Fungi and Actinobacteria: Alternative Probiotics for Sustainable Aquaculture
Abstract
:1. Introduction
2. Yeasts as Probiotics
2.1. Saccharomyces
2.2. Candida
2.3. Cryptococcus
2.4. Debaryomyces
2.5. Geotrichum
2.6. Leucosporidium
2.7. Pichia
2.8. Rhodosporidium
2.9. Rhodotorula
2.10. Sporidiobolus pararoseus
2.11. Sporobolomyces
2.12. Trichosporon
2.13. Yarrowia lipolytica
3. Mold (Aspergillus spp.) as Probiotics
3.1. Aspergillus niger
3.2. Aspergillus oryzae
4. Actinobacteria as Probiotics
5. Combined Application of Yeasts or Actinobacteria along with Other Probiotics
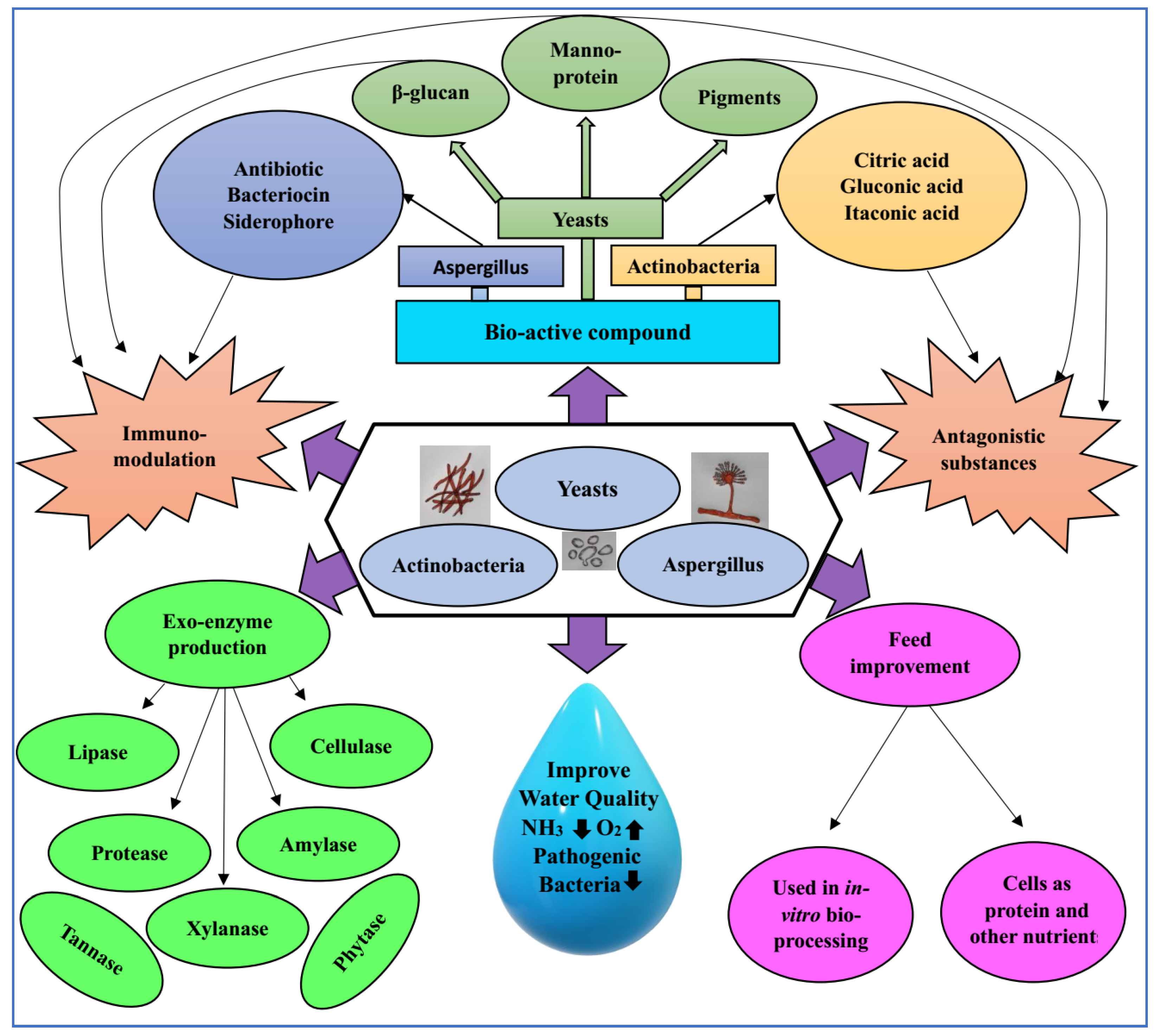
6. Mode of Actions of Probiotic Fungi and Actinobacteria
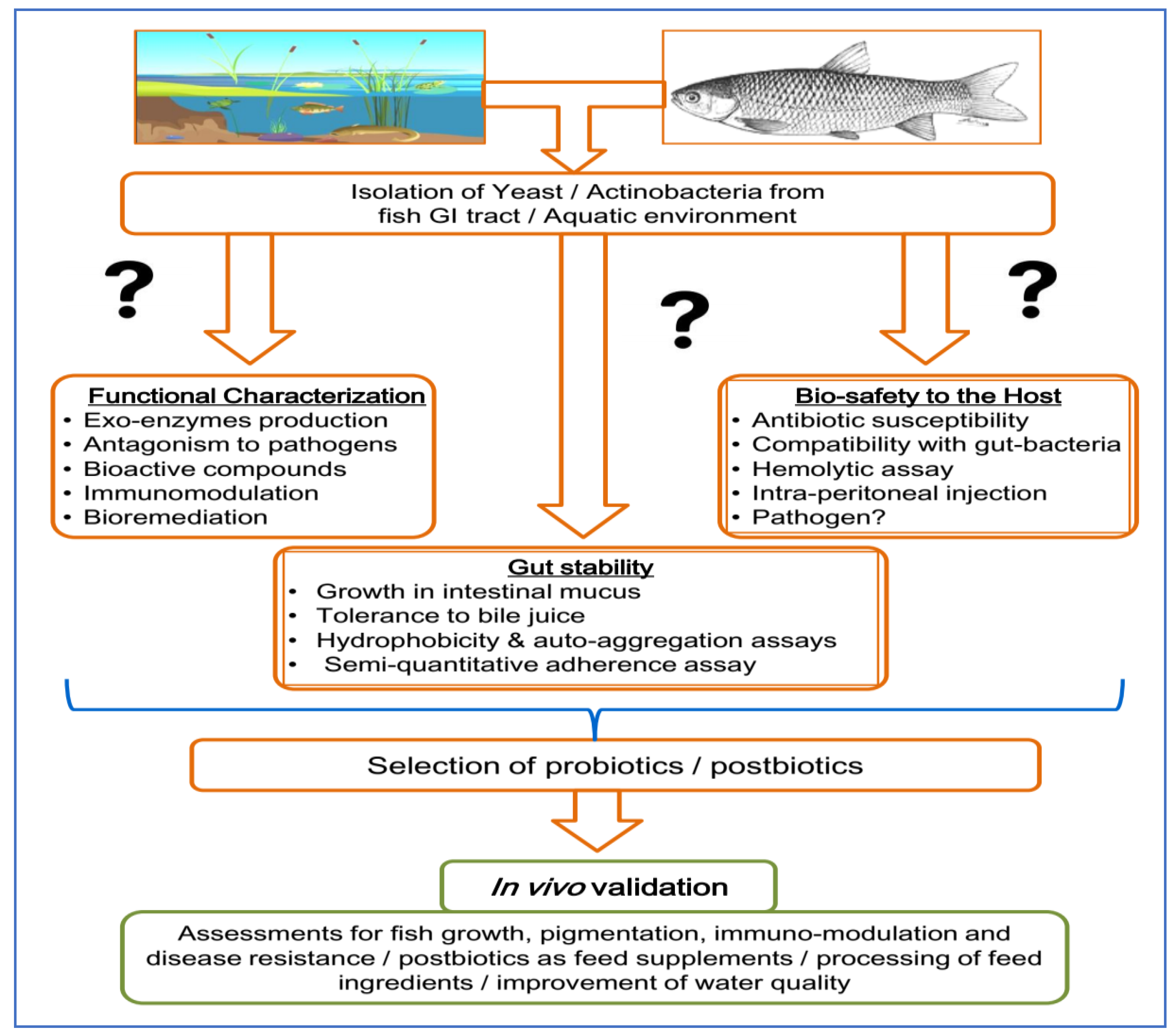
7. Selection of Fungi and Actinobacteria as Probiotics
- (a)
- Influence of temperature, pH and salt concentration (sodium chloride, NaCl) on growth;
- (b)
- Functional characterization, e.g., analyses of exo-enzyme production, antagonism against pathogenic bacteria, antioxidant activity, the production of short-chain fatty acids (SCFA) and vitamins, etc.;
- (c)
- Evaluation of growth and strain survivability against gut pH, pepsin, bile, and gut mucus;
- (d)
- Evaluation of colonization potential (co-cultivation with pathogens to test strain dominance and co-cultivation with other gut microorganisms to test strain compatibility, hydrophobicity, hydrophilicity and auto-aggregation assays);
- (e)
- Evaluation of safety assessment of strains through an antibiotic sensitivity test and hemolytic activity;
- (f)
- In vivo evaluation of the putative probiotic strains on the host via intra-peritoneal injection.
