Application of the Method Evaluation and Risk Assessment Tool for a Small-Scale Grouper Fishery in Indonesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Fishery
2.2. MERA Operation
2.2.1. MERA Questionnaire
2.2.2. Fishery Data
| Parameters | Value | References |
|---|---|---|
| Life history and stock | ||
| Maximum age (year) | 26 | Mathews and Samuels [31]; Agustina et al. [16,26,33] |
| M (year−1) | 0.16 | |
| Von Bertalanffy Linf parameter (cm) | 71.9 | |
| Von Bertalanffy k parameter | 0.12 | |
| Von Bertalanffy t0 parameter (year) | −1.17 | |
| Length-weight parameter a | 0.0182 | |
| Length-weight parameter b | 2.97 | |
| Length at 50% maturity (cm) | 38.8 | |
| Length at 95% maturity (cm) | 41.8 | |
| Length at first capture (cm) | 34.6 | |
| Stock depletion | 0.32 | Estimated from current SPR relative to general MSY equilibrium model (Goethel et al. [34]; Hoshino et al. [35]) |
| Catch data | ||
| Range of total annual catch (kg), from 2016 to 2021 | 3773.1–5629.4 | FIP2B fish landing monitoring data and analyses from 2016 to 2021 (unpublished) |
| Catch-at-length (cm), from 2016 to 2021 | Size class range: 20–64 cm; n = 4115 | |
2.2.3. Management Procedures
2.2.4. Evaluating the Performance and Selecting Management Procedures
2.2.5. Biomass and Yield Projections
2.2.6. Source of Uncertainties and Variation in Yield Projections
3. Results
3.1. Selection of Available Management Procedures
3.2. Yield–Biomass Trade-Offs
3.3. Biomass Projections
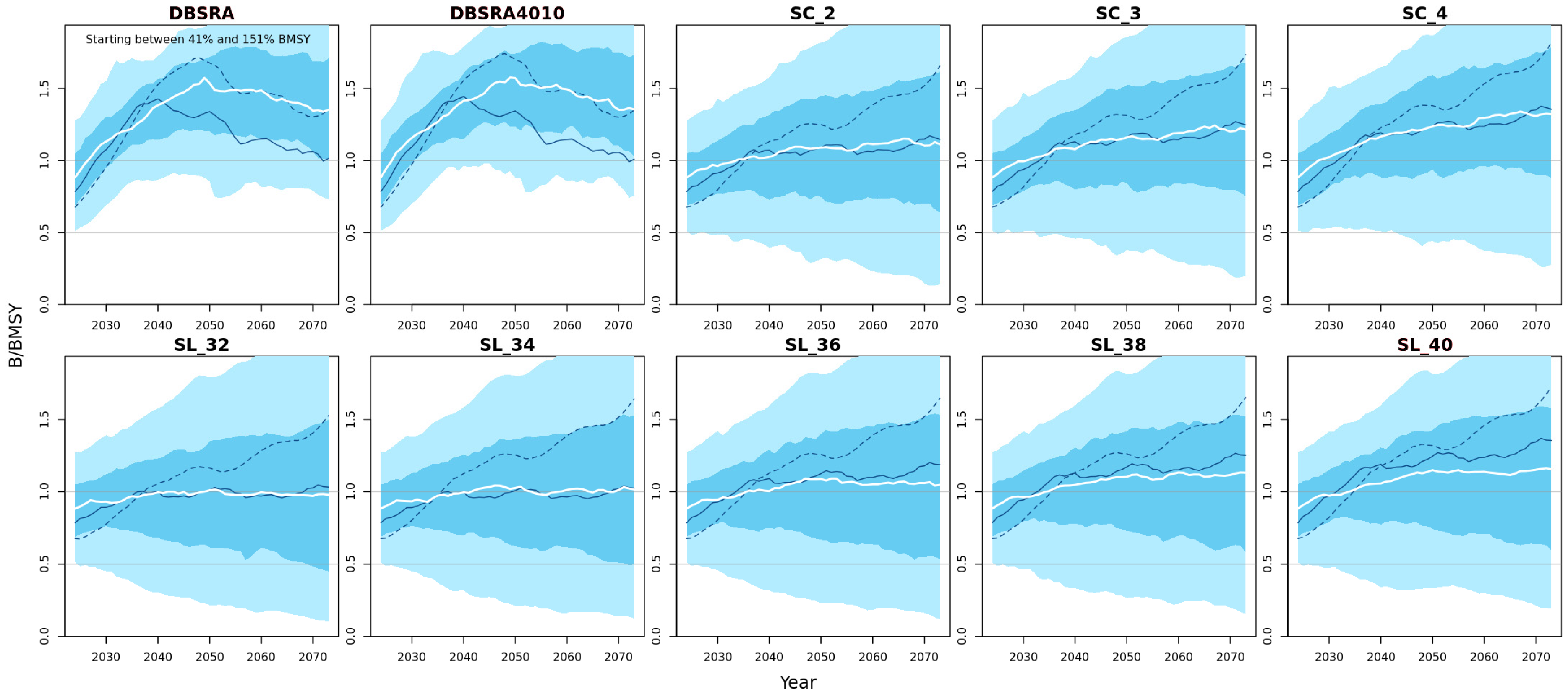
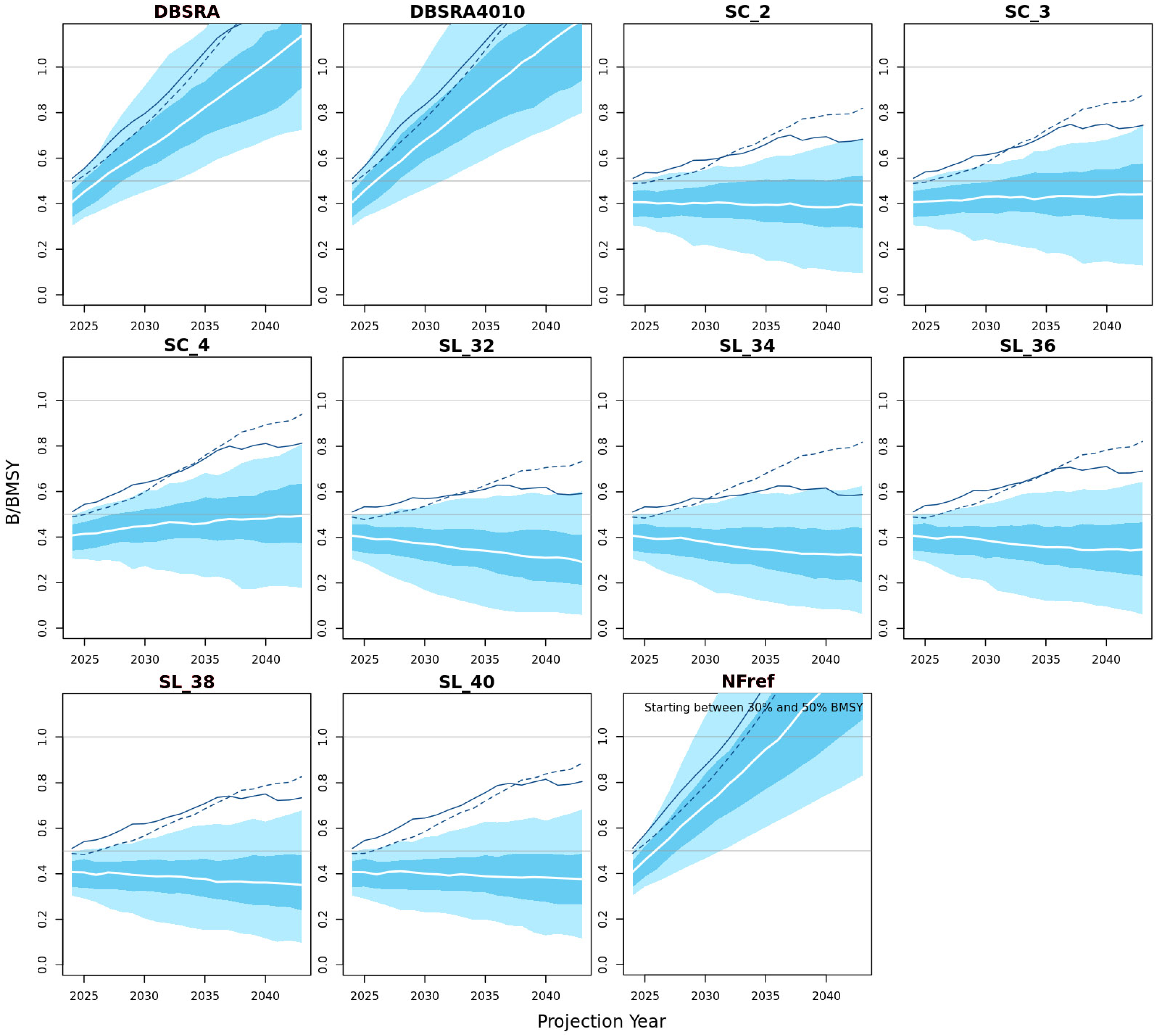
3.3.1. Biomass Probability Projections
3.3.2. Biomass Projections Plots
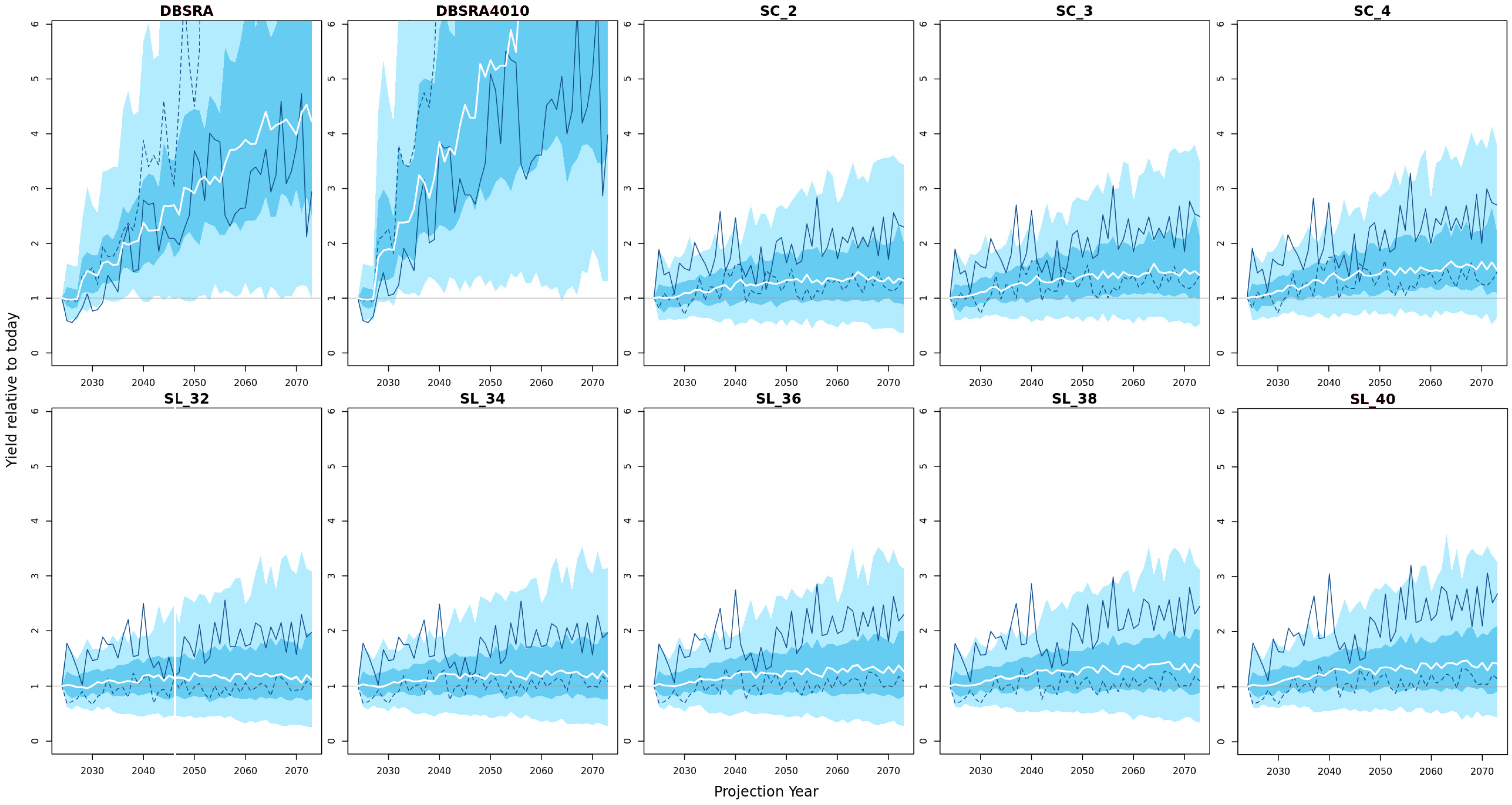
3.4. Yield Projections
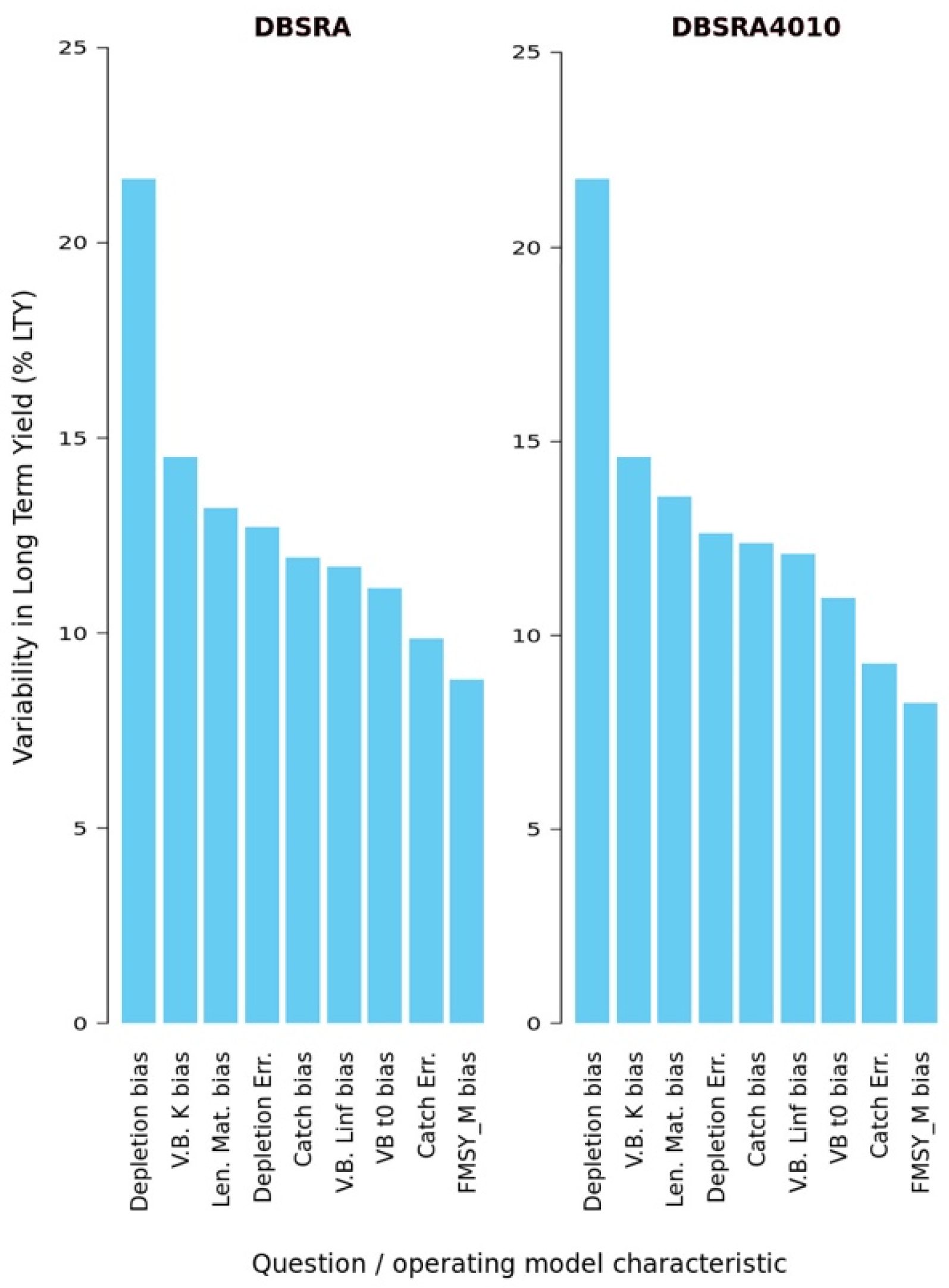
3.5. Sources of Uncertainty in Projections of the Long-Term-Yield
4. Discussion
4.1. Performance of Management Procedures
4.2. Selection of Management Procedures
4.3. Identification of Recommended Management Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amorim, P.; Sousa, P.; Jardim, E.; Menezes, G.M. Sustainability Status of Data-Limited Fisheries: Global Challenges for Snapper and Grouper. Front. Mar. Sci. 2019, 6, 654. [Google Scholar] [CrossRef]
- Amorim, P.; Westmeyer, M. Snapper and Grouper: SFP Fisheries Sustainability Overview 2015; Sustainable Fisheries Partnership: Honolulu, HI, USA, 2015; Available online: http://sfpcms.sustainablefish.org.s3.amazonaws.com/2016/03/07/SFP_Sectoral_Report_Snappers_Groupers_Final-99f93c03-d0ade6ae.pdf (accessed on 12 January 2023).
- Sadovy de Mitcheson, Y.J.; Linardich, C.; Barreiros, J.P.; Ralph, G.M.; Aguilar-Perera, A.; Afonso, P.; Erisman, B.E.; Pollard, D.A.; Fennessy, S.T.; Bertoncini, A.A.; et al. Valuable but Vulnerable: Over-Fishing and Under-Management Continue to Threaten Groupers so What Now? Mar. Policy 2020, 116, 103909. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations Fishery and Aquaculture Statistics; Global Capture Production 1950–2013; Updated 2022. Available online: https://www.fao.org/fishery/statistics-query/en/global_production/global_production_quantity (accessed on 22 March 2023).
