Unraveling the Peculiarities in the Temperature-Dependent Structural Evolution of Black Phosphorus
Abstract
:1. Introduction
2. Results
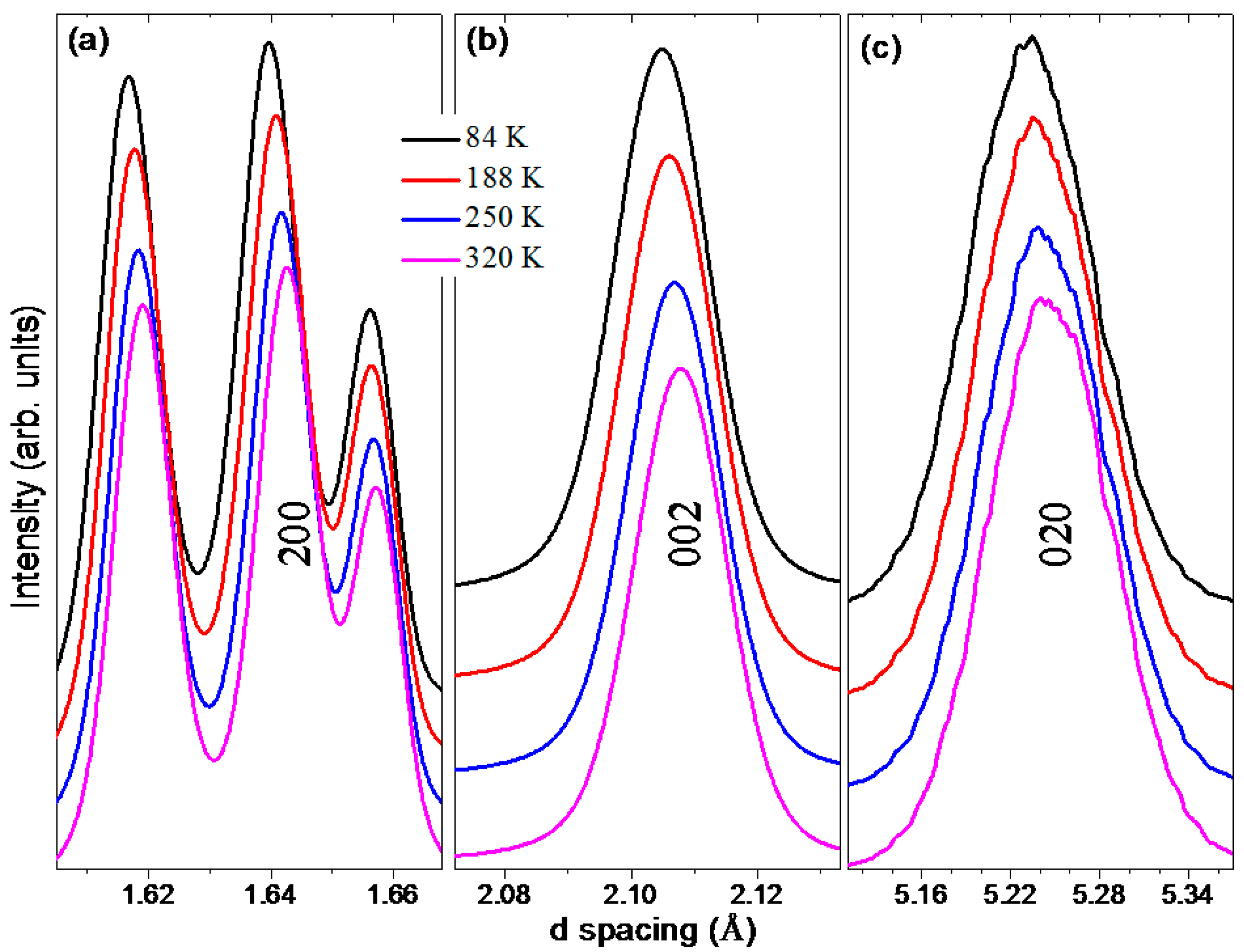
2.1. Preferred Orientation Effects in Black Phosphorus XRD Spectrum
2.2. Rietveld Refinement Results
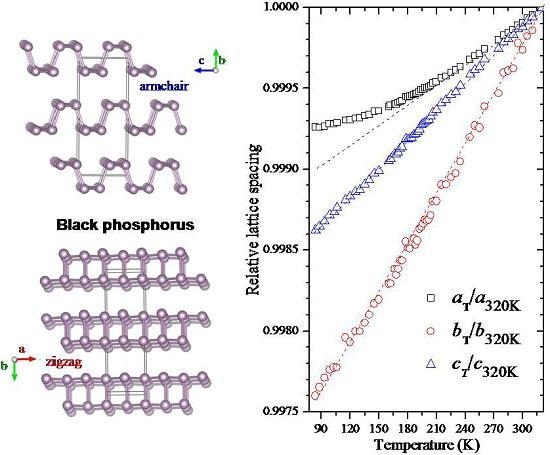
2.3. Temperature-Dependent Diffraction and Correlation between Structural and Macroscopic Properties of Black Phosphorus
3. Discussion
4. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poccia, N.; Fratini, M.; Ricci, A.; Campi, G.; Barba, L.; Vittorini-Orgeas, A.; Bianconi, G.; Aeppli, G.; Bianconi, A. Evolution and control of oxygen order in a cuprate superconductor. Nat. Mater. 2011, 10, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Poccia, N.; Campi, G.; Coneri, F.; Caporale, A.S.; Innocenti, D.; Burghammer, M.; Zimmermann, M.; Bianconi, A. Multiscale distribution of oxygen puddles in 1/8 doped YBa2Cu3O6.67. Sci. Rep. 2013, 3, 2383. [Google Scholar] [CrossRef] [PubMed]
- Campi, G.; Bianconi, A.; Poccia, N.; Bianconi, G.; Barba, L.; Arrighetti, G.; Innocenti, D.; Karpinski, J.; Zhigadlo, N.D.; Kazakov, S.M.; et al. Inhomogeneity of charge-density-wave order and quenched disorder in a high-Tc superconductor. Nature 2015, 525, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Schiavo, B. Effects of ball-milling on the hydrogen sorption properties of LaNi5. J. Alloys Compd. 2009, 480, 912–916. [Google Scholar] [CrossRef]
- Maugeri, L.; Simonelli, L.; Iadecola, A.; Joseph, B.; Okubo, M.; Honma, I.; Wadati, H.; Mizokawa, T.; Saini, N.L. Temperature dependent local structure of LiCoO2 nanoparticles determined by Co K-edge X-ray absorption fine structure. J. Power Sour. 2013, 229, 272–276. [Google Scholar] [CrossRef]
- Joseph, B.; Mohapatra, S.; Lenka, H.P.; Kuiri, P.K.; Mahapatra, D.P. Size saturation in low energy ion beam synthesized Au nanoclusters and their size redistribution with O irradiation. Thin Solid Films 2005, 492, 35–40. [Google Scholar] [CrossRef]
- Mathew, S.; Satpati, B.; Joseph, B.; Dev, B.N.; Nirmala, R.; Malik, S.K.; Kesavamoorthy, R. Magnetism in C60 films induced by proton irradiation. Phys. Rev. B 2007, 75, 075426. [Google Scholar] [CrossRef]
- Joseph, B.; Sandeep, C.S.S.; Sekhar, B.R.; Mahapatra, D.P.; Philip, R. Nonlinear optical properties of MeV and keV ion beam synthesized Ag nanoclusters. Nucl. Instrum. Methods Phys. Res. Sect. B 2007, 265, 631–636. [Google Scholar] [CrossRef]
- Iadecola, A.; Agrestini, S.; Filippi, M.; Simonelli, L.; Joseph, B.; Mahajan, D.; Saini, N.L. Local structure of ReFeAsO (Re = La, Pr, Nd, Sm) oxypnictides studied by Fe K-edge EXAFS. EPL 2009, 87, 26005. [Google Scholar] [CrossRef]