8. Conclusions and Further Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ringø, E.; Li, X.; Doan, H.V.; Ghosh, K. Interesting probiotic bacteria other than the more widely used lactic acid bacteria and bacilli in finfish. Front. Mar. Sci. 2022, 9, 848037. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.; El-Mageed, A.; Taia, A.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, L.; Esteban, M.Á. Administration of probiotics in the water in finfish aquaculture systems: A review. Fishes 2018, 3, 33. [Google Scholar] [CrossRef]
- Ringø, E.; van Doan, H.; Lee, S.H.; Soltani, M.; Hoseinifar, S.H.; Ramasamy, H.; Song, S.K. Probiotics, lactic acid bacteria and bacilli: Interesting supplementation for aquaculture. J. Appl. Microbiol. 2020, 129, 116–136. [Google Scholar] [CrossRef]
- Soltani, M.; Ghosh, K.; Hoseinifar, S.H.; Kumar, V.; Lymbery, A.; Roy, S.; Ringø, E. Genus Bacillus, promising probiotics in aquaculture: Aquatic animal origin, bio-active components, bioremediation in fish and shellfish. Rev. Fish. Sci. Aquacult. 2019, 27, 331–379. [Google Scholar] [CrossRef]
- Nayak, S.K. Multifaceted applications of probiotic bacillus species in aquaculture with special reference to Bacillus subtilis. Rev. Aquacult. 2021, 13, 862–906. [Google Scholar] [CrossRef]
- Gatesoupe, F.J. Live yeasts in the gut: Natural occurrence, dietary introduction, and their effects on fish health and development. Aquaculture 2007, 267, 20–30. [Google Scholar] [CrossRef]
- Navarrete, P.; Tovar-Ramírez, D. Use of yeasts as probiotics in fish aquaculture. Sustain. Aquac. Tech. 2014, 1, 135–172. [Google Scholar]
- Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Streptomyces bacteria as potential probiotics in aquaculture. Front. Microbial. 2016, 7, 79. [Google Scholar]
- Hayatgheib, N.; Moreau, E.; Calvez, S.; Lepelletier, D.; Pouliquen, H. A review of functional feeds and the control of Aeromonas infections in freshwater fish. Aquacult. Inter. 2020, 28, 1083–1123. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Sharin, M.G. Synbiotic effects of Aspergillus oryzae and beta-glucan on growth and oxidative and immune responses of Nile tilapia, Oreochromis niloticus. Prob. Antimic. Prot. 2020, 12, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Jasim, S.A.; Abdelbasset, W.K.; Shichiyakh, A.; Al-Shawi, S.G.; Jalil, A.T.; Karim, Y.S.; Mustafa, Y.F.; Norbakhsh, M. Probiotic effects of the fungi, Aspergillus niger on growth, immunity, haematology, intestine fungal load and digestive enzymes of the common carp, Cyprinus carpio. Aquacult. Res. 2022, 53, 3828–3840. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; Boutin, S.; Hoseinifar, S.H.; Derome, N. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 2014, 5, 207. [Google Scholar] [CrossRef]
- Kurtzman, C. Molecular taxonomy of the yeasts. Yeast 1994, 10, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Øverland, M.; Skrede, A. Yeast derived from lingocellulosic biomass as a sustainable feed resource for use in aquaculture. J. Sci. Food Agric. 2017, 97, 733–742. [Google Scholar] [CrossRef]
- Ceseña, C.E.; Vega-Villasante, F.; Aguirre-Guzman, G.; Luna-Gonzalez, A.; Campa-Cordova, A. Update on the use of yeast in shrimp aquaculture: A mini review. Int. Aquat. Res. 2021, 13, 1–16. [Google Scholar]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.I.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef]
- Shukry, M.; Abd El-Kader, M.F.; Hendam, B.M.; Dawood, M.A.; Farrag, F.A.; Aboelenin, S.M.; Soliman, M.M.; Abdel-Latif, H.M. Dietary Aspergillus oryzae modulates serum biochemical indices, immune responses, oxidative stress, and transcription of HSP70 and cytokine genes in Nile tilapia exposed to salinity stress. Animals 2021, 11, 1621. [Google Scholar] [CrossRef]
- Das, S.; Lyla, P.S.; Khan, A.S. Distribution and generic composition of culturable marine actinomycetes from the sediments of Indian continental slope of Bay of Bengal. Chin. J. Oceanol. Limnol. 2008, 26, 166–177. [Google Scholar] [CrossRef]
- Jensen, P.R.; Mincer, T.J.; Williams, P.G.; Fenical, W. Marine actinomycete diversity and natural product discovery. Antonie Van Leeuwenhoek 2005, 87, 43–48. [Google Scholar] [CrossRef]
- Adel, M.; Lazado, C.; Safari, R.; Yeganeh, S.; Zorriehzahra, M. Aqualase®, a yeast-based in-feed probiotic, modulates intestinal microbiota, immunity and growth of rainbow trout Oncorhynchus mykiss. Aquacult. Res. 2017, 48, 1815–1826. [Google Scholar] [CrossRef]
- Angulo, M.; Reyes-Becerril, M.; Medina-Córdova, N.; Tovar-Ramírez, D.; Angulo, C. Probiotic and nutritional effects of Debaryomyces hansenii on animals. Appl. Microbiol. Biotechnol. 2020, 104, 7689–7699. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Becerril, M.; Salina, I.; Cuesta, A.; Meseguer, J.; Tovar-Ramirez, D.; Ascencio-Valle, F.; Esteban, M. Oral delivery of live yeast Debaryomyces hansenii modulates the main innate immune parameters and the expression of immune-relevant genes in the gilthead seabream (Sparus aurata). Fish Shellfish Immunol. 2008, 25, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Ramirez, D.; Mazurais, D.; Gatesoupe, J.F.; Quazuguel, P.; Cahu, C.L.; Zambonino-Infante, J.L. Dietary probiotic live yeast modulates antioxidant enzyme activities and gene expression of sea bass (Dicentrarchus labrax) larvae. Aquaculture 2010, 300, 142–147. [Google Scholar] [CrossRef]
- Perdichizzi, A.; Meola, M.; Caccamo, L.; Caruso, G.; Gai, F.; Maricchiolo, G. Live Yeast (Saccharomyces cerevisiae var. boulardii) Supplementation in a European Sea Bass (Dicentrarchus labrax) Diet: Effects on the Growth and Immune Response Parameters. Animals 2023, 13, 3383. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Mousavi, S.M.; Ahmadmoradi, E.; Zakeri, M.; Jahedi, A. Effects of Saccharomyces cerevisiae on survival rate and growth performance of convict cichlid (Amatitlania nigrofasciata). Iran. J. Vet. Res. 2015, 16, 59–62. [Google Scholar]
- Waché, Y.; Auffray, F.; Gatesoupe, F.J.; Zambonino, J.; Gayet, V.; Labbé, L.; Quentel, C. Cross effects of the strain of dietary Saccharomyces cerevisiae and rearing conditions on the onset of intestinal microbiota and digestive enzymes in rainbow trout, Onchorhynchus mykiss, fry. Aquaculture 2006, 258, 470–478. [Google Scholar] [CrossRef]
- Essa, M.A.; Mabrouk, H.A.; Mohamed, R.A.; Michael, F.R. Evaluating different additive levels of yeast, Saccharomyces cerevisiae, on the growth and production performances of a hybrid of two populations of Egyptian African catfish, Clarias gariepinus. Aquaculture 2011, 320, 137–141. [Google Scholar] [CrossRef]
- Ran, C.; Huang, L.; Liu, Z.; Xu, L.; Yang, Y.; Tacon, P.; Auclair, E.; Zhou, Z. A comparison of the beneficial effects of live and heat-in activated baker`s yeast on Nile tilapia: Suggestions on the role and function of the secondary metabolites released from yeast. PLoS ONE 2015, 10, e0145448. [Google Scholar] [CrossRef]
- Ran, C.; Huang, L.; Hu, J.; Tacon, P.; He, S.; Li, Z.M.; Wang, Y.; Liu, Z.; Xu, L.; Yang, Y.L.; et al. Effects of dietary live and heat-inactivate baker`s yeast on growth, gut health, and disease resistance of Nile tilapia under high rearing density. Fish Shellfish Immunol. 2016, 56, 263–271. [Google Scholar] [CrossRef]
- Huyben, D.; Sun, L.; Moccia, R.; Kiessling, A.; Dicksved, J.; Lundh, T. Dietary live yeast and incresed water temperature influence the gut microbiota of rainbow trout. J. Appl. Microbiol. 2018, 124, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Salah, W.; Gafaar, A.; Tanekhy, M.; Alsenosy, A.; Elbialy, A.A.; Abaza, S.; Soliman, M. Efficacy of dietary Saccharomyces cerevisiae supplementation with inclusion of Q Z TossTM on Nile tilapia. Damanhour J. Vet. Sci. 2019, 2, 9–13. [Google Scholar] [CrossRef]
- Dias, M.K.R.; Yoshioka, E.T.O.; Rodriguez, A.F.R.; Ribeiro, R.A.; Faria, F.S.E.D.V.; Almeida Ozorio, R.O.A.; Tavares-Dias, M. Growth and hematological and immunological responses of Arapaima gigas fed diets supplemented with immunostimulant based on Saccharomyces cerevisiae and subjected to handling stress. Aquacult. Rep. 2020, 17, 100335. [Google Scholar]
- Hansen, J.Ø.; Lagos, L.; Lei, P.; Reveco-Urzua, F.E.; Morales-Lange, B.; Hansen, L.D.; Schiavone, M.; Mydland, L.T.; Arntzen, M.Ø.; Mercado, L.; et al. Down-stream processing of baker’s yeast (Saccharomyces cerevisiae)—Effect on nutrient digestibility and immune response in Atlantic salmon (Salmo salar). Aquaculture 2021, 530, 735707. [Google Scholar] [CrossRef]
- Jahan, N.; Islam, S.M.M.; Rohani, M.F.; Hossain, M.T.; Shahjahan, M. Probiotic yeast enhances growth performance of rohu (Labeo rohita) through upgrading hematology, and intestinal microbiota and morphology. Aquaculture 2021, 545, 737243. [Google Scholar] [CrossRef]
- El-Bab, A.F.F.; Saghir, S.A.M.; El-Naser, I.A.A.; El-Kheir, S.M.M.A.; Abdel-Kader, M.F.; Alruhaimi, R.S.; Alqhtani, H.A.; Mahmoud, A.M.; Naiel, M.A.E.; El-Raghi, A.A. The effect of dietary Saccharamyces cerevisiae on growth preformance, oxidative status, and immune response of sea bream (Sparus aurata). Life 2022, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhou, Z.; Meng, K.; Zhao, H.; Yao, B.; Ringø, E.; Yoon, I. Effects of dietary antibiotic growth promoter and Saccharomyces cerevisiae fermentation product on production, intestinal bacterial community, and nonspecific immunity of hybrid tilapia (Oreochromis niloticus female × Oreochromis aureus male). J. Anim. Sci. 2011, 89, 84–92. [Google Scholar] [CrossRef]
- Ortuño, J.; Cuesta, A.; Rodríguez, A.; Esteban, M.; Meseguer, J. Oral administration of yeast, Saccharomyces cerevisiae, enhances the cellular innate immune response of gilthead seabream (Sparus aurata L.). Vet. Immunol. Immunopathol. 2002, 85, 41–50. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M.; Mahmoud, R.; El-Araby, I.E. Effect of dietary yeast nucleotide on antioxidant activity, non-specific immunity, intestinal cytokines, and disease resistance in Nile tilapia. Fish Shellfish Immunol. 2018, 80, 281–290. [Google Scholar] [CrossRef]
