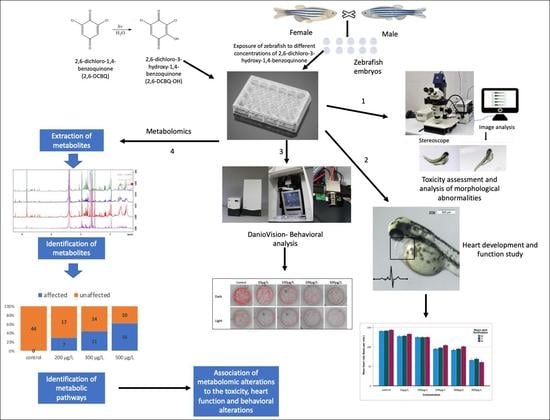
Untargeted Metabolomics Associated with Behavioral and Toxicological Studies Yield Insights into the Impact of 2,6-Dichloro-3-hydroxy-1,4-benzoquinone Disinfection By-Product on Zebrafish Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Housing and Husbandry
2.3. Zebrafish Toxicity Testing
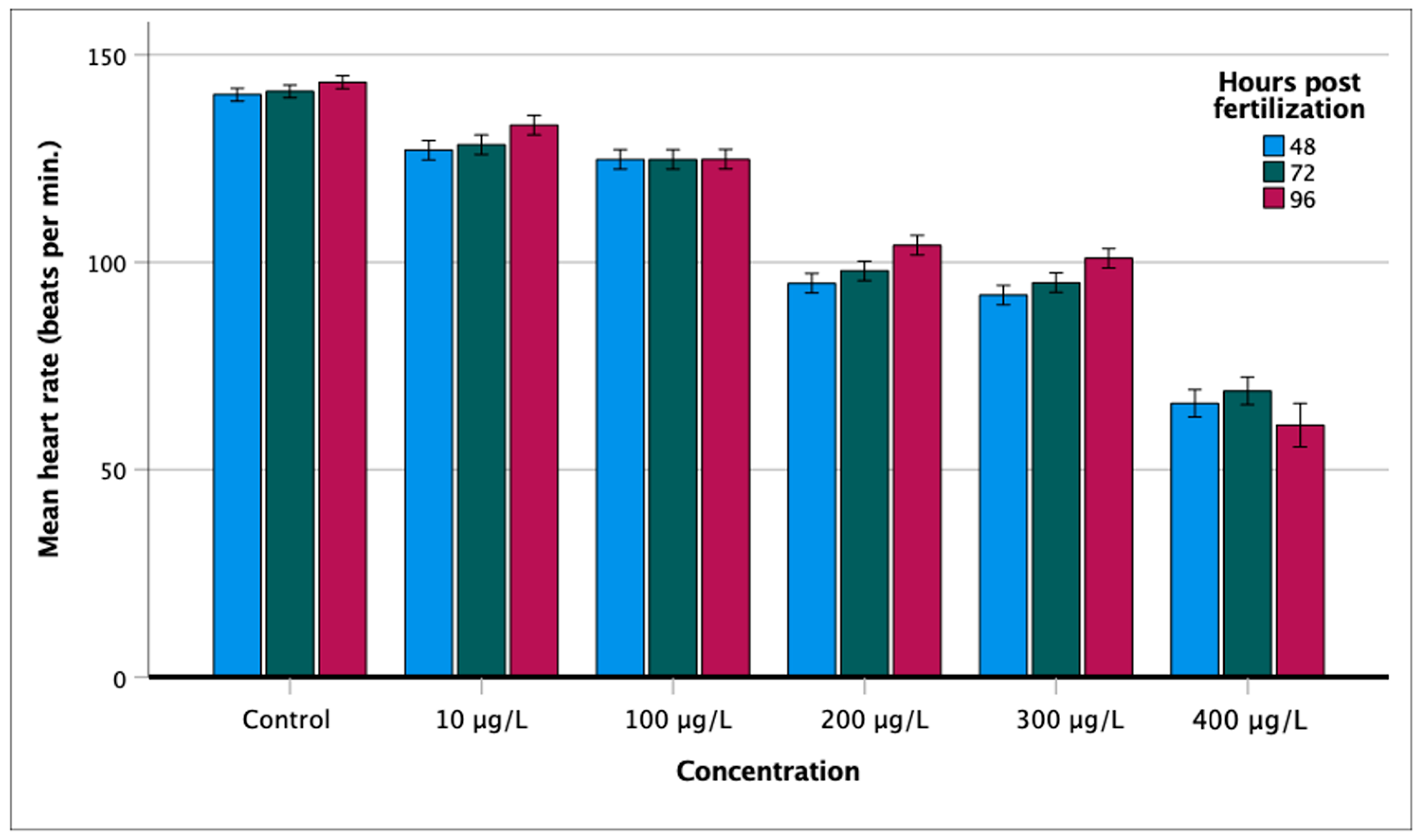
2.4. Heart Rate
2.5. Behavior Screening
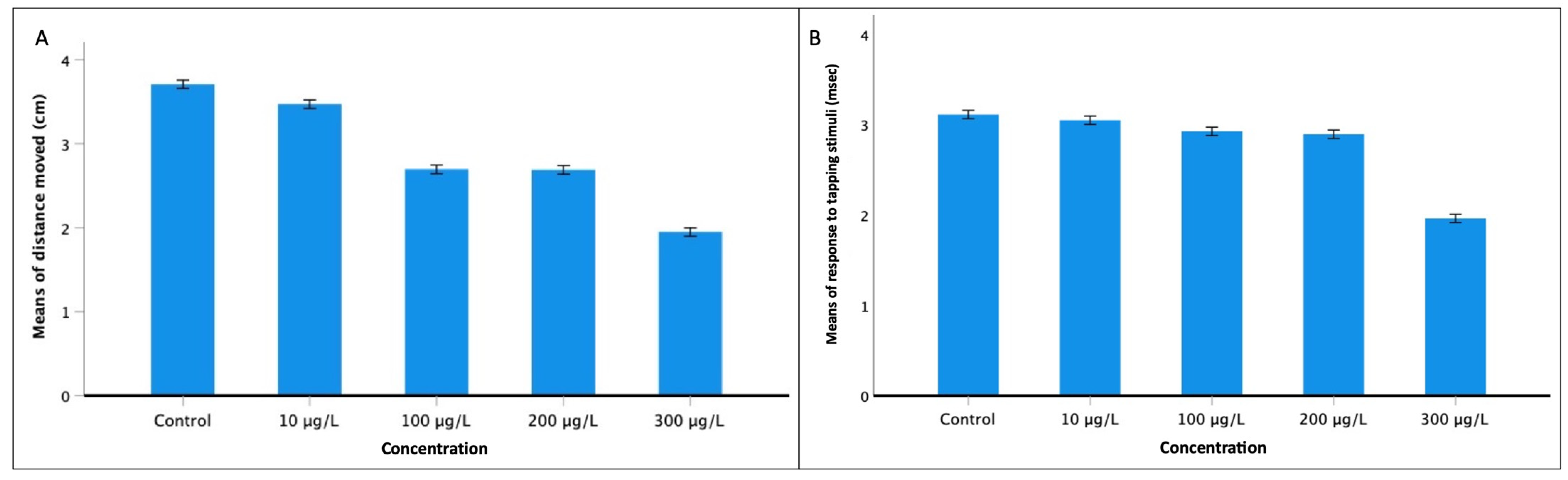
