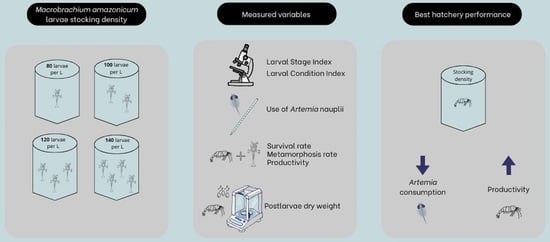
Intensification of Amazon River Prawn Hatchery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Larval Stage Index and Larval Condition Index
2.3. Use of Artemia nauplii
2.4. Survival, Metamorphosis Rate, Productivity and Dry Weight
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Troell, M.; Costa-Pierce, B.; Stead, S.; Cottrell, R.S.; Brugere, C.; Farmery, A.K.; Little, D.C.; Strand, Å.; Pullin, R.; Soto, D.; et al. Perspectives on aquaculture’s contribution to the improved human and planetary health. J. World Aquac. Soc. 2023, 54, 251–342. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; Mcnevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Maciel, C.R.; Valenti, W.C. Biology, fisheries and aquaculture of the Amazon River Prawn Macrobrachium amazonicum: A review. Nauplius 2009, 17, 61–79. [Google Scholar]
- Da Costa, T.V.; de Mattos, L.A.; Machado, N.d.J.B. Estrutura populacional de Macrobrachium amazonicum em dois lagos de várzea da Amazônia. Bol. Inst. Pesca 2016, 42, 281–293. [Google Scholar] [CrossRef]
- Costa e Silva, R.; Cunha, M.C.; Mossolin, E.C.; Jacobucci, G.B. Population structure of Macrobrachium amazonicum (Heller, 1862) (Decapoda: Palaemonidae) in Miranda Hydroelectric Plant Reservoir, Araguari river, Minas Gerais, Brazil. Acta Limnol. Bras. 2019, 31, e14. [Google Scholar] [CrossRef]
- Moraes-Valenti, P.; Valenti, W.C. Culture of the Amazon River Prawn Macrobrachium amazonicum. In Freshwater Prawns: Biology and Farming; New, M.B., Valenti, W.C., Tidwell, J.H., D’abramo, L.R., Kutty, M.N., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 485–501. [Google Scholar]
- Taddei, F.G.; Reis, S.D.S.; David, F.S.; Silva, T.E.D.; Fransozo, V.; Fransozo, A. Population structure, mortality, and recruitment of Macrobrachium amazonicum (Heller, 1862) (Caridea: Palaemonidae) in the eastern Amazon region, Brazil. J. Crust. Biol. 2017, 37, 131–141. [Google Scholar] [CrossRef]
- Valenti, W.C.; Daniels, W.H.; New, M.B.; Correia, E. Hatchery systems and management. In Freshwater Prawns: Biology and Farming; New, M.B., Valenti, W.C., Tidwell, J.H., D’Abramo, L.R., Kutty, M.N., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 55–85. [Google Scholar]
- Marques, H.L.A.; Barros, H.P.B.; Mallasen, M.; Boock, M.V.; Moraes-Valenti, P.M.C. Influence of stocking densities in the nursery phase on the growth of Macrobrachium amazonicum reared in net pens. Aquaculture 2012, 358–359, 240–245. [Google Scholar] [CrossRef]
- Rodrigues, C.G.; Garcia, B.F.; Verdegem, M.; Santos, M.R.; Amorim, R.V.; Valenti, W.C. Integrated culture of Nile tilapia and Amazon river prawn in stagnant ponds, using nutrient-rich water and substrates. Aquaculture 2019, 503, 111–117. [Google Scholar] [CrossRef]