- Pelusio, N.F.; Parma, L.; Volpe, E.; Ciulli, S.; Errani, F.; Natale, S.; De Cesare, A.; Indio, V.; Carcano, P.; Mordenti, O.; et al. Yeast-extracted nucleotides and nucleic acids as promising feed additives for European sea bass (Dicentrarchus labrax) juveniles. Front. Mar. Sci. 2023, 10, 1145660. [Google Scholar] [CrossRef]
- Barducci, R.S.; de Abreu, V.; Aparecido, A.; Santos, D.; Pacheco, L.G.; Koch, J.F.A.; Florencio, M.; Pilarski, F. Natural feed additive containing Saccharomyces cerevisiae-originated free nucleotides improves innate immunity, gut histology and disease resistance in Nile tilapia. Anim. Feed Sci. Technol. 2022, 289, 115337. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, R.; Mo, J.; Yang, S.; Gu, N.; Wu, Z.; Babu, V.S.; Li, J.; Huang, Y.; Lin, L. Effects of brewer`s yeast hydrolysate on the growth performance and the intestinal bacterial diversity of largemouth bass (Micropterus salmoides). Aquaculture 2018, 484, 139–144. [Google Scholar] [CrossRef]
- Vargas, O.; Gutiérrez, M.S.; Caruffo, M.; Valderrama, B.; Medina, D.A.; Garcia, K.; Reyes-Jara, A.; Toro, M.; Feijóo, C.G.; Navarrete, P. Probiotic yeasts and Vibrio anguillarum infection modify the microbiome of zebrafish larvae. Front. Microbiol. 2021, 12, 647977. [Google Scholar] [CrossRef] [PubMed]
- Sanahuja, I.; Ruiz, R.; Firmino, J.P.; Reyes-López, F.E.; Ortiz-Delgado, J.B.; Vallejos-Vidal, E.; Tort, L.; Tovar-Ramírez, D.; Cerezo, I.M.; Moriñigo, M.S.; et al. Debaryomyces hansenii supplementation in low fish meal diets promotes growth, modulates microbiota and enhances intestinal conditions in juvenile marine fish. J. Anim. Sci. Biotechnol. 2023, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, M.; Zuberi, A.; Amir, I.; Imran, M.; Noor, Z. Effect of probiotic Geotrichum candidum on early rearing of Labeo rohita (Hamilton, 1822). Turkish J. Fish. Aquatic Sci. 2017, 17, 1263–1270. [Google Scholar]
- Amir, I.; Zuberi, A.; Kamran, M.; Imran, M.; Murtaza, M.U.H. Evaluation of commercial application of dietary encapsulated probiotic (Geotrichum candidum QAUGC01): Effect on growth and immunological indices of rohu (Labeo rohita, Hamilton 1822) in semi-intensive culture system. Fish Shellfish Immunol. 2019, 95, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Ghori, I.; Tubassam, M.; Ahmad, T.; Zuberi, A.; Imran, M. Gut microbiome modulation mediated by probiotics: Positive impact on growth and health status of Labeo rohita. Front. Physiol. 2022, 13, 949559. [Google Scholar] [CrossRef]
- Huda, N.-U.; Liu, H.; Jin, J.; Zhu, X.; Han, D.; Yang, Y.; Xie, S. Dietary supplementation of Gentrichum candidum improves growth, gut microbiota, immune-related gene expression and disease resistance in gibel carp CAS III (Carassius auratus gibelio). Fish Shellfish Immunol. 2020, 99, 144–153. [Google Scholar]
- Van Doan, H.; Tapingkae, W.; Chaiyaso, T.; Wangkahart, E.; Panchan, R.; Sutthi, N. Effects of Red Yeast (Sporidiobolus pararoseus) on Growth, Innate Immunity, Expression of Immune-related Genes and Disease Resistance of Nile Tilapia (Oreochromis niloticus). Probiot. Antimicrob. Prot. 2022, 15, 1312–1326. [Google Scholar] [CrossRef]
- Agboola, J.O.; Mensah, D.D.; Hansen, J.Ø.; Lapeña, D.; Mydland, L.T.; Arntzen, M.Ø.; Horn, S.J.; Øyås, O.; Press, C.M.; Øverland, M. Effects of yeast species and processing on intestinal health and transcriptomic profiles of Atlantic salmon (Salmo salar) fed soybean meal-based diets in seawater. Int. J. Mol. Sci. 2022, 23, 1675. [Google Scholar] [CrossRef]
- Leeper, A.; Ekmay, R.; Knobloch, S.; Skirnisdóttir, S.; Varunjikar, M.; Dubois, M.; Smárason, B.O.; Arnason, J.; Koppe, W.; Benhaim, D. Torula yeast in the diet of Atlantic salmon Salmo salar and the impact on growth performance and gut microbiome. Sci. Rep. 2022, 12, 567. [Google Scholar] [CrossRef]
- Suguna, S.; Rajendran, K. Production of probiotics from Streptomyces sp. associated with fresh water fish and its growth evaluation on Xiphorous helleri. Int. J. Pharm. Biol. Sci. Arch. 2012, 3, 601–603. [Google Scholar]
- Selvakumar, D.; Jyothi, P.; Dhevendaran, K. Application of Streptomyces as a single cell protein to the juvenile fish Xiphophorus maculatus. World J. Fish Mar. Sci. 2013, 5, 582–586. [Google Scholar]
- Arghideh, M.; Hoseinifar, S.H.; Nasrabadi, R.G.; Mazandarani, M.; El-Haroun, E.; Van Doan, H. Evaluation of soil-derived Streptomyces chartreusis KU324443 effects as probiotic on growth performance, antioxidant enzyme activity, mucosal and serum immune parameters, and related gene expression in common carp (Cyprinus carpio) fingerlings. Aquacult. Nutr. 2022, 2022, 2278130. [Google Scholar] [CrossRef]
- Lewis, J.W.; Morley, N.J.; Drinkall, J.; Jamieson, B.J.; Wright, R.; Parry, J.D. Toxic effects of Streptomyces griseus spores and exudate on gill pathology of freshwater fish. Ecotoxicol. Environ. Saf. 2008, 72, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.F.; Hu, Y.; Zhu, B.; Wang, G.X. Antiviral activity of anisomycin against spring viraemia of carp virus in epithelioma papulosum cyprini cells and zebrafish. Virus Res. 2019, 268, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraj, S.; Dhevendaran, K. Evaluation of Streptomyces as a probiotic feed for the growth of ornamental fish Xiphophorus helleri. Food Technol. Biotechnol. 2010, 48, 497–504. [Google Scholar]
- Galao, R.P.; Scheller, N.; Alves-Rodrigues, I.; Breinig, T.; Meyerhans, A.; Díez, J. Saccharomyces cerevisiae: A versatile eukaryotic system in virology. Microb. Cell Fact. 2007, 6, 32. [Google Scholar] [CrossRef]
- Mandal, S.; Ghosh, K. Isolation of tannase producing microbiota from the gastrointestinal tracts of some freshwater fish. J. Appl. Ichthyol. 2013, 29, 145–153. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghosh, K. Enumeration of gut associated extracellular enzyme producing yeasts in some freshwater fishes. J. Appl. Ichthyol. 2014, 30, 986–993. [Google Scholar] [CrossRef]
- Das, P.; Ghosh, K. The presence of phytase in yeasts isolated from the gastrointestinal tract of four major carps [Labeo rohita (Hamilton, 1822), Catla catla (Hamilton, 1822), Cirrhinus mrigala (Hamilton, 1822), Hypophthalmichthys molitrix (Valenciennes, 1844)], climbing perch [Anabas testudineus (Bloch, 1792)] and Mozambique tilapia [Oreochromis mossambicus (Linnaeus, 1758)]. J. Appl. Ichthyol. 2014, 30, 403–407. [Google Scholar]
- del Valle, J.C.; Bonadero, M.C.; Gimenez, A.V.F. Saccharomyces cerevisiae as probiotic, prebiotic, synbiotic, postbiotics and parabiotics in aquaculture: An overview. Aquaculture 2023, 569, 739342. [Google Scholar] [CrossRef]
- Noh, H.; Han, K.I.; Won, T.H.; Choi, Y.J. Effect of antibiotics, enzymes, yeast culture and probiotics on the growth performance of Israeli carp. Korean J. Anim. Sci. 1994, 36, 480–486. [Google Scholar]
- Lara-Flores, M.; Olvera-Novoa, M.A.; Guzmán-Méndez, B.E.; López-Madrid, W. Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture 2003, 216, 193–201. [Google Scholar] [CrossRef]
- Gopalakannan, A.; Arul, V. Enhancement of the innate immune system and disease-resistant activity in Cyprinus carpio by oral administration of β-glucan and whole cell yeast. Aquacult. Nutr. 2010, 41, 884–892. [Google Scholar] [CrossRef]
- Chiu, C.H.; Cheng, C.H.; Gua, W.R.; Guu, Y.K.; Cheng, W. Dietary administration of the probiotic, Saccharomyces cerevisiae P13, enhanced the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol. 2010, 29, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Abdel-Rahman, A.M.; Ismael, N.E.M. Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture 2008, 280, 185–189. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, M.C.; Kim, J.S.; Balasundaram, C.; Heo, M.S. Immunomodulatory effect of probiotics enriched diets on Uronema marinum infected olive flounder. Fish Shellfish Immunol. 2011, 30, 964–971. [Google Scholar] [CrossRef]
- Li, P.; Gatlin, D.M., III. Evaluation of brewer’s yeast (Saccharomyces cerevisiae) as a feed supplement for hybrid striped bass (Morone chrysops × M. saxatilis). Aquaculture 2003, 219, 681–692. [Google Scholar] [CrossRef]
- Li, P.; Gatlin, D.M., III. Dietary brewer’s yeast and the prebiotic Grobiotic™ AE influence growth performance, immune responses and resistance of hybrid striped bass (Morone chrysops × M. saxatilis) to Streptococcus iniae infection. Aquaculture 2004, 231, 445–456. [Google Scholar] [CrossRef]
- Li, P.; Gatlin, D.M., III. Evaluation of the prebiotic GroBiotic®-A and brewer’s yeast as dietary supplements for sub-adult hybrid striped bass (Morone chrysops × M. saxatilis) challenged in situ with Mycobacterium marinum. Aquaculture 2005, 248, 197–205. [Google Scholar] [CrossRef]
- Pal, D.; Joardar, S.N.; Roy, B. Immunostimulatory effects of a yeast (Saccharomyces cerevisiae) cell wall feed supplement on rohu (Labeo rohita), an Indian major carp. Israeli J. Aquacult. Bamidgeh. 2007, 59, 175–181. [Google Scholar] [CrossRef]
- Ramakrishnan, C.M.; Haniffa, M.A.; Manohar, M.; Dhanaraj, M.; Arockiaraj, J.; Seetharaman, S.; Arunsingh, S.V. Effects of probiotics and spirulina on survival and growth of juvenile common carp (Cyprinus carpio). Israeli J. Aquacult. 2008, 60, 128–133. [Google Scholar] [CrossRef]
- El-Boshy, M.E.; Ahmed, M.; AbdelHamid, F.M.; Gadalla, H.A. Immunomodulatory effect of dietary Saccharomyces cerevisiae, β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2010, 28, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Tukmechi, A.; Andani, H.R.R.; Manaffar, R.; Sheikhzadeh, N. Dietary administration of beta-mercapto-ethanol treated Saccharomyces cerevisiae enhanced the growth, innate immune response and disease resistance of the rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 2011, 30, 923–928. [Google Scholar] [CrossRef]
