M, Brodeur N, LeBlanc F, Douglas S, Chamberland P, Guyondet T, Steeves R, Gagné N. eDNA and Acoustic Tag Monitoring Reveal Congruent Overwintering Distributions of Striped Bass in a Hydrologically Complex Estuarine Environment. Fishes. 2022; 7(4):183. https://doi.org/10.3390/fishes7040183
Chicago/Turabian StyleHarris, Michaela, Nathalie Brodeur, Francis LeBlanc, Scott Douglas, Paul Chamberland, Thomas Guyondet, Royce Steeves, and Nellie Gagné. 2022. "eDNA and Acoustic Tag Monitoring Reveal Congruent Overwintering Distributions of Striped Bass in a Hydrologically Complex Estuarine Environment" Fishes 7, no. 4: 183. https://doi.org/10.3390/fishes7040183