- Dantas, D.P.; Flickinger, D.L.; Costa, G.A.; Batlouni, S.R.; Moraes-Valenti, P.; Valenti, W.C. Technical feasibility of integrating Amazon river prawn culture during the first phase of tambaqui grow-out in stagnant ponds, using nutrient-rich water. Aquaculture 2020, 516, 734611. [Google Scholar] [CrossRef]
- Marques, A.M.; Boaratti, A.Z.; Belmudes, D.; Ferreira, J.R.C.; Mantoan, P.V.L.; Moraes-Valenti, P.; Valenti, W.C. Improving the Efficiency of Lambari Production and Diet Assimilation Using Integrated Aquaculture with Benthic Species. Sustainability 2021, 13, 10196. [Google Scholar] [CrossRef]
- Iitembu, J.A.; Fitzgerald, D.; Altintzoglou, T.; Boudry, P.; Britz, P.; Byron, C.J.; Delago, D.; Girard, S.; Hannon, C.; Kafensztok, M.; et al. Comparative Description and Analysis of Oyster Aquaculture in Selected Atlantic Regions: Production, Market Dynamics, and Consumption Patterns. Fishes 2023, 8, 584. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B. Recirculating Aquaculture Systems. In Aquaculture Production Systems; Tidwell, J.H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 245–277. [Google Scholar]
- Tierney, T.W.; Fleckenstein, L.J.; Ray, A.J. The effects of density and artificial substrate on intensive shrimp Litopenaeus vannamei nursery production. Aquac. Eng. 2020, 89, 102063. [Google Scholar] [CrossRef]
- Nhan, D.T.; Wille, M.; Hung, L.T.; Sorgeloos, P. Effects of larval stocking density and feeding regime on larval rearing of giant freshwater prawn (Macrobrachium rosenbergii). Aquaculture 2010, 300, 80–86. [Google Scholar] [CrossRef]
- David, F.S.; Cohen, F.P.A.; Valenti, W.C. Intensification of the Giant river prawn Macrobrachium rosenbergii hatchery production. Aquac. Res. 2016, 47, 3747–3752. [Google Scholar] [CrossRef]
- David, F.S.; Fonseca, T.; Bueno, G.W.; Valenti, W.C. Economic feasibility of intensification of Macrobrachium rosenbergii hatchery. Aquac. Res. 2018, 49, 3769–3776. [Google Scholar] [CrossRef]
- Marques, H.L.; New, M.B.; Boock, M.V.; Barros, H.P.; Mallasen, M.; Valenti, W.C. Integrated freshwater prawn farming: State-of-the-art and future potential. Rev. Fish. Sci. Aquac. 2016, 24, 264–293. [Google Scholar] [CrossRef]
- Medeiros, M.V.; Aubin, J.; Camargo, A.F. Life cycle assessment of fish and prawn production: Comparison of monoculture and polyculture freshwater systems in Brazil. J. Clean. Prod. 2017, 156, 528–537. [Google Scholar] [CrossRef]
- Peña-Herrejón, G.A.; Sánchez-Velázquez, J.; García-Trejo, J.F.; Soto-Zarazúa, G.M.; Rico-García, E. Effect of stocking density on growth and survival of the prawn Macrobrachium tenellum, cultured in a recirculating aquaculture system. Lat. Am. J. Aquat. Res. 2019, 47, 342–348. [Google Scholar] [CrossRef]
- Mallasen, M.; Valenti, W.C. Comparison of artificial and natural, new and reused brackish water for the larviculture of the freshwater prawn Macrobrachium rosenbergii in a recirculating system. J. World Aquac. Soc. 1998, 29, 345–350. [Google Scholar] [CrossRef]