- Welker, T.L.; Lim, C.; Yildirim-Aksoy, M.; Klesius, P.H. Effect of short-term feeding duration of diets containing commercial whole-cell yeast or yeast subcomponents on immune function and disease resistance in channel catfish, Ictalurus punctatus. J. Anim. Physiol. Anim. Nutr. 2012, 96, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Ciji, A.; Tripathi, P.H.; Sharma, P. Dietary β-glucan influences the expression of testicular aquaporins, antioxidative defence genes and sperm quality traits in endangered golden mahseer, Tor putitora (Hamilton, 1822). Int. J. Biol. Macromol. 2021, 193, 1286–1293. [Google Scholar] [CrossRef]
- Tewary, A.; Patra, B.C. Oral administration of baker’s yeast (Saccharomyces cerevisiae) acts as a growth promoter and immunomodulator in Labeo rohita (Ham.). J. Aquac. Res. Dev. 2011, 2, 109. [Google Scholar] [CrossRef]
- El-feky, M.M.; Essa, M.A.; Osman, A.G.M.; Shalaby, S.M.; Moustafa, A.M. Growth Performance of African Catfish Clarias gariepinus (Burchell, 1822) treated with Live Baker’s Yeast (Saccharomyces cerevisiae) in Egypt. Int. J. Biotech. Bioeng. 2017, 3, 176–187. [Google Scholar]
- Osman, H.A.M.; Ibrahim, T.B.; Soliman, W.E.; Monier, M.M. Influence of dietary commercial baker’s yeast, Saccharomyces cerevisiae on growth performance, survival and immunostimulation of Oreochromis niloticus challenged with Aeromonas hydrophila. Nat. Sci. 2010, 8, 96–103. [Google Scholar]
- Pooramini, M.; Kamali, A.; Hajimoradloo, A.; Alizadeh, M.; Ghorbani, R. Effect of using yeast (Saccharomyces cerevisiae) as probiotic on growth parameters, survival and carcass quality in rainbow trout Oncorhynchus mykiss fry. Int. Aquacult. Res. 2009, 1, 39–44. [Google Scholar]
- Abdel-Tawwab, M. Interactive effects of dietary protein and live bakery yeast, Saccharomyces cerevisiae on growth performance of Nile tilapia, Oreochromis niloticus (L.) fry and their challenge against Aeromonas hydrophila infection. Aquacult. Int. 2011, 20, 317–331. [Google Scholar] [CrossRef]
- Ozório, R.O.; Portz, L.; Borghesi, R.; Cyrino, J.E. Effects of dietary yeast (Saccharomyces cerevisia) supplementation in practical diets of tilapia (Oreochromis niloticus). Animals 2012, 2, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Pooramini, M.; Kamali, A.; Hajimoradloo, A.; Alizadeh, M.; Ghorbani, R.; Hatami, R.; Haghparast, S. The effects of different concentrations of probiotic Saccharomyces cerevisiae on growth performance and survival rate of rainbow trout (Oncorhynchus mykiss), fry and resistance against salinity. Afr. J. Biotechnol. 2014, 13, 1160–1168. [Google Scholar]
- Caruffo, M.; Navarrete, N.; Salgado, O.; Díaz, A.; López, P.; García, K.; Feijóo, C.G.; Navarrete, P. Potential probiotic yeasts isolated from the fish gut protect zebrafish (Danio rerio) from a Vibrio anguillarum challenge. Front. Microbiol. 2015, 6, 1093. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Mousavi, S.M.; Zakeri, M.; Ebtesam, A. Effect of dietary probiotic, Saccharomyces cerevisiae on growth performance, survival rate and body biochemical composition of three spot cichlid (Cichlasomatrim aculatum). AACL Bioflux 2016, 9, 451–457. [Google Scholar]
- Rachmawati, D.; Hutabarat, J.; Samidjan, I.; Herawati, V.E.; Windarto, S. The effects of Saccharomyces cerevisiae-enriched dieton feed usage efficiency, growth performance and survival rate in Java barb (Barbonymus gonionotus) fingerlings. AACL Bioflux 2019, 12, 1841–1849. [Google Scholar]
- Yasin, R.; Jabeen, F.; Ali, M.; Malik, K.S.; Hussain, S.M. Effects of yeast (Saccharomyces cerevisiae) on the intestinal microbiota of GIFT tilapia (Oreochromis mossambicus). Int. J. Biosci. 2018, 12, 283–291. [Google Scholar]
- Meena, D.K.; Das, P.; Kumar, S.; Mandal, S.C.; Prusty, A.K.; Singh, S.K.; Akhtar, M.S.; Behera, B.K.; Kumar, K.; Pal, A.K.; et al. Beta-glucan: An ideal immunostimulant in aquaculture (a review). Fish Physiol. Biochem. 2013, 39, 431–457. [Google Scholar] [CrossRef]
- Li, P.; Lewis, D.H.; Gatlin III, D.M. Dietary oligonucleotides from yeast RNA influence immune responses and resistance of hybrid striped bass (Morone chrysops × Morone saxatilis) to Streptococcus iniae infection. Fish Shellfish Immunol. 2004, 16, 561–569. [Google Scholar] [CrossRef]
- Abu-Elala, N.; Marzouk, M.; Moustafa, M. Use of different Saccharomyces cerevisiae biotic forms as immune-modulator and growth promoter for Oreochromis niloticus challenged with some fish pathogens. Int. J. Vet. Sci. Med. 2013, 1, 21–29. [Google Scholar] [CrossRef]
- Savin, V.; Patriche, N.; Mocanu, E.; Tenciu, M.; Lăcătuș, M.; Popa, D.; Savin, C. The effect of Saccharomyces boulardii yeast feed supplementation on growth parameters and biochemical composition of carp (Cyprinus carpio). USAMV Iasi 2019, 72, 188–192. [Google Scholar]
- Nematzadeh, K.; Ahmadifard, N.; Naser, S.; Agh, N.; Ghaderpour, S. The effects of zinc-enriched Saccharomyces cerevisiae on the growth and mineral composition of marine rotifer, Brachionus plicatilis. Int. J. Aquatic. Biol. 2018, 6, 88–94. [Google Scholar]
- Opiyo, M.A.; Jumbe, J.; Ngugi, C.C.; Charo-Karisa, H. Different levels of probiotics affect growth, survival and body composition of Nile tilapia (Oreochromis niloticus) cultured in low input ponds. Sci. Afr. 2019, 4, e00103. [Google Scholar] [CrossRef]
- Opiyo, M.A.; Jumbe, J.; Ngugi, C.C.; Charo-Karisa, H. Dietary administration of probiotics modulates non-specific immunity and gut microbiota of Nile tilapia (Oreochromis niloticus) cultured in low input ponds. Int. J. Vet. Sci. 2019, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Banu, M.R.; Akter, S.; Islam, M.R.; Mondol, M.N.; Hossain, M.A. Probiotic yeast enhanced growth performance and disease resistance in freshwater catfish gulsa tengra, Mystus cavasius. Aquacult. Rep. 2020, 16, 100237. [Google Scholar] [CrossRef]
- MajharulIslam, S.M.; Rohani, M.F.; Shahjahan, M. Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquacult. Rep. 2021, 21, 100800. [Google Scholar]
- Rawling, M.; Schiavone, M.; Apper, E.; Merrifield, D.L.; Castex, M.; Leclercq, E.; Foey, A. Yeast cell wall extracts from Saccharomyces cerevisiae varying in structure and composition differentially shape the innate immunity and mucosal tissue responses of the intestine of zebrafish (Danio rerio). Front. Immunol. 2023, 14, 1158390. [Google Scholar] [CrossRef]
- Andlid, T.; Vázquez-Juárez, R.; Gustafsson, L. Yeasts isolated from the intestine of rainbow trout adhere to and grow in intestinal mucus. Mol. Mar. Biol. Biotechnol. 1998, 7, 115–126. [Google Scholar]
- Siangpro, N.; Chuakrut, S.; Sirimanapong, W.; Tanasupawat, S.; Phongsopitanun, W.; Meksiriporn, B.; Boonnorat, J.; Sarin, S.; Kucharoenphaibul, S.; Jutakanoke, R. Lactiplantibacillus argentoratensis and Candida tropicalis Isolated from the Gastrointestinal Tract of Fish Exhibited Inhibitory Effects against Pathogenic Bacteria of Nile Tilapia. Vet. Sci. 2023, 10, 129. [Google Scholar] [CrossRef]
- Taha, M.D.; Didinen, B.I.; Onuk, E.E.; Metin, S.; Yilmaz, S.; Mohamed, A.A.; Pakır, S.; Gülşen, O.; Abdel-Latif, H.M. Identification of four autochthonous yeasts from the intestines of goldfish, Carassius auratus with potential probiotic properties and their effects on the most common fish bacterial pathogens. Microb. Pathog. 2023, 184, 106381. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, A.M.; Schafer, A.M.; Begerow, D. Leucoporidium drummii sp. nov., a member of the Microbotryomycetes isolated from soil. Int. J. Syst. Evol. Micr. 2012, 62, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Arthur, H.; Watson, K. Thermal adaptation in yeast: Growth temperatures, membrane lipid, and cytochrome composition of psychrophilic, mesophilic, and thermophilic yeasts. J. Bacteriol. 1976, 128, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Crooker, P.; Obreque-Contreras, J. Bioremediation of aquaculture wastes. Curr. Opin. Biotechnol. 2010, 21, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Summerbell, R.C. The heterobasidiomycetous yeast genus Leucosporidium in an area of temperate climate. Canad. J. Bot. 1983, 61, 1402–1410. [Google Scholar] [CrossRef]
- Turkiewicz, M.; Pazgier, M.; Donachie, S.P.; Kalinowska, H. Invertase and a-glucosidase production by the endemic Antarctic marine yeast Leucosporidium antarcticum. Pol. Polar Res. 2005, 26, 125–136. [Google Scholar]
- Shivaji, S.; Prasad, G.S. Antarctic yeasts: Biodiversity and potential applications. In Yeast Biotechnology: Diversity and Applications; Springer: Dordrecht, The Netherlands, 2009; pp. 3–18. [Google Scholar]
- Turkiewicz, M.; Pazgier, M.; Kalinowska, H.; Bielecki, S. A cold-adapted extracellular serine proteinase of the yeast Leucosporidium antarcticum. Extremophiles 2003, 7, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.W.F.; Bonugli-Santos, R.C.; Duarte, A.L.F.; Gomes, E.; Sette, L.D. Statistical experimental design applied to extracellular lipase production by the marine Antarctic yeast Leucosporidium scottii CRM 728. Biocatal. Agric. Biotechnol. 2021, 32, 101954. [Google Scholar] [CrossRef]
- Azhar, M.A. Cloning, Expression and Analysis of α-Amylase Gene from Psychrophilic Yeast Leucosporidium antarcticum PI12. Ph.D. Thesis, Universiti Teknologi Malaysia, Johor Bahru, Malaysia, 2012. [Google Scholar]
- Andlid, T.; Vázquez-Juárez, R.; Gustafsson, L. Yeast colonizing the intestine of rainbow trout (Salmo gairdneri) and turbot (Scophthalmus maximus). Microb. Ecol. 1995, 30, 321–334. [Google Scholar] [CrossRef]