- Halim, A.; Loneragan, N.R.; Wiryawan, B.; Hordyk, A.R.; Sondita, M.F.A.; Yulianto, I. Evaluating Data-Limited Fisheries for Grouper (Serranidae) and Snapper (Lutjanidae) in the Coral Triangle, Eastern Indonesia. Reg. Stud. Mar. Sci. 2020, 38, 101388. [Google Scholar] [CrossRef]
- Prince, J.; Victor, S.; Kloulchad, V.; Hordyk, A. Length Based SPR Assessment of Eleven Indo-Pacific Coral Reef Fish Populations in Palau. Fish. Res. 2015, 171, 42–58. [Google Scholar] [CrossRef]
- Mudjirahayu; Bawole, R.; Rembet, U.N.W.J.; Ananta, A.S.; Runtuboi, F.; Sala, R. Growth, Mortality and Exploitation Rate of Plectropomus maculatus and P. oligocanthus (Groupers, Serranidae) on Cenderawasih Bay National Park, Indonesia. Egypt. J. Aquat. Res. 2017, 43, 213–218. [Google Scholar] [CrossRef]
- Frisch, A.J.; Cameron, D.S.; Pratchett, M.S.; Williamson, D.H.; Williams, A.J.; Reynolds, A.D.; Hoey, A.S.; Rizzari, J.R.; Evans, L.; Kerrigan, B.; et al. Key Aspects of the Biology, Fisheries and Management of Coral Grouper. Rev. Fish Biol. Fish. 2016, 26, 303–325. [Google Scholar] [CrossRef]
- Andamari, R.; Moria, S.B.; Permana, G.N. Aspects of Leopard Coral Grouper (Plectropomus leopardus). Indones. Aquac. J. 2007, 2, 51–57. [Google Scholar]
- Craig, M.T.; de Mitcheson, Y.S.; Heemstra, P.C. Groupers of the World: A Field and Market Guide; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-920033-11-8. [Google Scholar]
- Herdiana, Y.; Agustina, S.; Hartati, I.D.; Utami, A.; de la Rosa, E.; Yulianto, I. Fisheries Management for the Leopard Coral Grouper (Plectropomus leopardus) in Saleh Bay, West Nusa Tenggara, Indonesia. In Proceedings from Workshops on Management Strategy Evaluation of Data-Limited Fisheries: Towards Sustainability—Applying the Method Evaluation and Risk Assessment Tool to Seven Indonesian Fisheries; Murdoch University: Perth, WA, Australia; IPB University: Bogor, Indonesia, 2021; pp. 129–150. ISBN 978-0-646-82951-7. [Google Scholar]
- Ministry of Marine Affairs and Fisheries. Pusat Data Statistik dan Informasi (Pusdatin) Data Produksi Perikanan 2023. Available online: https://statistik.kkp.go.id/home.php (accessed on 12 August 2022).
- Yulianto, I.; Kartawijaya, T.; Rafandi, M.T.; Agustina, S.; Pingkan, J.; Aminollah; Nurjamil; Sabariyono; Widodo; Ramadhan, S.; et al. Profil Perikanan Tangkap di Teluk Saleh dan Teluk Cempi Provinsi Nusa Tenggara Barat; Wildlife Conservation Society: Bogor, Indonesia, 2016. [Google Scholar]
- Efendi, D.S.; Adrianto, L.; Yonvitner; Wardiatno, Y. An Evaluation of Grouper and Snapper Fisheries Management Policy in Saleh Bay, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 744, 012013. [Google Scholar] [CrossRef]
- Retnoningtyas, H.; Yulianto, I.; Soemodinoto, A.; Herdiana, Y.; Kartawijaya, T.; Natsir, M.; Haryanto, J.T. Stakeholder Participation in Management Planning for Grouper and Snapper Fisheries in West Nusa Tenggara Province, Indonesia. Mar. Policy 2021, 128, 104452. [Google Scholar] [CrossRef]
- Agustina, S.; Natsir, M.; Gigentika, S.; Marliana, I.; Anas, A.; Rafandi, M.T.; Hernawati; Kartawijaya, T.; Prasetia, R. Telaah Ilmiah Pengelolaan Perikanan Kerapu Dan Kakap Berkelanjutan Di Nusa Tenggara Barat 2023; Forum Ilmiah Pengelolaan Perikanan Berkelanjutan (FIP2B Provinsi Nusa Tenggara Barat): Mataram, Indonesia, 2023. [Google Scholar]
- Fisheries Improvement Program. Saleh Bay Snapper and Grouper—Bottom Longline and Handline. Available online: https://fisheryprogress.org/fip-profile/indonesia-saleh-bay-snapper-and-grouper-bottom-longline-and-handline (accessed on 22 March 2023).
- Punt, A.E.; Butterworth, D.S.; de Moor, C.L.; De Oliveira, J.A.A.; Haddon, M. Management Strategy Evaluation: Best Practices. Fish Fish. 2016, 17, 303–334. [Google Scholar] [CrossRef]
- Carruthers, T.R.; Punt, A.E.; Walters, C.J.; MacCall, A.; McAllister, M.K.; Dick, E.J.; Cope, J. Evaluating Methods for Setting Catch Limits in Data-Limited Fisheries. Fish. Res. 2014, 153, 48–68. [Google Scholar] [CrossRef]
- Goethel, D.R.; Lucey, S.M.; Berger, A.M.; Gaichas, S.K.; Karp, M.A.; Lynch, P.D.; Walter, J.F. Recent Advances in Management Strategy Evaluation: Introduction to the Special Issue “Under Pressure: Addressing Fisheries Challenges with Management Strategy Evaluation”. Can. J. Fish. Aquat. Sci. 2019, 76, 1689–1696. [Google Scholar] [CrossRef]
- Carruthers, T.R. Customizing DLMtool for Canadian Fisheries; Technical Report for Fisheries and Oceans Canada; Fisheries and Oceans Canada: Vancouver, BC, Canada, 2017; Available online: https://www.datalimitedtoolkit.org/wp-content/uploads/2017/09/Customizing-DLMtool-for-Canadian-Fisheries-WEB.pdf (accessed on 25 March 2023).