- Guest, W.C. Laboratory Life History of the palaemonid shrimp Macrobrachium amazonicum (Heller) (Decapoda, Palaemonidae). Crustaceana 1979, 37, 141–152. [Google Scholar] [CrossRef]
- Manzi, J.J.; Maddox, M.B.; Sandifer, P.A. Algal supplement enhancement of Macrobrachium rosenbergii (De Man) larviculture. Proc. World Maric. Soc. 1977, 8, 207–223. [Google Scholar] [CrossRef]
- Tayamen, M.; Brown, J.H. A condition index for evaluating larval quality of Macrobrachium rosenbergii (De Man, 1879). Aquac. Res. 1999, 30, 917–922. [Google Scholar] [CrossRef]
- Maciel, C.R.; New, M.B.; Valenti, W.C. The predation of Artemia nauplii by the larvae of the Amazon River Prawn, Macrobrachium amazonicum (Heller, 1862), is affected by prey density, time of day, and ontogenetic development. J. World Aquac. Soc. 2012, 43, 659–669. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Dailey, W.H. Life history strategies, population dynamics, and consequences for supplemental stocking of tarpon. Contrib. Mar. Sci. 2002, 35, 81–94. [Google Scholar]
- Sheskin, D.J. Handbook of Parametric and Nonparametric Statistical Procedures, 5th ed.; CRC Press: Boca Raton, FL, USA, 2011; 1886p. [Google Scholar]
- Hayd, L.A.; Lemos, D.; Valenti, W.C. Effects of ambient nitrite on Amazon river prawn, Macrobrachium amazonicum, larvae. J. World Aquac. Soc. 2014, 45, 55–64. [Google Scholar] [CrossRef]
- McKay, H.; McAuliffe, W.; Waldhorn, D.R. Welfare Considerations for Farmed Shrimp; Rethink Priorities: San Francisco, CA, USA, 2023. [Google Scholar] [CrossRef]
- Barreto, A.V.; Soares, C.M.A. Produção de pós-larvas de Macrobrachium amazonicum (Heller, 1862) (Decapoda; Palaemonidae), sob condições controladas de laboratório. Rev. Bras. Zool. 1982, 1, 51–53. [Google Scholar] [CrossRef]
- Rahman, M.M.; Salin, K.R.; Tsusaka, T.W.; Anal, A.K.; Rahi, M.L.; Yakupitiyage, A. Effect of stocking density on growth performance and gonadal maturity of all-female giant freshwater prawn, Macrobrachium rosenbergii. J. World Aquac. Soc. 2022, 53, 1120–1133. [Google Scholar] [CrossRef]
- Arifin, O.Z.; Prakoso, V.A.; Subagja, J.; Kristanto, A.H.; Pouil, S.; Slembrouck, J. Effects of stocking density on survival, food intake and growth of giant gourami (Osphronemus goramy) larvae reared in a recirculating aquaculture system. Aquaculture 2019, 509, 159–166. [Google Scholar] [CrossRef]
- Coelho-Filho, P.A.; Gonçalvez, A.P.; Barros, H.P. Artemia nauplii intake by Macrobrachium carcinus at different larval stages in laboratory. Aquaculture 2018, 484, 333–337. [Google Scholar] [CrossRef]
- Nhan, D.T. Evaluation of different diets to replace Artemia nauplii for larval rearing of giant freshwater prawn (Macrobrachium rosenbergii). Agric. Dev. Mag. 2018, 17, 35–43. [Google Scholar] [CrossRef]
- Barros, H.P.; Valenti, W.C. Food intake of Macrobrachium rosenbergii during larval development. Aquaculture 2003, 216, 165–176. [Google Scholar] [CrossRef]