2.5.1. Touching Motor Response (TMR)
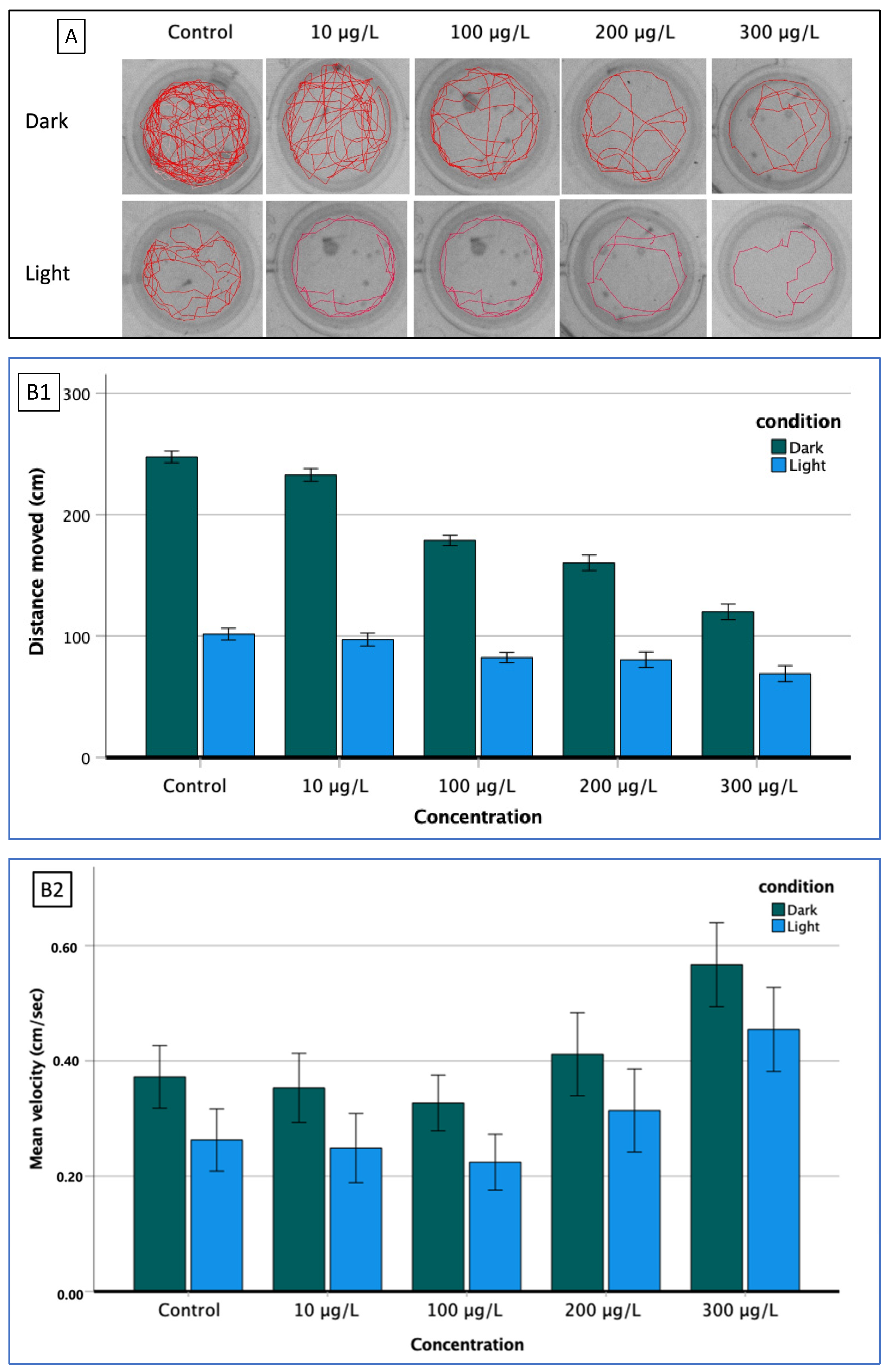
2.5.2. Locomotor Activity
2.6. Metabolomic Study and Data Processing
2.6.1. Metabolite Extraction
2.6.2. Metabolome Study and Data Processing
2.7. Statistical Analysis
3. Results
3.1. Morphological Abnormalities
3.2. Effect on Heart Rate
3.3. Behavioral Analysis
3.3.1. Larval Activity (BLA, VMR)
3.3.2. Touch Motor Response (TMR) and Vibrational Startle Response (VSR)
3.4. Metabolomic Study
- ✓
- The metabolism of essential amino acids in exposed individuals is downregulated. Glutathione metabolism is upregulated and biosynthesis of arginine is activated in specimens exposed to 2,6-DCBQ-OH.
- ✓
- The metabolic pathway of the synthesis of unsaturated fatty acids appeared only in the control group (Table 1). The pathway of elongation of fatty acids and the butyric acid metabolism pathway are downregulated in specimens exposed to 2,6-DCBQ-OH.