- Hordyk, A.; Newman, D.; Carruthers, T.R.; Suatoni, L. Applying Management Strategy Evaluation to California Fisheries: Case Studies and Recommendations. 2017. Available online: https://www.datalimitedtoolkit.org/wp-content/uploads/2017/07/Applying-MSE-to-CA-Fisheries-Case-Studies-Recommendations.pdf (accessed on 25 March 2023).
- Loneragan, N.; Wiryawan, B.; Hordyk, A.; Halim, A.; Proctor, C.; Satria, F.; Yulianto, I. Proceedings from Workshops on Management Strategy Evaluation of Data-Limited Fisheries: Towards Sustainability—Applying the Method Evaluation and Risk Assessment Tool to Seven Indonesian Fisheries; Murdoch University: Perth, WA, Australia; IPB University: Bogor, Indonesia, 2021; ISBN 978-0-646-82951-7. [Google Scholar]
- Carruthers, T.R.; Huynh, Q.C.; Hordyk, A.R.; Newman, D.; Smith, A.D.M.; Sainsbury, K.J.; Stokes, K.; Morison, A.; Agnew, D.; Parma, A.; et al. Method Evaluation and Risk Assessment: A Framework for Evaluating Management Strategies for Data-limited Fisheries. Fish Fish. 2023, 24, 279–296. [Google Scholar] [CrossRef]
- Hordyk, A.R.; Carruthers, T.R. A Quantitative Evaluation of a Qualitative Risk Assessment Framework: Examining the Assumptions and Predictions of the Productivity Susceptibility Analysis (PSA). PloS ONE 2018, 13, e0198298. [Google Scholar] [CrossRef] [PubMed]
- Agustina, S.; Panggabean, A.S.; Natsir, M.; Retroningtyas, H.; Yulianto, I. Yield-per-Recruit Modeling as Biological Reference Points to Provide Fisheries Management of Leopard Coral Grouper (Plectropomus leopardus) in Saleh Bay, West Nusa Tenggara. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012005. [Google Scholar] [CrossRef]
- Yulius, Y.; Ramdhan, M.; Ardiansyah, A. Characteristic of Saleh Bay Coastline. Segara 2018, 14, 117. [Google Scholar] [CrossRef]
- Khasanah, M.; Nurdin, N.; Sadovy de Mitcheson, Y.; Jompa, J. Management of the Grouper Export Trade in Indonesia. Rev. Fish. Sci. Aquac. 2020, 28, 1–15. [Google Scholar] [CrossRef]
- Khasanah, M.; Kadir, N.N.; Jompa, J. Reproductive Biology of Three Important Threatened/Near-Threatened Groupers (Plectropomus leopardus, Epinephelus polyphekadion and Plectropomus areolatus) in Eastern Indonesia and Implications for Management. Animals 2019, 9, 643. [Google Scholar] [CrossRef]
- Ebisawa, A. Life History Traits of Leopard Coralgrouper Plectropomus leopardus in the Okinawa Islands, Southwestern Japan. Fish. Sci. 2013, 79, 911–921. [Google Scholar] [CrossRef]
- Mathews, C.P.; Samuel, M. Growth, Mortality and Assessment for Groupers from Kuwait. Kuwait Bull. Mar. Sci. 1997, 9, 173–191. [Google Scholar]
- Efendi, D.S.; Daud, A.R.; Utomo, D.S.; Muawanah, U.; Adrianto, L. Dampak kebijakan perikanan kerapu dan kakap terhadap keberlanjutan stok sumber daya di Teluk Saleh. In Prosiding Seminar Nasional Riset dan Kebijakan Sosial Ekonomi Kalautan dan Perikanan Tahun 2020; AMAFRAD Press: Jakarta, Indonesia, 2020; pp. 153–162. ISBN 978-623-7651-68-0. [Google Scholar]
- Agustina, S.; Natsir, M.; Panggabean, A.S.; Hartati, I.D.; Rosdiana, A.; Qonita, Y.; Rafandi, M.T.; Hernawati; Yulianto, I. Laporan Teknis: Evaluasi Implementasi Rencana Aksi Pengelolaan Perikanan Kerapu dan Kakap Berkelanjutan (P2K2B) Provinsi Nusa Tenggara Barat Tahun ke-2 2021; Wildlife Conservation Society: Bogor, Indonesia, 2022. [Google Scholar]
- Goethel, D.R.; Smith, M.W.; Cass-Calay, S.L.; Porch, C.E. Establishing Stock Status Determination Criteria for Fisheries with High Discards and Uncertain Recruitment. N. Am. J. Fish. Manag. 2018, 38, 120–139. [Google Scholar] [CrossRef]
- Hoshino, E.; Pascoe, S.; Hutton, T.; Kompas, T.; Yamazaki, S. Estimating Maximum Economic Yield in Multispecies Fisheries: A Review. Rev. Fish. Biol. Fish. 2018, 28, 261–276. [Google Scholar] [CrossRef]
- DKP. Dinas Kelautan dan Perikanan. Rencana Pengelolaan dan Zonasi Kawasan Konservasi Perairan, Suaka Alam Perairan Pulau Lipan dan Pulau Rakit Kabupaten Sumbawa, Provinsi Nusa Tenggara Barat 2018; Dinas Kelautan dan Perikanan Provinsi Nusa Tenggara Barat: Mataram, Indonesia, 2018. [Google Scholar]
- DKP. Dinas Kelautan dan Perikanan. Rencana Pengelolaaan dan Zonasi Taman Wisata Perairan Pulau Liang dan Pulau Ngali 2018–2038; Dinas Kelautan dan Perikanan Provinsi Nusa Tenggara Barat: Mataram, Indonesia, 2018. [Google Scholar]
- Matley, J.; Heupel, M.; Simpfendorfer, C. Depth and Space Use of Leopard Coralgrouper Plectropomus leopardus Using Passive Acoustic Tracking. Mar. Ecol. Prog. Ser. 2015, 521, 201–216. [Google Scholar] [CrossRef]
- Zeller, D. Home Range and Activity Patterns of the Coral Trout Plectropomus leopardus (Serranidae). Mar. Ecol. Prog. Ser. 1997, 154, 65–77. [Google Scholar] [CrossRef]
- Natsir, M.; Ruchimat, T.; Agustina, S.; Yulianto, I. Application of Global Positioning System Tracker to Detect the Fishing Ground Location and Effective Effort in Artisanal Fishery. Sens. Mater. 2019, 31, 803. [Google Scholar] [CrossRef]
- Dowling, N.A.; Dichmont, C.M.; Venables, W.; Smith, A.D.M.; Smith, D.C.; Power, D.; Galeano, D. From Low- to High-Value Fisheries: Is It Possible to Quantify the Trade-off between Management Cost, Risk and Catch? Mar. Policy 2013, 40, 41–52. [Google Scholar] [CrossRef]
- Punt, A.E. Strategic Management Decision-Making in a Complex World: Quantifying, Understanding, and Using Trade-Offs. ICES J. Mar. Sci. 2017, 74, 499–510. [Google Scholar] [CrossRef]
- Dick, E.J.; MacCall, A.D. Depletion-Based Stock Reduction Analysis: A Catch-Based Method for Determining Sustainable Yields for Data-Poor Fish Stocks. Fish. Res. 2011, 110, 331–341. [Google Scholar] [CrossRef]
- Carruthers, T.R.; Huynh, Q.; Hordyk, A. Data-Limited Methods Reference (DLMtool Functions 6.0.6). 2022. Available online: https://dlmtool.openmse.com/ (accessed on 15 July 2023).