- Gomes, J.N.; Abrunhosa, F.A.; Costa, A.K.; Maciel, C.R. Feeding and larval growth of an exotic freshwater prawn Macrobrachium equidens (Decapoda: Palaemonidae), from Northeastern Pará, Amazon Region. An. Acad. Bras. Ciên. 2014, 86, 1525–1536. [Google Scholar] [CrossRef]
- Coyle, S.D.; Tidwell, J.H.; Danaher, J.; Yasharian, D.K.; Bright, L.A. The Effect of Biomass Density, Salinity, and Substrate on Transport Survival of Juvenile Freshwater Prawns Macrobrachium rosenbergii in Continuously Oxygenated, Vented Containers. N. Am. J. Aquac. 2006, 68, 271–275. [Google Scholar] [CrossRef]
- Araujo, M.C.; Valenti, W.C. Effects of feeding strategy on larval development of the Amazon River prawn Macrobrachium amazonicum. Rev. Bras. Zoot. 2017, 46, 85–90. [Google Scholar] [CrossRef]
- Araujo, M.C.; Valenti, W.C. Efeito da intensidade luminosa no desenvolvimento larval do Macrobrachium amazonicum. Bol. Inst. Pesca 2011, 37, 155–164. [Google Scholar]
- Maciel, C.R.; Valenti, W.C. Assessing the potential of partial replacing of Artemia by practical inert diet in the larviculture of the Amazon River Prawn. Bol. Inst. Pesca 2014, 40, 69–78. [Google Scholar]
- New, M.W. Freshwater prawn farming: Global status, recent research and a glance at the future. Aquac. Res. 2005, 36, 210–230. [Google Scholar] [CrossRef]

| Variables | Stocking Density (Larvae L−1) | |||
|---|---|---|---|---|
| 80 | 100 | 120 | 140 | |
| Temperature (°C) | 29.8 ± 0.1 | 29.8 ± 0.1 | 29.9 ± 0.1 | 29.9 ± 0.1 |
| Recirculation rate (% day−1) | 22.4 ± 0.4 | 25.0 ± 6.0 | 21.5 ± 2.9 | 22.5 ± 3.5 |
| TAN (mg L−1) | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.24 ± 0.07 | 0.25 ± 0.05 |
| N-NO2 (mg L−1) | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.03 | 0.04 ± 0.01 |
| DO (mg L−1) | 7.84 ± 0.17 | 8.08 ± 0.15 | 7.83 ± 0.60 | 7.84 ± 0.13 |
| DO Saturation (%) | 103.3 ± 4.0 | 106.3 ± 1.1 | 103.1 ± 4.6 | 106.8 ± 4.4 |
| Salinity | 10.2 ± 0.4 | 10.4 ± 0.5 | 10.1 ± 0.2 | 9.9 ± 0.4 |
| pH | 7.71 ± 0.56 | 7.71 ± 0.58 | 7.76 ± 0.56 | 7.75 ± 0.53 |
| Rearing Time (Days) | Stocking Density (Larvae L−1) | ||||||
|---|---|---|---|---|---|---|---|
| 80 | 100 | 120 | 140 | F-Value | p-Value | CV (%) | |
| 2 | 1.1 ± 0.1 | 1.4 ± 0.4 | 1.3 ± 0.5 | 1.1 ± 0.0 | 0.54 | 0.66 | 27.5 |
| 4 | 2.8 ± 0.2 | 2.8 ± 0.5 | 2.7 ± 0.1 | 2.7 ± 0.5 | 0.04 | 0.98 | 14.2 |
| 6 | 4.5 ± 0.3 | 4.4 ± 0.4 | 4.7 ± 0.2 | 4.5 ± 0.2 | 0.64 | 0.60 | 6.2 |
| 8 | 5.5 ± 0.3 | 5.2 ± 0.2 | 5.4 ± 0.4 | 5.5 ± 0.3 | 0.86 | 0.49 | 6.0 |
| 10 | 6.3 ± 1.0 | 6.5 ± 0.6 | 6.8 ± 0.4 | 6.6 ± 0.2 | 0.40 | 0.75 | 9.6 |
| 12 | 7.9 ± 0.3 | 7.6 ± 0.2 | 8.0 ± 0.3 | 7.8 ± 0.5 | 0.76 | 0.54 | 4.7 |
| 14 | 8.4 ± 0.2 | 8.3 ± 0.3 | 8.3 ± 0.4 | 8.3 ± 0.2 | 0.18 | 0.91 | 3.35 |
| 16 | 8.6 ± 0.3 | 8.6 ± 0.1 | 8.7 ± 0.4 | 8.5 ± 0.3 | 0.28 | 0.83 | 3.8 |
| 18 | 8.7 ± 0.2 | 9.0 ± 0.1 | 8.6 ± 0.2 | 8.6 ± 0.5 | 0.52 | 0.68 | 2.8 |