- ✓
- The pathway of linoleic acid is detected only in the individuals that were not exposed to 2,6-DCBQ-OH.
4. Discussion
4.1. Developmental and Behavioral Alterations
4.2. Metabolic Alterations
4.2.1. Beta-Alanine Metabolism
4.2.2. Glutathione Metabolism
4.2.3. Biosynthesis of Unsaturated Fatty Acids and Glycerophospholipids
4.2.4. Elongation of Fatty Acids
4.2.5. Metabolism of Linoleic Acid
4.2.6. Butanoate (Butyric Acid) Metabolism
4.2.7. Other Metabolic Changes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, H.; Li, J.; Moe, B.; McGuigan, C.F.; Shen, S.; Li, X.-F. Cytotoxicity and Oxidative Damage Induced by Halobenzoquinones to T24 Bladder Cancer Cells. Environ. Sci. Technol. 2013, 47, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, W.; Moe, B.; Wang, H.; Li, X.F. Chemical and Toxicological Characterization of Halobenzoquinones, an Emerging Class of Disinfection Byproducts. Chem. Res. Toxicol. 2015, 28, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Anichina, J.; Lu, X.; Bull, R.J.; Krasner, S.W.; Hrudey, S.E.; Li, X.F. Occurrence and Formation of Chloro- and Bromo-Benzoquinones during Drinking Water Disinfection. Water Res. 2012, 46, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qian, Y.; Li, J.; Moe, B.; Huang, R.; Zhang, H.; Hrudey, S.E.; Li, X.F. Analytical and Toxicity Characterization of Halo-Hydroxyl-Benzoquinones as Stable Halobenzoquinone Disinfection Byproducts in Treated Water. Anal. Chem. 2014, 86, 4982–4988. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.; Mohan, A.; Reckhow, D.A.; Godri Pollitt, K.J. Assessment of the in Vitro Toxicity of the Disinfection Byproduct 2,6-Dichloro-1,4-Benzoquinone and Its Transformed Derivatives. Chemosphere 2019, 234, 902–908. [Google Scholar] [CrossRef]
- Wu, H.; Long, K.; Sha, Y.; Lu, D.; Xia, Y.; Mo, Y.; Yang, Q.; Zheng, W.; Yang, M.; Wei, X. Occurrence and Toxicity of Halobenzoquinones as Drinking Water Disinfection Byproducts. Sci. Total Environ. 2021, 770, 145277. [Google Scholar] [CrossRef]
- Wang, W.; Qian, Y.; Boyd, J.M.; Wu, M.; Hrudey, S.E.; Li, X.F. Halobenzoquinones in Swimming Pool Waters and Their Formation from Personal Care Products. Environ. Sci. Technol. 2013, 47, 3275–3282. [Google Scholar] [CrossRef]
- Prochazka, E.; Escher, B.I.; Plewa, M.J.; Leusch, F.D.L. In Vitro Cytotoxicity and Adaptive Stress Responses to Selected Haloacetic Acid and Halobenzoquinone Water Disinfection Byproducts. Chem. Res. Toxicol. 2015, 28, 2059–2068. [Google Scholar] [CrossRef]
- Hung, S. Evaluating the Toxicity and Formation of Halobenzoquinones in Point-of-Use Chlorinated Drinking Water. Masters Theses 2018, 734, 87. [Google Scholar] [CrossRef]
- Spitsbergen, J.M.; Kent, M.L. The State of the Art of the Zebrafish Model for Toxicology and Toxicologic Pathology Research--Advantages and Current Limitations. Toxicol. Pathol. 2007, 31, 62–87. [Google Scholar] [CrossRef]
- Kang, Y.-F.; Li, Y.-H.; Fang, Y.-W.; Xu, Y.; Wei, X.-M.; Yin, X.-B. Carbon Quantum Dots for Zebrafish Fluorescence Imaging. Sci. Rep. 2015, 5, 11835. [Google Scholar] [CrossRef] [Green Version]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation. Science 2010, 327, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Wixon, J. Danio rerio, the Zebrafish. Int. J. Genom. 2000, 17, 225–231. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, Tissue Distribution, and Toxicity of Polystyrene Nanoparticles in Developing Zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Wiecinski, P.N. Toxicity of Intact and Weathered Nanomaterials to Zebrafish. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2012. [Google Scholar]