- Zhou, S.; Punt, A.E.; Ye, Y.; Ellis, N.; Dichmont, C.M.; Haddon, M.; Smith, D.C.; Smith, A.D. Estimating Stock Depletion Level from Patterns of Catch History. Fish Fish. 2017, 18, 742–751. [Google Scholar] [CrossRef]
- Pacific Fishery Management Council. Pacific Coast Groundfish Fishery Management Plan for the California, Oregon, and Washington Groundfish Fishery; Pacific Fishery Management Council: Portland, OR, USA, 2022; Available online: https://www.pcouncil.org/documents/2022/08/pacific-coast-groundfish-fishery-management-plan.pdf/ (accessed on 15 July 2023).
- Williams, E.H.; Shertzer, K.W. Implications of Life-History Invariants for Biological Reference Points Used in Fishery Management. Can. J. Fish. Aquat. Sci. 2003, 60, 11. [Google Scholar] [CrossRef]
- Carruthers, T.R.; Hordyk, A.R. The Data-Limited Methods Toolkit (DLM Tool): An R Package for Informing Management of Data-limited Populations. Methods Ecol. Evol. 2018, 9, 2388–2395. [Google Scholar] [CrossRef]
- Sadovy De Mitcheson, Y.; Craig, M.T.; Bertoncini, A.A.; Carpenter, K.E.; Cheung, W.W.L.; Choat, J.H.; Cornish, A.S.; Fennessy, S.T.; Ferreira, B.P.; Heemstra, P.C.; et al. Fishing Groupers Towards Extinction: A Global Assessment of Threats and Extinction Risks in a Billion Dollar Fishery. Fish Fish. 2013, 14, 119–136. [Google Scholar] [CrossRef]
- Sadovy De Mitcheson, Y.; Colin, P.L.; Lindfield, S.J.; Bukurrou, A. A Decade of Monitoring an Indo-Pacific Grouper Spawning Aggregation: Benefits of Protection and Importance of Survey Design. Front. Mar. Sci. 2020, 7, 571878. [Google Scholar] [CrossRef]
- Erisman, B.; Heyman, W.; Kobara, S.; Ezer, T.; Pittman, S.; Aburto-Oropeza, O.; Nemeth, R.S. Fish Spawning Aggregations: Where Well-placed Management Actions Can Yield Big Benefits for Fisheries and Conservation. Fish Fish. 2017, 18, 128–144. [Google Scholar] [CrossRef]
- Bejarano Chavarro, S.; Mumby, P.J.; Golbuu, Y. Changes in the Spear Fishery of Herbivores Associated with Closed Grouper Season in Palau, Micronesia: Spear Fishery of Herbivores during Closed Grouper Season. Anim. Conserv. 2014, 17, 133–143. [Google Scholar] [CrossRef]
- Lindley, J. Fishing Non-Compliance and Culture. Mar. Policy 2023, 152, 105581. [Google Scholar] [CrossRef]
- Halim, A.; Wiryawan, B.; Loneragan, N.R.; Hordyk, A.; Sondita, M.F.A.; White, A.T.; Koeshendrajana, S.; Ruchimat, T.; Pomeroy, R.S.; Yuni, C. Developing a Functional Definition of Small-Scale Fisheries in Support of Marine Capture Fisheries Management in Indonesia. Mar. Policy 2019, 100, 238–248. [Google Scholar] [CrossRef]
- Oyanedel, R.; Gelcich, S.; Milner-Gulland, E.J. Motivations for (Non-)Compliance with Conservation Rules by Small-scale Resource Users. Conserv. Lett. 2020, 13, 1120–1134. [Google Scholar] [CrossRef]
- Prince, J.; Creech, S.; Madduppa, H.; Hordyk, A. Length Based Assessment of Spawning Potential Ratio in Data-Poor Fisheries for Blue Swimming Crab (Portunus spp.) in Sri Lanka and Indonesia: Implications for Sustainable Management. Reg. Stud. Mar. Sci. 2020, 36, 101309. [Google Scholar] [CrossRef]
- Oyanedel, R.; Gelcich, S.; Milner-Gulland, E.J. A Synthesis of (Non-)Compliance Theories with Applications to Small-scale Fisheries Research and Practice. Fish Fish. 2020, 21, 1120–1134. [Google Scholar] [CrossRef]
- Marshall, D.J.; Gaines, S.; Warner, R.; Barneche, D.R.; Bode, M. Underestimating the Benefits of Marine Protected Areas for the Replenishment of Fished Populations. Front. Ecol. Env. 2019, 17, 407–413. [Google Scholar] [CrossRef]
- Hall, A.; Cameron, D.; Kingsford, M. Prohibiting Spearfishing Boosts Conservation Outcomes for Partially Protected Areas. Biol. Conserv. 2022, 272, 109662. [Google Scholar] [CrossRef]
- Balbar, A.C.; Metaxas, A. The Current Application of Ecological Connectivity in the Design of Marine Protected Areas. Glob. Ecol. Conserv. 2019, 17, e00569. [Google Scholar] [CrossRef]