- Tovar, D.; Zambonino, J.; Cahu, C.; Gatesoupe, F.J.; Vázquez-Juárez, R.; Lésel, R. Effect of live yeast incorporation in compound diet on digestive enzyme activity in sea bass (Dicentrarchus labrax) larvae. Aquaculture 2002, 204, 113–123. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Adeola, O. Carbohydrases, protease, and phytase have an additive beneficial effect in nutritionally marginal diets for broiler chicks. Poult. Sci. 2005, 84, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Fru-Nji, F.; Kluenter, A.M.; Fischer, M.; Pontoppidan, K. A feed serine protease improves broiler performance and increases protein and energy digestibility. J. Poult. Sci. 2011, 48, 239–246. [Google Scholar] [CrossRef]
- Ogata, K.; Nishikawa, H.; Ohsugi, M. A yeast capable of utilizing methanol. Agr. Biol. Chem. 1969, 33, 1519–1520. [Google Scholar] [CrossRef]
- Rebnegger, C.; Vos, T.; Graf, A.B.; Valli, M.; Pronk, J.T.; Daran-Lapujade, P.; Mattanovich, D. Pichia pastoris exhibits high viability and a low maintenance energy requirement at near-zero specific growth rates. Appl. Environ. Microbiol. 2016, 82, 4570–4583. [Google Scholar] [CrossRef] [PubMed]
- Cregg, J.M.; Barringer, K.J.; Hessler, A.Y.; Madden, K.R. Pichia pastoris as a host system for transformations. Mol. Cell Biol. 1985, 5, 3376–3385. [Google Scholar]
- Passoth, V.; Fredlund, E.; Druvefors, U.Ä.; Schnürer, J. Biotechnology, physiology and genetics of the yeast Pichia anomala. FEMS Yeast Res. 2005, 6, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Banerjee, S.; Moon, U.M.; Khan, H.A.; Dutta, D. Evaluation of gut associated extracellular enzyme-producing and pathogen inhibitory microbial community as potential probiotics in Nile tilapia, Oreochromis niloticus. Int. J. Aquacult. 2017, 7, 143–158. [Google Scholar] [CrossRef]
- Acosta, J.; Morales, R.; Morales, A.; Alonso, M.; Estrada, M.P. Pichia pastoris expressing recombinant tilapia growth hormone accelerates the growth of tilapia. Biotechnol. Lett. 2007, 29, 1671–1676. [Google Scholar] [CrossRef]
- Yu-Fang, S.; Yan, T.; Jing, X.; Yun-Fang, Q. Biosynthesis of Crucian Carp (Carassius auratus) c-type Lysozyme Based on Recombinant Pichia pastoris and Its Characteristics. J. Agric. Biotechnol. 2021, 27, 2177–2188. [Google Scholar]
- Pinto, V.B.; Costenaro-Ferreira, C.; Oliveira, P.L.S.; Oliveira, R.R.B.D.; Piedras, S.R.N.; Pouey, J.L.O.F. Performance of jundiá larvae, Rhamdia quelen, fed on probiotic supplemented diets. Acta Scientiarum. Anim. Sci. 2015, 37, 215–220. [Google Scholar] [CrossRef]
- Islam, F.; Salam, M.A.; Rahman, M.A.; Paul, S.I.; Das, T.R.; Rahman, M.M.; Shaha, D.C.; Gupta, D.R.; Alam, M.S.; Islam, T. Plant endophytic yeasts Pichia fermentans and Meyerozyma caribbica improve growth, biochemical composition, haematological parameters and morphology of internal organs of premature Barbonymus gonionotus. Aquacult. Rep. 2021, 19, 100575. [Google Scholar] [CrossRef]
- Ghosh, K.; Mandal, S. Nutritional evaluation of groundnut oil cake in formulated diets for rohu, Labeo rohita (Hamilton) fingerlings after solid state fermentation with a tannase producing yeast, Pichia kudriavzevii (GU939629) isolated from fish gut. Aquac. Rep. 2015, 2, 82–90. [Google Scholar] [CrossRef]
- Mandal, S.; Ghosh, K. Utilization of fermented Pistia leaves in the diet of rohu, Labeo rohita (Hamilton): Effects on growth, digestibility and whole body composition. Waste Biomass Valorization 2019, 10, 3331–3342. [Google Scholar] [CrossRef]
- Sealey, W.M.; Conley, Z.B.; Hinman, B.T.; O’Neill, T.J.; Bowzer, J.; Block, S.S. Evaluation of the ability of Pichia guilliermondii to improve growth performance and disease resistance in rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2022, 53, 411–423. [Google Scholar] [CrossRef]
- Harnpicharnchai, P.; Sornlake, W.; Tang, K.; Eurwilaichitr, L.; Tanapongpipat, S. Cell-surface phytase on Pichia pastoris cell wall offers great potential as a feed supplement. FEMS Microbiol. Lett. 2010, 302, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ganapathiwar, S.; Bhukya, B. In vitro assessment for the probiotic potential of Pichia kudriavzevii. Bioinformation 2023, 19, 441. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Lopez, P.; Diaz, A.; Carrasco, D.; Silva, A.; Velez, A.; Opazo, R.; Magne, F.; Navarrete, P.A. Debaryomyces hansenii and Rhodotorula mucilaginosa comprised the yeast core gut microbiota of wild and reared carnivorous salmonids, croaker and yellowtail. Environ. Microbiol. 2014, 16, 2791–2803. [Google Scholar] [CrossRef]
- Bass, D.; Howe, A.; Brown, N.; Barton, H.; Demidova, M.; Michelle, H.; Li, L.; Sanders, H.; Watkinson, S.C.; Willock, S.; et al. Yeast forms dominate fungal diversity in the deep oceans. Proc. Royal. Soc. B 2007, 274, 3069–3077. [Google Scholar] [CrossRef]
- Chen, Y.S.; Yanagida, F.; Chen, L.Y. Isolation of marine yeasts from coastal waters of northeastern Taiwan. Aqua. Biol. 2009, 8, 55–60. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Z.B.; Bai, F.W. Optimization of culture conditions for lipid production by Rhodosporidium toruloides. J. Biotechnol. 2006, 22, 650–656. [Google Scholar] [CrossRef]
- Scholz, U.; Diaz, G.G.; Ricque, D.; Suarez, L.E.C.; Albores, F.V.; Larchford, J. Enhancement of vibrios resistance in juvenile Penaeus vannamei by supplementation of diets with different yeast products. Aquaculture 1999, 176, 271–283. [Google Scholar] [CrossRef]
- Barron, C.C.; Sponagle, B.J.; Arivalagan, P.; D’Cunha, G.B. Optimization of oligomeric enzyme activity in ionic liquids using Rhodotorula glutinis yeast phenylalanine ammonia lyase. Enzyme Microb. Technol. 2017, 96, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cui, W.; Fang, Y.; Liu, Y.; Gao, X.; Zhou, Z. Cloning, expression and characterization of phenylalanine ammonia-lyase from Rhodotorula glutinis. Biotechnol. Lett. 2013, 35, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.T.; Hernandez, R.; Holmes, W.E.; Claupein, W. Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Kolouchová, I.; Schreiberová, O.; Sigler, K.; Masák, J.; Řezanka, T. Biotransformation of volatile fatty acids by oleaginous and non-oleaginous yeast species. FEMS Yeast Res. 2015, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Fazeli, G.; Di Micco, L.; Autore, G.; Adesso, S.; Dal Piaz, F.; Heidland, A.; Di Iorio, B. Supplementation of short-chain fatty acid, sodium propionate, in patients on maintenance hemodialysis: Beneficial effects on inflammatory parameters and gut-derived uremic toxins, a pilot study (PLAN Study). J. Clin. Med. 2018, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Jiang, Y.; Gong, C. Exogenous xylanase expression simultaneously with the indigenous cellulase to increase the cellulose hydrolysis efficiency. Int. Biodeterior. Biodegrad. 2019, 140, 126–132. [Google Scholar] [CrossRef]
- Korhola, M.; Hakonen, R.; Juuti, K.; Edelmann, M.; Kariluoto, S.; Nyström, L.; Sontag-Strohm, T.; Piironen, V. Production of folate in oat bran fermentation by yeasts isolated from barley and diverse foods. J. Appl. Microbiol. 2014, 117, 679–689. [Google Scholar] [CrossRef]
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal fungi in health and disease. Cell Host Microbe 2017, 22, 156–165. [Google Scholar] [CrossRef]
- Xue, S.J.; Chi, Z.; Zhang, Y.; Li, Y.F.; Liu, G.L.; Jiang, H.; Hu, Z.; Chi, Z.M. Fatty acids from oleaginous yeasts and yeast-like fungi and their potential applications. Crit. Rev. Biotechnol. 2018, 38, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Hof, H. Rhodotorula spp. in the gut–foe or friend? GMS Infect. Dis. 2019, 7. [Google Scholar]
- Yang, J.; Wang, Y.; Jiang, T.; Lv, Z. Novel branch patterns and anticoagulant activity of glycosaminoglycan from sea cucumber Apostichopus japonicus. Int. J. Biol. Macromol. 2015, 72, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Zhao, W.; Xie, S.W.; Xie, J.J.; Zhang, Z.H.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 90, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lin, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Cloning and expression analysis of c-type lysozyme gene in golden pompano, Trachinotus ovatus. Fish Shellfish Immunol. 2016, 54, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Rekha, R.; Nimsi, K.A.; Manjusha, K.; Sirajudheen, T.K. Marine yeast Rhodotorula paludigena VA 242 a pigment enhancing feed additive for the ornamental fish koi carp. Aquac. Fish. 2024, 9, 66–70. [Google Scholar] [CrossRef]
- Krinsky, N.I. Carotenoid antioxidants. Nutrition 2001, 17, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.; Martelli, H.L.; Da Silva, I.M.; Pomeroy, D. Production of β-carotene by a Rhodotorula strain. Biotechnol. Lett. 2005, 9, 373–375. [Google Scholar]
- Iriani, R.M.; Adilma, R.P.; Scamparini.; Delia, B.; Rodriguez, A. Selection and characterization of carotenoid producing yeasts from Campinas region. Braz. J. Microbiol. 2005, 40, 2985–2991. [Google Scholar]
- Wang, B.; Liu, Y.; Luo, K.; Zhang, S.; Wei, C.; Wang, L.; Qiu, Y.; Tian, X. ‘Biotic’potential of the red yeast Rhodotorula mucilaginosa strain JM-01 on the growth, shell pigmentation, and immune defense attributes of the shrimp, Penaeus vannamei. Aquaculture 2023, 572, 739543. [Google Scholar] [CrossRef]
- Nimrat, S.; Khaopong, W.; Sangsong, J.; Boonthai, T.; Vuthiphandchai, V. Dietary administration of Bacillus and yeast probiotics improves the growth, survival, and microbial community of juvenile white leg shrimp, Litopenaeus vannamei. J. Appl. Aquac. 2021, 33, 15–31. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Yao, M.; Gu, X.; Li, B.; Liu, H.; Ding, M.; Xiao, W.; Yuan, Y. Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol. Biofuels 2018, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, X.Q.; Tian, L.X.; Liu, Y.J.; Niu, J. Enhanced intestinal health, immune responses and ammonia resistance in Pacific white shrimp (Litopenaeus vannamei) fed dietary hydrolyzed yeast (Rhodotorula mucilaginosa) and Bacillus licheniformis. Aquac. Rep. 2020, 17, 100385. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D. Exploitation of genus Rhodosporidium for microbial lipid production. World J. Microbiol. Biotechnol. 2017, 33, 54. [Google Scholar] [CrossRef] [PubMed]