- Bendele, M.; Marini, C.; Joseph, B.; Pierantozzi, G.M.; Caporale, A.S.; Bianconi, A.; Pomjakushina, E.; Conder, K.; Krzton-Maziopa, A.; Irifune, T.; et al. Interplay of electronic and lattice degrees of freedom in A1−xFe2−ySe2 superconductors under pressure. Phys. Rev. B 2013, 88, 180506. [Google Scholar] [CrossRef]
- Malavasi, L.; Artioli, G.A.; Kim, H.; Maroni, B.; Joseph, B.; Ren, Y.; Proffen, T.; Billinge, S.J.L. Local structural investigation of SmFeAsO1−xFx high temperature superconductors. J. Phys. Condens. Matter 2011, 23, 272201. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Marcelli, A.; Joseph, B.; Iadecola, A.; Chu, W.S.; Gioacchino, D.D.; Bianconi, A.; Wu, Z.Y.; Saini, N.L. Local structural disorder in REFeAsO oxypnictides by RE L3-edge XANES. J. Phys. Condens. Matter 2010, 22, 125701. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Subbarao, U.; Joseph, B.; Peter, S.C. Mixed valence and metamagnetism in a metal flux grown compound Eu2Pt3Si5. J. Solid State Chem. 2015, 225, 181–186. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, S.; Halappa, P.; Kalsi, D.; Mumbaraddi, D.; Ghara, S.; Pati, S.K.; Sundaresan, A.; da Silva, I.; Rayaprol, S.; et al. Synthetically tuned structural variations in CePdxGe2−x (x = 0.21, 0.32, 0.69) towards diverse physical properties. Inorg. Chem. Front. 2017, 4, 241–255. [Google Scholar] [CrossRef]
- Nandi, P.; Giri, C.; Joseph, B.; Rath, S.; Manju, U.; Topwal, D. CH3NH3PbI3, a potential solar cell candidate: Structural and spectroscopic investigations. J. Phys. Chem. A 2016, 120, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M.S. The renaissance of black phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Gomez, A. Black phosphorus: Narrow gap, wide applications. J. Phys. Chem. Lett. 2015, 6, 4280–4291. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, S.Y.; Jang, H.W. Black phosphorus: Critical review and potential for water splitting photocatalyst. Nanomaterials 2016, 6, 194. [Google Scholar] [CrossRef]
- Bridgman, P.W. Two new modifications of phosphorus. J. Am. Chem. Soc. 1914, 36, 1344–1363. [Google Scholar] [CrossRef]
- Brown, A.; Rundqvist, S. Refinement of the crystal structure of black phosphorus. Acta. Cryst. 1965, 19, 684–685. [Google Scholar] [CrossRef]
- Qiao, J.; Kong, X.; Hu, Z.-X.; Yang, F.; Ji, W. High mobility transport anisotropy and linear dichroism in few-layer black phosphorus. Nat. Comm. 2014, 5, 4475. [Google Scholar] [CrossRef] [PubMed]
- Keyes, R.W. The electrical properties of black phosphorus. Phys. Rev. B 1953, 92, 580–584. [Google Scholar] [CrossRef]