- Chatzimitakos, T.G.; Pliatsika, C.; Chousidis, I.; Leonardos, I.D.; Stalikas, C.D. Metabolomic Profiling Unveils the Impact of Non-Doped and Heteroatom-Doped Carbon Nanodots on Zebrafish (Danio rerio) Embryos. Nanomaterials 2021, 11, 483. [Google Scholar] [CrossRef]
- Robertson, D.G. Metabonomics in Toxicology: A Review. Toxicol. Sci. 2005, 85, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Morello, J.; Derks, R.J.E.; Lopes, S.S.; Steenvoorden, E.; Monteiro, E.C.; Mayboroda, O.A.; Pereira, S.A. Zebrafish Larvae Are a Suitable Model to Investigate the Metabolic Phenotype of Drug-Induced Renal Tubular Injury. Front. Pharmacol. 2018, 9, 1193. [Google Scholar] [CrossRef] [Green Version]
- Bouhifd, M.; Hartung, T.; Hogberg, H.T.; Kleensang, A.; Zhao, L. Review: Toxicometabolomics. J. Appl. Toxicol. 2013, 33, 1365–1383. [Google Scholar] [CrossRef]
- Chousidis, I.; Chatzimitakos, T.; Leonardos, D.; Filiou, M.D.; Stalikas, C.D.; Leonardos, I.D. Cannabinol in the Spotlight: Toxicometabolomic Study and Behavioral Analysis of Zebrafish Embryos Exposed to the Unknown Cannabinoid. Chemosphere 2020, 252, 126417. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Chousidis, I.; Leonardos, D.; Stalikas, C.; Leonardos, I. In the Swim of Cannabis: Developmental Toxicity and Metabolomic Pathway Alterations of Zebrafish Larvae Exposed to THC for the Assessment of Its Potential Environmental and Human Health Impact. Molecules 2022, 27, 5506. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Zhang, Y.; Zhang, J.Y.; Lin, H.; Chen, J.; Hong, H. The Toxicity of 2,6-Dichlorobenzoquinone on the Early Life Stage of Zebrafish: A Survey on the Endpoints at Developmental Toxicity, Oxidative Stress, Genotoxicity and Cytotoxicity. Environ. Pollut. 2019, 245, 719–724. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Zheng, Q.; Moe, B.; Li, X.F. Halobenzoquinone-Induced Developmental Toxicity, Oxidative Stress, and Apoptosis in Zebrafish Embryos. Environ. Sci. Technol. 2018, 52, 10590–10598. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Xiang, P.; Tang, M.-H.; Sun, L.; Ma, L.Q. Arsenic Impacted the Development, Thyroid Hormone and Gene Transcription of Thyroid Hormone Receptors in Bighead Carp Larvae (Hypophthalmichthys nobilis). J. Hazard. Mater. 2016, 303, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; Haffter, P.; Odenthal, J.; Vogelsang, E.; Brand, M.; van Eeden, F.J.; Furutani-Seiki, M.; Granato, M.; Hammerschmidt, M.; Heisenberg, C.P.; et al. Mutations Affecting the Cardiovascular System and Other Internal Organs in Zebrafish. Development 1996, 123, 293–302. [Google Scholar] [CrossRef]
- Costa, B.P.D.; Moura, L.A.; Pinto, S.A.G.; Lima-Maximino, M.; Maximino, C. Zebrafish Models in Neural and Behavioral Toxicology across the Life Stages. Fishes 2020, 5, 23. [Google Scholar] [CrossRef]
- Levin, E.D.; Sledge, D.; Roach, S.; Petro, A.; Donerly, S.; Linney, E. Persistent Behavioral Impairment Caused by Embryonic Methylphenidate Exposure in Zebrafish. Neurotoxicol. Teratol. 2011, 33, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in Energy Metabolism: There Is Still More to Learn. Trends Endocrinol. Metab. 2021, 32, 159–169. [Google Scholar] [CrossRef]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring Anxiety in Zebrafish: A Critical Review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef]