- Nelis, H.J.; De Leenheer, A.P. Microbial sources of carotenoid pigments used in food and feeds. J. Appl. Bacteriol. 1991, 70, 181–191. [Google Scholar] [CrossRef]
- Maldonade, I.R.; Rodriguezamaya, D.B.; Scamparini, A.R. Statistical optimisation of cell growth and carotenoid production by Rhodotorula mucilaginosa. Braz. J. Microbiol. 2012, 43, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Squina, F.M.; Yamashita, F.; Pereira1, J.L.; Mercadante, A.Z. Production of carotenoids by Rhodotorula rubra and R. glutinis in culture medium supplemented with sugar cane juice. Food Biotechnol. 2002, 16, 227–235. [Google Scholar] [CrossRef]
- Aksu, Z.; Eren, A.T. Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as a carbon source. Process Biochem. 2005, 40, 2985–2991. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Yang, C.F. Using strain Rhodotorula mucilaginosa to produce carotenoids using food wastes. J. Taiwan Inst. Chem. Eng. 2016, 61, 270–275. [Google Scholar] [CrossRef]
- Srinual, O.; Moonmanee, T.; Lumsangkul, C.; Doan, H.V.; Punyatong, M.; Yachai, M.; Chaiyaso, T.; Kongtong, K.; Tapingkae, W. Can red yeast (Sporidiobolus pararoseus) be used as a novel feed additive for mycotoxin binders in broiler chickens? Toxins 2022, 14, 678. [Google Scholar] [CrossRef]
- Tapingkae, W.; Srinual, O.; Lumsangkul, C.; Doan, H.V.; Chiang, H.I.; Manowattana, A.; Boonchuay, P.; Chaiyaso, T. Industrial-Scale Production of Mycotoxin Binder from the Red Yeast Sporidiobolus pararoseus KM281507. J. Fungi 2022, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, H.; Du, C.; Zhang, W.; Qian, H. Tentative identification of torulene cis/trans geometrical isomers isolated from Sporidiobolus pararoseus by high-performance liquid chromatography-diode array detection-mass spectrometry and preparation by column chromatography. Anal. Sci. 2013, 29, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Maldonade, I.R.; Rodriguez-Amaya, D.B.; Scamparini, A.R. Carotenoids of yeasts isolated from the Brazilian ecosystem. Food Chem. 2008, 107, 145–150. [Google Scholar] [CrossRef]
- Ianiri, G.; Idnurm, A.; Wright, S.A.; Durán-Patrón, R.; Mannina, L.; Ferracane, R.; Ritieni, A.; Castoria, R. Searching for genes responsible for patulin degradation in a biocontrol yeast provides insight into the basis for resistance to this mycotoxin. Appl. Environ. Microbiol. 2013, 79, 3101–3115. [Google Scholar] [CrossRef] [PubMed]
- Burgents, J.E.; Burnett, K.G.; Burnett, L.E. Disease resistance of Pacific white shrimp, Litopenaeus vannamei, following the dietary administration of a yeast culture food supplement. Aquaculture 2004, 231, 1–8. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W.; Huang, X.; Guo, T.; Wen, W.; Feng, L.; Wei, L. The effect of replacement of fish meal by yeast extract on the digestibility, growth and muscle composition of the shrimp Litopenaeus vannamei. Aquac. Res. 2017, 48, 311–320. [Google Scholar] [CrossRef]
- Xiong, J.; Dai, W.; Qiu, Q.; Zhu, J.; Yang, W.; Li, C. Response of host–bacterial colonization in shrimp to developmental stage, environment and disease. Mol. Ecol. 2018, 27, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Middlehoven, W.J.; Scorzetti, G.; Fell, J.W. Systematics of the anamorphic basidiomycetous yeast genus Trichosporon Behrend with the description of five novel species: Trichosporon vadense, T. smithiae, T. dehoogii, T. scarabaeorum, and T. gamsii. Int. J. Syst. Evol. Biol. 2004, 54, 975–986. [Google Scholar] [CrossRef]
- Bergauer, P.; Fonteyne, P.A.; Nolard, N.; Schinner, F.; Margesin, R. Biodegradation of phenol and phenol-related compounds by psychrophilic and cold-tolerant alpine yeasts. Chemosphere 2005, 59, 909–918. [Google Scholar] [CrossRef]
- Godjevargova, T.; Ivanova, D.; Alexieva, Z.; Dimova, N. Biodegradation of toxic organic components from industrial phenol production waste waters by free and immobilized Trichosporon cutaneum. Proc. Biochem. 2003, 38, 9150920. [Google Scholar] [CrossRef]
- Middlehoven, W.J. Catabolism of benzen compounds by ascomycetous and basidiomycetous yeasts and yeast-like fungi—A literature review and an experimental approach. Ant. LeeuwInt. J. Gen. Appl. Mol. Microbiol. 1993, 63, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Gupta, R. Novel S-enantioselective lipase TALipB from Trichosporon asahii MSR54: Heterologous expression, characterization, conformational stability and homology modeling. Enzyme Microb. Technol. 2016, 83, 29–39. [Google Scholar] [CrossRef]
- Valle, R.S.; Ramos, L.S.; Reis, V.J.; Ziccardi, M.; Dornelas-Ribeiro, M.; Sodré, C.L.; Branquinha, M.H.; Santos, A.L. Trichosporon asahii secretes a 30-kDa aspartic peptidase. Microbiol. Res. 2017, 205, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Yang, R.; Wang, C. Study on antioxidant enzymatic activities of Trichosporon asahii. Indian J. Microbiol. 2016, 56, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.N.; Li, H.T.; Yong, R.Y.; Ao, J.H.; Wang, W.L.; Zhu, H.; Cong, L.; Wang, C.M. Experimental study on oxidant sensitivity of Trichosporona sahii. J. Pract. Dermatol. 2012, 5, 65–70. [Google Scholar]
- Dawood, M.A.; Magouz, F.I.; Essa, M.; Mansour, M. Impact of yeast fermented poultry by-product meal on growth, digestive enzyme activities, intestinal morphometry and immune response traits of common carp (Cyprinus carpio). Ann. Anim. Sci. 2020, 20, 939–959. [Google Scholar] [CrossRef]
- Dawood, M.A.; Eweedah, N.M.; Moustafa, E.M.; Farahat, E.M. Probiotic effects of Aspergillus oryzae on the oxidative status, heat shock protein, and immune related gene expression of Nile tilapia (Oreochromis niloticus) under hypoxia challenge. Aquaculture 2020, 520, 734669. [Google Scholar] [CrossRef]
- Salawudeen, M.T.; Kazeem, H.M.; Raji, M.A.; Oniye, S.J.; Kwanashie, C.N.; Ibrahim, M.J. Isolation and identification of fungi from apparently healthy and diseased Clarias gariepinus from freshwater in Zaria, Kaduna State, Nigeria. Microbiol. Res. J. Int. 2017, 5, 8–15. [Google Scholar] [CrossRef]
- Saleemi, M.K.; Ashraf, K.; Gul, S.T.; Naseem, M.N.; Sajid, M.S.; Mohsin, M.; He, C.; Zubair, M.; Khan, A. Toxicopathological effects of feeding aflatoxins B1 in broilers and its ameliosration with indigenous mycotoxin binder. Ecotoxicol. Environ. Saf. 2020, 187, 109712. [Google Scholar] [CrossRef]
- Marijani, E.; Kigadye, E.; Okoth, S. Occurrence of fungi and mycotoxins in fish feeds and their impact on fish health. Int. J. Microbiol. 2019, 2019, 6743065. [Google Scholar] [CrossRef]
- Sharma, M.K.; White, D.L.; Singh, A.K.; Liu, H.; Tan, Z.; Peng, X.; Kim, W.K. Effect of dietary supplementation of probiotic Aspergillus niger on performance and cecal microbiota in hy-line W-36 laying hens. Animals 2022, 12, 2406. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, A.H.; Jafaryan, H.; Khosravi, A.; Arpanahi, D.A. The probiotic effects of dietary Saccharomyces cerevisiae and Aspergillus niger on the growth and some immunity factors of cultured juvenile beluga sturgeon (Huso huso). ISFJ 2014, 23, 21–33. [Google Scholar]
- Frisvad, J.C.; Larsen, T.O.; Thrane, U.; Meijer, M.; Varga, J.; Samson, R.A.; Nielsen, K.F. Fumonisin and ochratoxin production in industrial Aspergillus niger strains. PLoS ONE 2011, 6, e23496. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, M.K.P.; Nakandakare, I.B.; Terhune, J.S.; Wood, T.; Ranzani-Paiva, M.J.T. Dietary supplementation with Bacillus subtilis, Saccharomyces cerevisiae and Aspergillus oryzae enhance immunity and disease resistance against Aeromonas hydrophila and Streptococcus iniae infection in juvenile tilapia Oreochromis niloticus. Fish Shellfish Immunol. 2015, 43, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Eweedah, N.M.; Moustafa, E.M.; Shahin, M.G. Effects of feeding regimen of dietary Aspergillus oryzae on the growth performance, intestinal morphometry and blood profile of Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2019, 25, 1063–1072. [Google Scholar] [CrossRef]
- Dawood, M.A.; Eweedah, N.M.; Khalafalla, M.M.; Khalid, A. Evaluation of fermented date palm seed meal with Aspergillus oryzae on the growth, digestion capacity and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2020, 26, 828–841. [Google Scholar] [CrossRef]
- Hedayati, S.A. Growth performances and hemato-immunological responses of common carp (Cyprinus carpio Linnaeus, 1758) to fermented Aspergillus oryzae. Iran. J. Fish. Sci. 2020, 19, 1749–1756. [Google Scholar]
- Kurtböke, D.İ. Ecology and habitat distribution of actinobacteria. In Biology and Biotechnology of Actinobacteria; Springer: Cham, Switzerland, 2017; pp. 123–149. [Google Scholar]
- James, G.; Geetha, P.P.; Puthiyedathu, S.T.; Jayadradhan, R.K.V. Applications of Actinobacteria in aquaculture: Prospects and challenges. 3 Biotech 2023, 13, 42. [Google Scholar] [CrossRef]
- Tarazona, U.; León, J.; Galindo, N.; Vallejo, M.; Marguet, E. Characterization of Actinomycetes of marine sediment and their antagonistic activity against Vibrio sp. isolated from “white leg shrimp” Litopenaeus vannamei (BOONE, 1931). Revista Investig. Vet. Peru. 2018, 29, 676–691. [Google Scholar] [CrossRef]