- Lee, S.; Yang, F.; Suh, J.; Yang, S.; Lee, Y.; Guo, L.; Choe, H.S.; Suslu, A.; Chen, Y.; Ko, C.; et al. Anisotropic in-plane thermal conductivity of black phosphorus nanoribbons at temperatures higher than 100 K. Nat. Comm. 2015, 6, 8573. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.P.; Sakar, S.; Mathew, S.; Zhu, J.-T.; Gopinadhan, K.; Venkatesan, T.; Ang, K.-W. Black phosphorus transistors with near band edge contact Schottky barrier. Sci. Rep. 2015, 5, 18000. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhu, C.; You, L.; Liang, S.-J.; Zheng, S.; Zhou, J.; Fu, Q.; He, Y.; Zeng, Q.; Fan, H.J.; et al. 2D Black Phosphorus/SrTiO3-Based Programmable Photoconductive Switch. Adv. Mater. 2016, 28, 7768–7773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, G.; Hou, Z.; Yang, B.; Zhang, X.; Liu, E.; Xi, X.; Liu, Z.; Zeng, Z.; Wang, W.; et al. Large anisotropic thermal transport properties observed in bulk single crystal black phosphorus. Appl. Phys. Lett. 2016, 108, 092102. [Google Scholar] [CrossRef]
- Villegas, C.E.P.; Rocha, A.R.; Marini, A. Anomalous temperature dependence of the band-gap in black phosphorus. Nano Lett. 2016, 16, 5095–5101. [Google Scholar] [CrossRef] [PubMed]
- Marakatti, V.S.; Sarma, S.C.; Joseph, B.; Banerjee, D.; Peter, S.C. Synthetically tuned atomic ordering in PdCu nanoparticles with enhanced catalytic activity toward solvent-free Benzylamine oxidation. ACS Appl. Mater. Interfaces 2017, 9, 3602–3615. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Marini, C.; Demitri, N.; Capitani, F.; Bernasconi, A.; Zhou, W.; Xing, X.; Shi, Z. Temperature dependent structural modulation in Ca0.82La0.18FeAs2 pnictide supercondcutors. Supercond. Sci. Technol. 2015, 28, 092001. [Google Scholar] [CrossRef]
- Cartz, L.; Srinivasa, S.R.; Riedner, R.J.; Jorgensen, J.D.; Worlton, T.G. Effect of pressure on bonding in black phosphorus. J. Chem. Phys. 1979, 71, 1718–1721. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-dimensional detector software: From real detector to idealized image or two-theta scan. J. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Larson, A.C.; von Dreele, R.B. General Structure Analysis System (GSAS). Los Alamos Natl. Lab. Rep. 2000, LAUR, 86–748. [Google Scholar]




© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, B.; Demitri, N.; Lotti, P.; Lausi, A.; Dore, P. Unraveling the Peculiarities in the Temperature-Dependent Structural Evolution of Black Phosphorus. Condens. Matter 2017, 2, 11. https://doi.org/10.3390/condmat2010011
Joseph B, Demitri N, Lotti P, Lausi A, Dore P. Unraveling the Peculiarities in the Temperature-Dependent Structural Evolution of Black Phosphorus. Condensed Matter. 2017; 2(1):11. https://doi.org/10.3390/condmat2010011
Chicago/Turabian StyleJoseph, Boby, Nicola Demitri, Paolo Lotti, Andrea Lausi, and Paolo Dore. 2017. "Unraveling the Peculiarities in the Temperature-Dependent Structural Evolution of Black Phosphorus" Condensed Matter 2, no. 1: 11. https://doi.org/10.3390/condmat2010011
APA StyleJoseph, B., Demitri, N., Lotti, P., Lausi, A., & Dore, P. (2017). Unraveling the Peculiarities in the Temperature-Dependent Structural Evolution of Black Phosphorus. Condensed Matter, 2(1), 11. https://doi.org/10.3390/condmat2010011