- Williamson, D.H.; Harrison, H.B.; Almany, G.R.; Berumen, M.L.; Bode, M.; Bonin, M.C.; Choukroun, S.; Doherty, P.J.; Frisch, A.J.; Saenz-Agudelo, P.; et al. Large-scale, Multidirectional Larval Connectivity among Coral Reef Fish Populations in the Great Barrier Reef Marine Park. Mol. Ecol. 2016, 25, 6039–6054. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, R.; Micheli, F.; De Leo, G.A. Integrating Marine Protected Areas with Catch Regulation. Can. J. Fish. Aquat. Sci. 2006, 63, 642–649. [Google Scholar] [CrossRef]
- Halim, A.; Loneragan, N.R.; Wiryawan, B.; Fujita, R.; Adhuri, D.S.; Hordyk, A.R.; Sondita, M.F.A. Transforming Traditional Management into Contemporary Territorial-Based Fisheries Management Rights for Small-Scale Fisheries in Indonesia. Mar. Policy 2020, 116, 103923. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J.; et al. Global Conservation Outcomes Depend on Marine Protected Areas with Five Key Features. Nature 2014, 506, 216–220. [Google Scholar] [CrossRef]
- Kininmonth, S.; Blenckner, T.; Niiranen, S.; Watson, J.; Orio, A.; Casini, M.; Neuenfeldt, S.; Bartolino, V.; Hansson, M. Is Diversity the Missing Link in Coastal Fisheries Management? Diversity 2022, 14, 90. [Google Scholar] [CrossRef]





| Management Procedures | Procedures Evaluated |
|---|---|
| MERA’s Default MPs (n = 20) | |
| Total Allowable Catch (TAC, 13) | DBSRA (Depletion-Based Stock Reduction Analysis) DBSRA_40 (Depletion-Based Stock Reduction Analysis 40) DBSRA4010 (Depletion-Based Stock Reduction Analysis 4010) DCAC (Depletion Corrected Average Catch) DCAC_40 (Depletion Corrected Average Catch 40) DD (Delay-Difference Stock Assessment) DD4010 (Delay-Difference Stock Assessment 4010) MCD (Mean Catch Depletion) MCD4010 (Mean Catch Depletion 4010) Fratio (F and M ratio) HDAAC (Hybrid Depletion Adjusted Average Catch) IT10 (Iterative Index Target 10%) IT5 (Iterative Index Target 5%) |
| Total Allowable Effort (TAE, 3) | DDe (Effort-based Delay-Difference Stock Assessment) DDe75 (Effort-based Delay-Difference Stock Assessment 75%) ITe10 (Index Target Effort-Based 10%) |
| Size Limit (2) | Matlenlim (Size limit at length-at-maturity) Matlenlim2 (Size limit at 110% length-at-maturity) |
| Spatial Closure/Marine Protected Area (2) | MRnoreal (Spatial closure—no reallocation of effort) MRreal (Spatial closure—with reallocation of effort) |
| Custom MPs (n = 19) | |
| Total Allowable Catch (TAC; 4) | Index_10_TAC (Reduction of 10% from current catch) Index_15_TAC (Reduction of 15% from current catch) Index_20_TAC (Reduction of 20% from current catch) Index_25_TAC (Reduction of 25% from current catch) |
| Total Allowable Effort (TAE; 4) | Index_10_Eff (Reduction of 10% from current effort) Index_15_Eff (Reduction of 15% from current effort) Index_20_Eff (Reduction of 20% from current effort) Index_25_Eff (Reduction of 25% from current effort) |
| Size Limit (8) | SL_25 (set catch size limit at 25 cm) SL_28 to SL_40 (set catch size limit at between 28 to 40 cm, with 2 cm increment) |
| Seasonal closure (3) | SC_2 (no fishing for 2 month) SC_3 (no fishing for 3 month) SC_4 (no fishing for 4 month) |
| MP | MP Type | MP Group | Simulation for Current Fishery Condition | Simulation for Stock Rebuilding Scenario | ||||
|---|---|---|---|---|---|---|---|---|
| Mean Prob. Biomass > 50%BMSY (Year 1–10) | Mean Prob. Biomass > BMSY (Year 1–10) | Achieve B/BMSY > 1 in year 46–50 (1 = Yes; 0 = No) * | Achieve Yt/Ycurr = 1 in Year 46–50 (1 = Yes; 0 = No) * | Achieve B/BMSY > 1 in year 1–10 or short-term HCR (1 = Yes; 0 = No) | Achieve B/BMSY > 1 in year 46–50 or long-term HCR (1 = Yes; 0 = No) * | |||
| DBSRA4010 | TAC | Default | 98.7 | 27.1 | 1 | 1 | 1 | 1 |
| DD4010 | TAC | Default | 98.7 | 28.4 | 1 | 1 | 1 | 1 |
| MCD4010 | TAC | Default | 98.7 | 30.1 | 1 | 1 | 1 | 1 |
| DBSRA | TAC | Default | 98.6 | 20.3 | 1 | 1 | 1 | 1 |