- Butt, U.D.; Khan, S.; Liu, K.; Sharma, A.; Zhang, K.; Wu, B. Present status, limitations, and prospects of using Streptomyces bacteria as a potential probiotic agent in aquaculture. Probiot. Antimicrob. Prot. 2023, 1–17. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Othman, E.M.; Kampik, D.; Stopper, H.; Hentschel, U.; Ziebuhr, W.; Oelschlaeger, T.A.; Abdelmohsen, U.R. Marine sponge-derived Streptomyces sp. SBT343 extract inhibits Staphylococcal biofilm formation. Front. Microbiol. 2017, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Chater, K.F.; Biro, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The complex extracellular biology of Streptomyces. FEMS Microbiol.Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Saldaña, O.F.; Barboza-Corona, J.E.; Bideshi, D.K.; Casados-Vázquez, L.E. New bacteriocin-like substances produced by Streptomyces species with activity against pathogens. Folia Microbiol. 2020, 65, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Balagurunathan, R.; Radhakrishnan, M.; Shanmugasundaram, T.; Gopikrishnan, V.; Jerrine, J. Evaluation of Actinobacteria for aquaculture applications. In Protocols in Actinobacterial Research; Springer: New York, NY, USA, 2020; pp. 181–188. [Google Scholar]
- Gozari, M.; Mortazavi, M.S.; Bahador, N.; Rabbaniha, M. Isolation and screening of antibacterial and enzyme producing marine actinobacteria to approach probiotics against some pathogenic vibrios in shrimp Litopenaeus vannamei. Iran. J. Fish. Sci. 2016, 15, 630–644. [Google Scholar]
- Augustine, D.; Jacob, J.C.; Philip, R. Exclusion of Vibrio spp. by an antagonistic marine actinomycete Streptomyces rubrolavendulae M56. Aquacult. Res. 2016, 47, 2951–2960. [Google Scholar] [CrossRef]
- García-Bernal, M.; Medina-Marrero, R.; Rodríguez-Jaramillo, C.; Campa-Córdova, Á.I.; Mazón-Suástegui, J.M. Probiotic effect of Streptomyces strains alone or in combination with Bacillus and Lactobacillus in juveniles of the white shrimp Litopenaeus vannamei. Aquacult. Int. 2017, 25, 927–939. [Google Scholar] [CrossRef]
- Sheeja, M.S.; Selvakumar, D.; Dhevendaran, K. Antagonistic potential of Streptomyces associated with the gut of marine ornamental fishes. Middle East J. Sci. Res. 2011, 7, 327–334. [Google Scholar]
- Barakat, K.M.; Beltagy, E.A. Bioactive phthalate from marine Streptomyces ruber EKH2 against virulent fish pathogens. Egypt. J. Aquat. Res. 2015, 41, 49–56. [Google Scholar] [CrossRef]
- Subramanian, D.; Kim, M.S.; Kim, D.H.; Heo, M.S. Isolation, characterization, antioxidant, antimicrobial and cytotoxic effect of marine actinomycete, Streptomyces carpaticus MK-01, against fish pathogens. Braz. Arch. Biol. Technol. 2017, 60, e17160539. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Liang, Q.; Pan, G.; Zhao, J.; Cui, M.; Zhao, X.; Zhang, Q.; Xu, D. Antagonistic activity of marine Streptomyces sp. S073 on pathogenic Vibrio parahaemolyticus. Fish Sci. 2019, 85, 533–543. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, G.; Guo, Z.; Wang, Y.; Xu, Z.; Ren, Y.; Zhang, Q.; Cui, M.; Zhao, X.; Xu, D. Application of potential probiotic strain Streptomyces sp. SH5 on anti-Aeromonas infection in zebrafish larvae. Fish Shellfish Immunol. 2022, 127, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Kurnianto, M.A.; Kusumaningrum, H.D.; Lioe, H.N. Characterization of Streptomyces isolates associated with estuarine fish Chanos chanos and profiling of their antibacterial metabolites-crude-extract. Int. J. Microbiol. 2020, 2020, 8851947. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, S.; Bharathi, S.; Uttra, V.; Thirunavukkarasu, R.; Nainangu, P.; Krishnan, V.G.; Renuga, P.S.; Wilson, A.; Balaraman, D. Bioactive metabolites produced from Streptomyces enissocaesilis SSASC10 against fish pathogens. Biocatal. Agric. Biotechnol. 2020, 29, 101802. [Google Scholar] [CrossRef]
- Das, S.; Mondal, K.; Sengupta, C. Evaluation of the probiotic potential of Streptomyces antibioticus and Bacillus cereus on growth performance of freshwater catfish Heteropneustes fossilis. Aquac. Rep. 2021, 20, 100752. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, X.H.; Boon, N.; Bossier, P. Probiotics in aquaculture of China—Current state, problems and prospect. Aquaculture 2009, 290, 15–21. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Yang, H.L.; Ma, R.L.; Lin, W.Y. Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol. 2010, 29, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Rodiles, A.; Rawling, M.D.; Peggs, D.L.; do Vale Pereira, G.; Voller, S.; Yomla, R.; Standen, B.T.; Bowyer, P.; Merrifield, D.L. Probiotic applications for finfish aquaculture. In Probiotics and Prebiotics in Animal Health and Food Safety; Springer: Cham, Switzerland, 2018; pp. 197–217. [Google Scholar]
- Digutä, C.F.; Mihai, C.; Toma, R.C.; Cimpeanu, C.; Matei, F. In vitro assessment of yeast strains with probiotic attributes for aquaculture use. Foods 2022, 12, 124. [Google Scholar] [CrossRef]
- Lin, H.L.; Shiu, Y.L.; Chiu, C.S.; Huang, S.L.; Liu, C.H. Screening probiotic candidates for a mixture of probiotics to enhance the growth performance, immunity, and disease resistance of Asian seabass, Lates calcarifer (Bloch), against Aeromonas hydrophila. Fish Shellfish Immunol. 2017, 60, 474–482. [Google Scholar] [CrossRef]
- Mohapatra, S.; Chakraborty, T.; Prusty, A.K.; Kumar, K.; Prasad, K.P.; Mohanta, K.N. Fenvalerate induced stress mitigation by dietary supplementation of multispecies probiotic mixture in a tropical freshwater fish, Labeo rohita (Hamilton). Pestic. Biochem. Physiol. 2012, 104, 28–37. [Google Scholar] [CrossRef]
- Cerezuela, R.; Guardiola, F.A.; González, P.; Meseguer, J.; Esteban, M.Á. Effects of dietary Bacillus subtilis, Tetraselmis chuii, and Phaeodactylum tricornutum, singularly or in combination, on the immune response and disease resistance of sea bream (Sparus aurata L.). Fish Shellfish Immunol. 2012, 33, 342–349. [Google Scholar] [CrossRef]
- Geng, X.; Dong, X.H.; Tan, B.P.; Yang, Q.H.; Chi, S.Y.; Liu, H.Y.; Liu, X.Q. Effects of dietary probiotic on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Aquac. Nutr. 2012, 18, 46–55. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chandra, G.; Ghosh, K. Single or conjoint application of autochthonous Bacillus strains as potential probiotics: Effects on growth, feed utilization, immunity and disease resistance in Rohu, Labeo rohita (Hamilton). Aquaculture 2019, 512, 734302. [Google Scholar] [CrossRef]
- Mohanty, S.N.; Swain, S.K.; Tripathi, S.D. Rearing of catla (Catla catla Ham.) spawn on formulated diets. J. Aquac. Trop. 1996, 11, 253–258. [Google Scholar]
- Bogut, L.; Milakovic, Z.; Bukvic, Z.; Brkic, S.; Zimmer, R. Influence of probiotics (Streptococcus faecium M74) on growth and content of intestinal micro flora in carp (Cyprinus carpio). Czech J. Anim. Sci. 1998, 43, 231–235. [Google Scholar]
- Choudhury, T.G.; Kamilya, D. Paraprobiotics: An aquaculture perspective. Rev. Aquac. 2019, 11, 1258–1270. [Google Scholar] [CrossRef]
- Tran, N.T.; Yang, W.; Nguyen, X.T.; Zhang, M.; Ma, H.; Zheng, H.; Zhang, Y.; Chan, K.G.; Li, S. Application of heat-killed probiotics in aquaculture. Aquaculture 2022, 548, 737700. [Google Scholar] [CrossRef]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. In vitro evaluation of single-and multi-strain probiotics: Inter-species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe 2012, 18, 405–413. [Google Scholar] [CrossRef]
- Siddik, M.A.; Foysal, M.J.; Fotedar, R.; Francis, D.S.; Gupta, S.K. Probiotic yeast Saccharomyces cerevisiae coupled with Lactobacillus casei modulates physiological performance and promotes gut microbiota in juvenile barramundi, Lates calcarifer. Aquaculture 2022, 546, 737346. [Google Scholar] [CrossRef]
- Dennis, E.U.; Uchenna, O.J. Use of probiotics as first feed of larval African catfish Clarias gariepinus (Burchell 1822). Annu. Res. Rev. Biol. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Liu, Z.; Qiuqian, L.; Yao, Z.; Wang, X.; Huang, L.; Zheng, J.; Wang, K.; Li, L.; Zhang, D. Effects of a commercial microbial agent on the bacterial communities in shrimp culture system. Front. Microbiol. 2018, 9, 2430. [Google Scholar] [CrossRef]
- Ma, Y.X.; Li, L.Y.; Bao, P.Y.; Li, M.; Chen, W.; Chang, Y.Q. Effects of combined dietary administration of Rhodotorula sp. H26 and Bacillus sp. BC26 on growth, immunity and intestinal microbiota in juvenile sea cucumber, Apostichopus japonicus. Aquac. Res. 2018, 49, 3792–3803. [Google Scholar] [CrossRef]
- Machuca, C.; Méndez-Martínez, Y.; Reyes-Becerril, M.; Angulo, C. Yeast β-Glucans as Fish Immunomodulators: A Review. Animals 2022, 12, 2154. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.; Jamal, M.T.; Al-Harb, M.; Al-Mur, B.A.; Haque, M.F. Use of yeasts in aquaculture nutrition and immunostimulation: A review. J. Appl. Biol. Biotechnol. 2022, 10, 59–65. [Google Scholar] [CrossRef]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Beniwal, A.; Semwal, A.; Navani, N.K. Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front. Microbiol. 2019, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Dutta, D.; Banerjee, S.; Ringø, E.; Breines, E.M.; Hareide, E.; Chandra, G.; Ghosh, K. Potential probiotics from Indian major carp, Cirrhinus mrigala. Characterization, pathogen inhibitory activity, partial characterization of bacteriocin and production of exoenzymes. Res. Vet. Sci. 2016, 108, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.; Colom, F.; Frasés, S.; Mulet, E.; Abad, J.L.; Alió, J.L. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8 S ribosomal DNA typing in ocular infections. J. Clin. Microbiol. 2001, 39, 2873–2879. [Google Scholar] [CrossRef]
- Thejaswini, S.; Jojy, S.; Vijayan, A.; Paul, A.M. Isolation of gut Actinobacteria from fishes. In Methods in Actinobacteriology; Springer: New York, NY, USA, 2022; pp. 61–73. [Google Scholar]
- Vignesh, A.; Ayswarya, S.; Gopikrishnan, V.; Radhakrishnan, M. Bioactive potential of Actinobacteria isolated from the gut of marine fishes. Indian J. Mar. Sci. 2019, 48, 1280–1285. [Google Scholar]