- Costa, B.; Ferreira, S.; Póvoa, V.; Cardoso, M.J.; Vieira, S.; Stroom, J.; Fidalgo, P.; Rio-Tinto, R.; Figueiredo, N.; Parés, O.; et al. Developments in Zebrafish Avatars as Radiotherapy Sensitivity Reporters—Towards Personalized Medicine. EBioMedicine 2020, 51, 102578. [Google Scholar] [CrossRef]
- Maximino, C.; da Silva, A.W.B.; Gouveia, A.; Herculano, A.M. Pharmacological Analysis of Zebrafish (Danio rerio) Scototaxis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, F.; Noldus, L.P.J.J.; Tegelenbosch, R.A.J.; Richardson, M.K. Zebrafish Embryos and Larvae in Behavioural Assays. Behaviour 2012, 149, 1241–1281. [Google Scholar] [CrossRef]
- Tzima, E.; Serifi, I.; Tsikari, I.; Alzualde, A.; Leonardos, I.; Papamarcaki, T. Transcriptional and Behavioral Responses of Zebrafish Larvae to Microcystin-LR Exposure. Int. J. Mol. Sci. 2017, 18, 365. [Google Scholar] [CrossRef] [Green Version]
- Noyes, P.D.; Haggard, D.E.; Gonnerman, G.D.; Tanguay, R.L. Advanced Morphological—Behavioral Test Platform Reveals Neurodevelopmental Defects in Embryonic Zebrafish Exposed to Comprehensive Suite of Halogenated and Organophosphate Flame Retardants. Toxicol. Sci. 2015, 145, 177–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, J.; Oliveri, A.; Levin, E.D. Zebrafish Model Systems for Developmental Neurobehavioral Toxicology. Birth Defects Res. Part C—Embryo Today Rev. 2013, 99, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Chousidis, I.; Stalikas, C.D.; Leonardos, I.D. Induced Toxicity in Early-Life Stage Zebrafish (Danio rerio) and Its Behavioral Analysis after Exposure to Non-Doped, Nitrogen-Doped and Nitrogen, Sulfur-Co Doped Carbon Quantum Dots. Environ. Toxicol. Pharmacol. 2020, 79, 103426. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Patterson, J.; Kimmel, R.O. The Development and Behavioral Characteristics of the Startle Response in the Zebra Fish. Dev. Psychobiol. 1974, 7, 47–60. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kowalski, M.L.; Carson, R.P.; Bridges, L.R.; Ess, K.C. Heterozygous Inactivation of Tsc2 Enhances Tumorigenesis in P53 Mutant Zebrafish. Dis. Model. Mech. 2013, 6, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Kataba, A.; Botha, T.L.; Nakayama, S.M.M.; Yohannes, Y.B.; Ikenaka, Y.; Wepener, V.; Ishizuka, M. Acute Exposure to Environmentally Relevant Lead Levels Induces Oxidative Stress and Neurobehavioral Alterations in Larval Zebrafish (Danio rerio). Aquat. Toxicol. 2020, 227, 105607. [Google Scholar] [CrossRef]
- Artioli, G.G.; Gualano, B.; Smith, A.; Stout, J.; Lancha, A.H.J. Role of Beta-Alanine Supplementation on Muscle Carnosine and Exercise Performance. Med. Sci. Sport. Exerc. 2010, 42, 1162–1173. [Google Scholar] [CrossRef]
- Quesnele, J.J.; Laframboise, M.A.; Wong, J.J.; Kim, P.; Wells, G.D. The Effects of Beta-Alanine Supplementation on Performance: A Systematic Review of the Literature. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 14–27. [Google Scholar] [CrossRef]