| Rearing Time (Days) | Stocking Densities (Larvae L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 80 | 100 | 120 | 140 | |||||
| Nauplii mL−1 | Nauplii Larvae−1 | Nauplii mL−1 | Nauplii Larvae−1 | Nauplii mL−1 | Nauplii Larvae−1 | Nauplii mL−1 | Nauplii Larvae−1 | |
| 2 | 1.6 ± 2.3 | 20 ± 29 | 1.3 ± 1.1 | 14 ± 11 | 1.9 ± 2.1 | 16 ± 19 | 3.0 ± 1.0 | 22 ± 7 |
| 3 | 3.6 ± 0.7 | 46 ± 14 | 1.8 ± 2.7 | 19 ± 29 | 3.7 ± 1.1 | 32 ± 10 | 4.3 ± 1.8 | 32 ± 14 |
| 4 | 4.5 ± 1.3 | 59 ± 26 | 4.6 ± 1.0 | 49 ± 11 | 5.0 ± 1.2 | 45 ± 11 | 4.2 ± 1.2 | 32 ± 9 |
| 5 | 5.0 ± 1.5 | 66 ± 15 | 5.1 ± 1.5 | 55 ± 16 | 5.7 ± 1.8 | 52 ± 15 | 5.5 ± 1.3 | 42 ± 10 |
| 6 | 5.1 ± 0.8 | 68 ± 8 | 5.0 ± 1.8 | 55 ± 20 | 5.5 ± 0.8 | 52 ± 12 | 5.7 ± 1.7 | 45 ± 13 |
| 7 | 5.9 ±1.0 | 79 ± 13 | 5.1 ± 1.1 | 57 ± 12 | 6.4 ± 1.7 | 61 ± 18 | 5.8 ± 1.6 | 47 ± 14 |
| 8 | 4.9 ± 1.9 | 67 ± 27 | 5.5 ± 0.6 | 63 ± 6 | 6.0 ± 2.7 | 57 ± 24 | 5.2 ± 0.9 | 42 ± 8 |
| 9 | 5.4 ± 2.1 | 74 ± 23 | 4.3 ± 1.9 | 49 ± 21 | 6.0 ± 1.5 | 58 ± 10 | 6.1 ± 0.9 | 50 ± 7 |
| 10 | 5.7 ± 2.9 | 80 ± 31 | 6.0 ± 1.6 | 71 ± 17 | 6.1 ± 0.9 | 61 ± 7 | 6.9 ± 1.9 | 57 ± 16 |
| 11 | 6.3 ± 4.5 | 91 ± 53 | 6.4 ± 1.3 | 77 ± 18 | 5.6 ± 1.2 | 57 ± 12 | 5.2 ± 1.1 | 44 ± 9 |
| 12 | 5.1 ± 2.3 | 75 ± 59 | 5.1 ± 2.3 | 62 ± 29 | 4.8 ± 1.1 | 51 ± 16 | 5.5 ± 0.7 | 47 ± 7 |
| 13 | 3.9 ± 0.8 | 56 ± 16 | 5.9 ± 3.1 | 74 ± 41 | 3.9 ± 1.8 | 42 ± 20 | 6.6 ± 2.3 | 57 ± 20 |
| 14 | 4.3 ± 1.9 | 63 ± 23 | 5.0 ± 1.3 | 63 ± 18 | 5.3 ± 2.4 | 60 ± 36 | 6.1 ± 2.2 | 54 ± 19 |
| 15 | 5.3 ± 2.0 | 79 ± 27 | 5.0 ± 1.4 | 65 ± 19 | 5.4 ± 2.5 | 59 ± 27 | 6.8 ± 1.9 | 60 ± 15 |
| 16 | 7.3 ± 5.0 | 110 ± 59 | 6.5 ± 1.6 | 85 ± 21 | 7.4 ± 2.5 | 80 ± 21 | 7.3 ± 3.0 | 67 ± 29 |
| 17 | 7.2 ± 1.6 | 108 ± 25 | 7.7 ± 1.6 | 101 ± 17 | 7.6 ± 1.6 | 88 ± 30 | 8.7 ± 1.9 | 80 ± 15 |
| 18 | 6.5 ± 2.0 | 9.4 ± 2.5 | 8.6 ± 2.7 | 6.1 ± 0.6 | ||||
| Nauplii PL−1 | 1559 ± 497 a | 1237 ± 185 b | 1230 ± 423 b | 1206 ± 261 b | ||||
| Variables | Stocking Densities (Larvae L−1) | |||
|---|---|---|---|---|
| 80 | 100 | 120 | 140 | |
| Survival (%) | 80 ± 5 | 72 ± 6 | 72 ± 15 | 75 ± 5 |
| Metamorphosis rate (%) | 67 ± 13 | 65 ± 7 | 62 ± 15 | 57 ± 12 |
| Productivity (PL L−1) | 54 ± 11 a | 65 ± 7 ab | 75 ± 18 b | 80 ± 17 b |
| PL dry weight (mg) | 1.29 ± 0.10 | 1.21 ± 0.20 | 1.15 ± 0.20 | 1.23 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetorelli, M.P.; Rodrigues, L.A.; Kimpara, J.M.; Valenti, W.C. Intensification of Amazon River Prawn Hatchery. Fishes 2024, 9, 82. https://doi.org/10.3390/fishes9030082
Vetorelli MP, Rodrigues LA, Kimpara JM, Valenti WC. Intensification of Amazon River Prawn Hatchery. Fishes. 2024; 9(3):82. https://doi.org/10.3390/fishes9030082
Chicago/Turabian StyleVetorelli, Michelle Pinheiro, Laurindo André Rodrigues, Janaina Mitsue Kimpara, and Wagner C. Valenti. 2024. "Intensification of Amazon River Prawn Hatchery" Fishes 9, no. 3: 82. https://doi.org/10.3390/fishes9030082