- Hariharan, S.; Dharmaraj, S. Selection of new probiotics: The case of Streptomyces. In Therapeutic, Probiotic, and Unconventional Foods; Academic Press: Cambridge, MA, USA, 2018; pp. 27–54. [Google Scholar]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef]
| Species | Isolated from | Doses and Duration | Finfish Species Investigated | Parameters Investigated | References |
|---|---|---|---|---|---|
| Saccharomyces cerevisiae strain NCYC Sc 47 (Biosaf_ Sc 47) | Commercial strain | 106 CFU g−1, 31 days | Rainbow trout, fry | ↑ brush border enzymes, gut microbiota (inclusive of probiont colonization) → enzymes like alkaline phosphatase (AP), γ-glutamyl-transpeptidase (GGT) and leucine-amino-peptidase N (LAP) activities | [27] |
| S. cerevisiae | NI | 1.0, 1.5 and 2.0%, 214 days | Egyptian African catfish, 24.3 ± 1.4 g | ↑ growth performance at 2% inclusion | [28] |
| S. cerevisiae | Commercial strain | 1 g kg−1, 8 weeks | Nile tilapia, 0.66 g | ↑ growth performance, microvilli length, hsp70 expression in intestine and head kidney, intestinal Lactococcus spp. ↓ gut alkaline phosphatase | [29] |
| S. cerevisiae | Commercial strain | 1 g kg−1, 8 weeks | Nile tilapia, ~9.8 g | ↑ growth performance, microvilli length, hsp70 expression in intestine and head kidney and resistance towards A. hydrophila Alleviates negative effects induced via crowding stress ↓ gut alkaline phosphatase | [30] |
| S. cerevisiae | Commercial strain donated by Jastbolaget AB, Sollentuna, Sweden | ~log 8 of yeast per g, 6 weeks | Rainbow trout, ~130 g | Yeast-fed fish revealed similar bacterial diversity and lower abundances of Leuconostocaceae and Photobacterium compared to fish-fed fish meal | [31] |
| S. cerevisiae | Commercial strain | 250 g ton−1, 30 days | Nile tilapia, 50 ± 5 g | ↑ growth performance, white blood cell counts and histopathology | [32] |
| S. cerevisiae | Commercial strain | 4, 6 or 8 g kg−1, 30 days | Pirarucu, 9.8 ± 1.4 g | ↑ growth performance ↓ cholesterol | [33] |
| S. cerevisiae | Commercial strain, Idun industry, Norway | 300 g kg−1, 21 days | Atlantic salmon, 114 g | ↑ nutrient digestibility and immune responses | [34] |
| S. cerevisiae | NI | 0, 1, 2 or 4 g kg−1, 90 days | Rohu, 5.69 ± 0.02 g | ↑ growth performance, feed utilization and hematobiochemical indices Variations in intestinal microbiota (total viable and LAB counts) and intestinal morphology | [35] |
| S. cerevisiae | NI | 0, 1, 2 (SC2) or 4 (SC4) g kg−1, 16 weeks | Sea bream, 31.23 ± 1.2 g | ↑ growth performance parameters via SC2 and SC4 feeding and intestinal morphology SC4 feeding boosted innate immune response | [36] |
| S. cerevisiae fermentation product (DVAQUA) | Commercial fermentation product | 0.5 g kg−1, 16 weeks | Hybrid tilapia, ~47 g | ↑ non-specific immunity and increased intestinal bacterial count and bacterial diversity | [37] |
| Lyophilized whole yeast, S. cerevisiae | Commercial strain | 1, 5 or 10 g kg−1, 4 weeks | Gilthead seabream, 166 ± 16 g | ↑ phagocytic activity, respiratory burst activity, complement activity and myeloperoxidase activity | [38] |
| S. cerevisiae, nucleotides | NI | 0.5, 1.5, and 2.5 g kg−1, 30 days | Nile tilapia, 42.9 ± 0.14 g | ↑ blood proteins, leukocytes, antioxidant activity, non-specific immunity, cytokine gene expression and disease resistance against Aeromonas sobria | [39] |
| S. cerevisiae, nucleotides | NI | 500 mg kg−1, 80 days | European sea bass, 14.33 ± 0.18 g | ↑ growth performance, lipid efficiency and anti-inflammatory TGF-b Promoted beneficial lactic acid bacteria Weissella and Leuconostoc | [40] |
| S. cerevisae-orginated free nucleotides | Commercial product | 170, 320 or 470 ppm, 60 days | Nile tilapia, ~7.8 g | ↑ complement hemolytic activity Serum lysozyme concentration, intestinal villi height and density and survival toward A. hydrophila via N470 feeding → growth performance | [41] |
| S. cerevisiae, hydrolysate | Commercial hydrolysate (Sintun Aquatic Technology Co., Ltd.) | 0.1 and 0.2%, 8 weeks | Large mouth bass, 34 g | → growth performance, hepatosomic index and organ coefficient Modulation of gut microbiota ↓ Fusobacteria, Cyanobacteria, Tenericutes and Actinobacteria via 0.2% inclusion | [42] |
| Debaryomyces hansenii 97 | Fish intestine | 5 × 106 CFU mL−1, 3 days | Zebrafish larvae | ↑ survival against Vibrio anguillarum and the modulation of gut microbiota and metabolic pathways | [43] |
| D. hansenii | NI | 1.1% of D. hansenii (1.7 × 106 CFU), 70 days | Gilthead seabream | ↑ of somatic growth and improvement in feed conversion Modulation of gut microbiota, characterized by reduction in abundances of several Proteobacteria, especially opportunistic bacteria | [44] |
| Geotrichum candidum | Fermented milk | 109 CFU L−1, 70 days | Rohu larvae | ↑ growth performance, protease, amylase and cellulase activities and survival after Staphylococcus aureus challenge | [45] |
| Geotrichum candidum QAUGC01 | Commercial dairy product yogurt | 109 CFU g−1, un-encapsulated and encapsulated, 11 weeks | Rohu, 20 ± 2.34 g | ↑ growth rate, protease, amylase, cellulase, RBCs, Hb, HCT, WBCs, MCHC, respiratory bursts and phagocytic activity, total protein, lysozyme and IgM Upregulation of heat shock protein 70 gene in muscle, intestine and liver ↓ serum AST and ALT activities, total cholesterol and triglyceride Encapsulated diet revealed best results | [46] |
| Local fermented milk product of curd | 109 CFU g−1, 90 days | Rohu fingerings | ↑ growth performance, hematological profile and digestive enzymes Modulated the gut microbiota | [47] | |
| Grapes from Hubei Center for Industrial Culture Collection and Research | C: 0, T1:106, T2:107, T3:108, T4:109, T5:1010, T6:1011 CFU kg−1, 60 days | Gibel carp | ↑ feed utilization; α-amylase activity immunity; expression of immune related genes; il-1β, tnf-α, hsp70 and tlr-2 in liver; and disease resistance against Aeromonas hydrophila Modulation of the gut microbiota | [48] | |
| Sporidiobolus pararoseus | By-product of the biodiesel production process | T1 (control), T2 (5), T3 (10), and T4 (20) g kg−1, 90 days | Nile tilapia | ↑ growth performance (T3 and T4 diets). All treatments improved immune response and disease response against S. agalactiae. | [49] |
| Cyberlindnera jadinii | NI | 10% inclusion, 42 days | Atlantic salmon, 136 ± 0.25g | ↓ inflammation and enterocyte histology | [50] |
| Torula yeast (Cyberlindnera jadinii) | Commersial product, Arbiom Inc. (Durham, NC, USA) | Inclusion level (0, 10 and 20%), 35 days | Atlantic salmon, 1.14 g | → growth performance (20% inclusion) Modulated the gut microbiota (decreasing Tepidmicrobium and Lactobacillus, but a slight increase in Weisella was noted with increasing torula levels) | [51] |
| Yarrowia lipolytica | Fish intestine | 5 × 106 mL−1, 3 days | Zebrafish larvae | ↑ survival against V. anguillarum and the modulation of the gut microbiota and metabolic pathways | [43] |
| Aspergillus oryzae | No further information was given, Bio’c company, Uchida, Japan | 1 g kg−1, 60 days | Nile tilapia | ↑ growth performance, antioxidative enzymes, GPX and immunity Modulation of hematocrit, hemoglobin, red blood cells, white blood cells, total protein, and digestive enzymes | [11] |
| Aspergillus niger | Laboratory strain, no further information was given | 0, 103, 106 CFU g−1, 60 days | Common carp | ↑ Growth performance, protein efficiency ratio and lipid efficiency ratio, plasma levels of lysozyme and total immunoglobulin, red blood cell counts, haemoglobin concentrations, mean corpuscular haemoglobin, mean corpuscular volume values and lymphocyte counts | [12] |
| Streptomyces sp. | Catlaintestine | Dose not given, 15 days | Swordtail, 0.4 g | ↑ growth and food conversion | [52] |
| Streptomyces sp. | Sediment | Dose not specified, 15 days | Common platy, 0.4 g. | ↑ food conversion rate, food conversion efficiency and growth | [53] |
| Streptomyces chartreusis | Soil ecosystem | Control (0), 105 (S1), 106 (S2), and 107 (S3) CFU g−1, 2 months | Common carp, ~14 g | ↑ growth performance parameters, regardless of inclusion levels Different doses of S. chartreusis increased serum total Ig and lysozyme activity compared to those fed the control diet → serum antioxidant enzyme activity (CAT, SOD and GPx) | [54] |
| Streptomyces griseus | Field sites | Exposed to 102–106 spores mL−1 for up to 96 h. | Fish gill pathology, with bream and rainbow trout being more sensitive than carp, trench and roach | Elicits pathological changes to the gills These changes include hyperplasia, leading to the fusion of the secondary lamellae and loss of microridging on the filament epithelium of the primary lamellae | [55] |
| Anisomycin (Ani), a metabolite produced by Streptomyces griseolus | NI | Dose NI, 7 days | Zebrafish, 3.20 ± 0.15 cm | Ani showed strong anti-SVCV activity in vivo, as indicated by inhibiting viral gene expression and the increased survival of zebrafish | [56] |
| Streptomyces fradiae and Streptomyces sp. | Marine sponges, Callyspongia diffusa, Mycale mytilorum, Tedaniaanhelans and Dysidea fragilis | Dose NI, 50 days | Swordtail, ~0.6 g | ↑ growth | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, K.; Harikrishnan, R.; Mukhopadhyay, A.; Ringø, E. Fungi and Actinobacteria: Alternative Probiotics for Sustainable Aquaculture. Fishes 2023, 8, 575. https://doi.org/10.3390/fishes8120575
Ghosh K, Harikrishnan R, Mukhopadhyay A, Ringø E. Fungi and Actinobacteria: Alternative Probiotics for Sustainable Aquaculture. Fishes. 2023; 8(12):575. https://doi.org/10.3390/fishes8120575
Chicago/Turabian StyleGhosh, Koushik, Ramasamy Harikrishnan, Abhisek Mukhopadhyay, and Einar Ringø. 2023. "Fungi and Actinobacteria: Alternative Probiotics for Sustainable Aquaculture" Fishes 8, no. 12: 575. https://doi.org/10.3390/fishes8120575