- Massarsky, A.; Kozal, J.S.; Di Giulio, R.T. Glutathione and Zebrafish: Old Assays to Address a Current Issue. Chemosphere 2017, 168, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Gong, Z.; Kelly, B.C. Assessing Biological Effects of Fluoxetine in Developing Zebrafish Embryos Using Gas Chromatography-Mass Spectrometry Based Metabolomics. Chemosphere 2017, 188, 157–167. [Google Scholar] [CrossRef]
- Zeituni, E.M.; Farber, S.A. Studying Lipid Metabolism and Transport during Zebrafish Development. Methods Mol. Biol. 2016, 1451, 237–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, S.; Lee, J.N.; Kim, Y., II; Nam, I.K.; Kim, S.J.; Kim, S.J.; Kwak, S.A.; Oh, G.S.; Kim, H.J.; Yoo, H.J.; et al. The Fatty Acid Chain Elongase, Elovl1, Is Required for Kidney and Swim Bladder Development during Zebrafish Embryogenesis. Organogenesis 2016, 12, 78–93. [Google Scholar] [CrossRef] [Green Version]
- Tocher, D.R. Glycerophospholipid Metabolism. In Biochemistry and Molecular Biology of Fishes; Hochachka, P.W., Mommsen, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 4, pp. 119–157. [Google Scholar]
- Pirro, V.; Guffey, S.C.; Sepúlveda, M.S.; Mahapatra, C.T.; Ferreira, C.R.; Jarmusch, A.K.; Cooks, R.G. Lipid Dynamics in Zebrafish Embryonic Development Observed by DESI-MS Imaging and Nanoelectrospray-MS. Mol. Biosyst. 2016, 12, 2069–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Örn, S. The Zebrafish as a Model Organism for Evaluation of Endocrine Disrupters. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2006; 36p. [Google Scholar]
- Watkins, P.A. Fatty Acyl-CoA Synthetases. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, UK, 2013; pp. 290–295. ISBN 978-0-12-378631-9. [Google Scholar]
- Segner, H. Zebrafish (Danio rerio) as a Model Organism for Investigating Endocrine Disruption. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 187–195. [Google Scholar] [CrossRef]
- Henderson, F.; Johnston, H.R.; Badrock, A.P.; Jones, E.A.; Forster, D.; Nagaraju, R.T.; Evangelou, C.; Kamarashev, J.; Green, M.; Fairclough, M.; et al. Enhanced Fatty Acid Scavenging and Glycerophospholipid Metabolism Accompany Melanocyte Neoplasia Progression in Zebrafish. Cancer Res. 2019, 79, 2136–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agaba, M.; Tocher, D.R.; Dickson, C.A.; Dick, J.R.; Teale, A.J. Zebrafish CDNA Encoding Multifunctional Fatty Acid Elongase Involved in Production of Eicosapentaenoic (20:5n-3) and Docosahexaenoic (22:6n-3) Acids. Mar. Biotechnol. 2004, 6, 251–261. [Google Scholar] [CrossRef] [PubMed]
- D’Rozario, M.; Monk, K.R.; Petersen, S.C. Analysis of Myelinated Axon Formation in Zebrafish. Methods Cell Biol. 2017, 138, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Goldman, D. Retina Regeneration in Zebrafish. Curr. Opin. Genet. Dev. 2016, 40, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlegel, A.; Stainier, D.Y.R. Microsomal Triglyceride Transfer Protein Is Required for Yolk Lipid Utilization and Absorption of Dietary Lipids in Zebrafish Larvae. Biochemistry 2006, 45, 15179–15187. [Google Scholar] [CrossRef] [PubMed]
- Şahan, U.; Ipek, A.; Sozcu, A. Yolk Sac Fatty Acid Composition, Yolk Absorption, Embryo Development, and Chick Quality during Incubation in Eggs from Young and Old Broiler Breeders. Poult. Sci. 2014, 93, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, H. The Roles of Anabolic and Catabolic Reactions in the Synthesis and Recycling of Polyunsaturated Fatty Acids. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 79–83. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, K.; Lee, J.; Lee, J.; Lee, J.; Kim, S.; Lee, S.E.; Kim, J.H. Targeted Toxicometabolomics of Endosulfan Sulfate in Adult Zebrafish (Danio rerio) Using GC-MS/MS in Multiple Reaction Monitoring Mode. J. Hazard. Mater. 2020, 389, 122056. [Google Scholar] [CrossRef]