| DCAC_40 | TAC | Default | 98.6 | 22.2 | 1 | 1 | 1 | 1 |
| DCAC | TAC | Default | 98.6 | 22.8 | 1 | 1 | 1 | 1 |
| MCD | TAC | Default | 98.6 | 26.5 | 1 | 1 | 1 | 1 |
| HDAAC | TAC | Default | 98.6 | 27.1 | 1 | 1 | 1 | 1 |
| DD | TAC | Default | 97.8 | 11.0 | 1 | 1 | 0 | 1 |
| DBSRA_40 | TAC | Default | 97.4 | 11.4 | 1 | 1 | 0 | 1 |
| SC_4 | TAE | Custom | 94.59 | 3.53 | 1 | 1 | 0 | 0 |
| SC_3 | TAE | Custom | 91.36 | 2.81 | 1 | 1 | 0 | 0 |
| SC_2 | TAE | Custom | 89.48 | 2.4 | 1 | 1 | 0 | 0 |
| SL_40 | SzLim | Custom | 91.05 | 3.22 | 1 | 1 | 0 | 0 |
| SL_38 | SzLim | Custom | 88.76 | 3.01 | 1 | 1 | 0 | 0 |
| SL_36 | SzLim | Custom | 88.25 | 2.81 | 1 | 1 | 0 | 0 |
| SL_34 | SzLim | Custom | 86.35 | 2.7 | 1 | 1 | 0 | 0 |
| SL_32 | SzLim | Custom | 85.53 | 2.2 | 1 | 1 | 0 | 0 |
| MP | MP Type | MP Group | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | 2031 | 2032 | 2033 | |||
| DBSRA | TAC | Default | 95.8 | 97.9 | 97.9 | 97.9 | 100 | 100 | 100 | 100 | 100 | 100 |
| DBSRA4010 | TAC | Default | 95.8 | 97.9 | 99 | 99 | 100 | 100 | 100 | 100 | 100 | 100 |
| SC_2 | TAE | Custom | 95.8 | 94.8 | 94.8 | 95.8 | 92.7 | 92.7 | 92.7 | 92.7 | 92.7 | 91.7 |
| SC_3 | TAE | Custom | 95.8 | 94.8 | 94.8 | 95.8 | 94.8 | 95.8 | 95.8 | 95.8 | 95.8 | 94.8 |
| SC_4 | TAE | Custom | 95.8 | 95.8 | 95.8 | 96.9 | 95.8 | 96.9 | 96.9 | 97.9 | 96.9 | 95.8 |
| SL_32 | SzLim | Custom | 95.8 | 94.8 | 94.8 | 94.8 | 93.8 | 90.6 | 91.7 | 89.6 | 89.6 | 87.5 |
| SL_34 | SzLim | Custom | 95.8 | 94.8 | 94.8 | 94.8 | 93.8 | 90.6 | 91.7 | 89.6 | 89.6 | 89.6 |
| SL_36 | SzLim | Custom | 95.8 | 94.8 | 94.8 | 94.8 | 93.8 | 91.7 | 91.7 | 91.7 | 90.6 | 90.6 |
| SL_38 | SzLim | Custom | 95.8 | 94.8 | 94.8 | 94.8 | 93.8 | 93.8 | 92.7 | 92.7 | 91.7 | 91.7 |
| SL_40 | SzLim | Custom | 95.8 | 94.8 | 94.8 | 94.8 | 94.8 | 93.8 | 94.8 | 93.8 | 92.7 | 91.7 |
| MP | MP Type | MP Group | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | 2031 | 2032 | 2033 | |||
| DBSRA | TAC | Default | 32.3 | 36.5 | 46.9 | 56.2 | 58.3 | 66.7 | 67.7 | 74 | 80.2 | 84.4 |
| DBSRA4010 | TAC | Default | 32.3 | 36.5 | 49 | 57.3 | 62.5 | 68.8 | 72.9 | 79.2 | 86.5 | 86.5 |
| SC_2 | TAE | Custom | 32.3 | 33.3 | 35.4 | 36.5 | 38.5 | 43.8 | 46.9 | 47.9 | 46.9 | 44.8 |
| SC_3 | TAE | Custom | 32.3 | 33.3 | 35.4 | 38.5 | 42.7 | 46.9 | 49 | 50 | 52.1 | 58.3 |
| SC_4 | TAE | Custom | 32.3 | 34.4 | 38.5 | 42.7 | 51 | 54.2 | 55.2 | 54.2 | 60.4 | 61.5 |
| SL_32 | SzLim | Custom | 32.3 | 34.4 | 33.3 | 37.5 | 36.5 | 38.5 | 40.6 | 42.7 | 43.8 | 44.8 |
| SL_34 | SzLim | Custom | 32.3 | 34.4 | 34.4 | 37.5 | 36.5 | 40.6 | 40.6 | 43.8 | 43.8 | 44.8 |
| SL_36 | SzLim | Custom | 32.3 | 35.4 | 36.5 | 37.5 | 39.6 | 43.8 | 44.8 | 43.8 | 45.8 | 46.9 |
| SL_38 | SzLim | Custom | 32.3 | 35.4 | 37.5 | 38.5 | 41.7 | 44.8 | 45.8 | 45.8 | 46.9 | 49 |
| SL_40 | SzLim | Custom | 32.3 | 35.4 | 37.5 | 38.5 | 42.7 | 47.9 | 47.9 | 45.8 | 49 | 47.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herdiana, Y.; Wiryawan, B.; Wisudo, S.H.; Tweedley, J.R.; Yulianto, I.; Natsir, M.; Agustina, S.; Hordyk, A.; Loneragan, N.R. Application of the Method Evaluation and Risk Assessment Tool for a Small-Scale Grouper Fishery in Indonesia. Fishes 2023, 8, 498. https://doi.org/10.3390/fishes8100498
Herdiana Y, Wiryawan B, Wisudo SH, Tweedley JR, Yulianto I, Natsir M, Agustina S, Hordyk A, Loneragan NR. Application of the Method Evaluation and Risk Assessment Tool for a Small-Scale Grouper Fishery in Indonesia. Fishes. 2023; 8(10):498. https://doi.org/10.3390/fishes8100498
Chicago/Turabian StyleHerdiana, Yudi, Budy Wiryawan, Sugeng H. Wisudo, James R. Tweedley, Irfan Yulianto, Mohamad Natsir, Siska Agustina, Adrian Hordyk, and Neil R. Loneragan. 2023. "Application of the Method Evaluation and Risk Assessment Tool for a Small-Scale Grouper Fishery in Indonesia" Fishes 8, no. 10: 498. https://doi.org/10.3390/fishes8100498