| Metabolic Pathway | Control | 200 μg/L | 300 μg/L | 500 μg/L |
|---|---|---|---|---|
| Aminoacyl-tRNA biosynthesis | ✓ | ✓ | ✓ | ✓ |
| Histidine metabolism | ✓ | ✓ | ✓ | ✓ |
| Purine metabolism | ✓ | ✓ | ✓ | ✓ |
| Retinol metabolism | ✓ | ✓ | ✓ | ✓ |
| Selenocompound metabolism | ✓ | ✓ | ✓ | ✓ |
| Steroid hormone biosynthesis | ✓ | ✓ | ✓ | ✓ |
| Tyrosine metabolism | ✓ | ✓ | ✓ | ✓ |
| Valine, leucine and isoleucine biosynthesis | ✓ | ✓ | ✓ | ✓ |
| Valine, leucine and isoleucine degradation | ✓ | ✓ | ✓ | ✓ |
| Phenylalanine metabolism | ✓ | ✓ | ✓ | ✓ |
| Fatty acid biosynthesis | ✓ | ✓ | ✓ | |
| Pantothenate and CoA biosynthesis | ✓ | ✓ | ✓ | |
| Arginine biosynthesis | ✓ | ✓ | ✓ | |
| Pyrimidine metabolism | ✓ | ✓ | ✓ | |
| One carbon pool by folate | ✓ | ✓ | ||
| Pyruvate metabolism | ✓ | ✓ | ||
| Folate biosynthesis | ✓ | ✓ | ||
| Beta-Alanine metabolism | ✓ | |||
| Biosynthesis of unsaturated fatty acids | ✓ | |||
| Butanoate metabolism | ✓ | |||
| Fatty acid elongation | ✓ | |||
| Alanine, aspartate and glutamate metabolism | ✓ | |||
| Linoleic acid metabolism | ✓ | |||
| Sphingolipid metabolism | ✓ | |||
| Glutathione metabolism | ✓ | ✓ | ✓ | |
| Amino sugar and nucleotide sugar metabolism | ✓ | ✓ | ✓ | |
| Cysteine and methionine metabolism | ✓ | ✓ | ✓ | |
| Fructose and mannose metabolism | ✓ | ✓ | ✓ | |
| Glycine, serine, and threonine metabolism | ✓ | ✓ | ✓ | |
| Tryptophan metabolism | ✓ | ✓ | ||
| Propanoate metabolism | ✓ | ✓ | ||
| Vitamin B6 metabolism | ✓ | |||
| Arginine and proline metabolism | ✓ | ✓ | ||
| Pentose phosphate pathway | ✓ | ✓ | ||
| Porphyrin and chlorophyll metabolism | ✓ | ✓ | ||
| Primary bile acid biosynthesis | ✓ | ✓ | ||
| Thiamine metabolism | ✓ | ✓ | ||
| Glycerolipid metabolism | ✓ | |||
| Glycerophospholipid metabolism | ✓ | |||
| Nicotinate and nicotinamide metabolism | ✓ | |||
| Phenylalanine, tyrosine, and tryptophan biosynthesis | ✓ | |||
| Starch and sucrose metabolism | ✓ | |||
| Steroid biosynthesis | ✓ | |||
| Taurine and hypotaurine metabolism | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chousidis, I.; Chatzimitakos, T.; Stalikas, C.; Leonardos, I. Untargeted Metabolomics Associated with Behavioral and Toxicological Studies Yield Insights into the Impact of 2,6-Dichloro-3-hydroxy-1,4-benzoquinone Disinfection By-Product on Zebrafish Larvae. Fishes 2022, 7, 368. https://doi.org/10.3390/fishes7060368
Chousidis I, Chatzimitakos T, Stalikas C, Leonardos I. Untargeted Metabolomics Associated with Behavioral and Toxicological Studies Yield Insights into the Impact of 2,6-Dichloro-3-hydroxy-1,4-benzoquinone Disinfection By-Product on Zebrafish Larvae. Fishes. 2022; 7(6):368. https://doi.org/10.3390/fishes7060368
Chicago/Turabian StyleChousidis, Ieremias, Theodoros Chatzimitakos, Constantine Stalikas, and Ioannis Leonardos. 2022. "Untargeted Metabolomics Associated with Behavioral and Toxicological Studies Yield Insights into the Impact of 2,6-Dichloro-3-hydroxy-1,4-benzoquinone Disinfection By-Product on Zebrafish Larvae" Fishes 7, no. 6: 368. https://doi.org/10.3390/fishes7060368