A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO
Abstract
1. Introduction
2. Computational Method
3. Results and Discussion
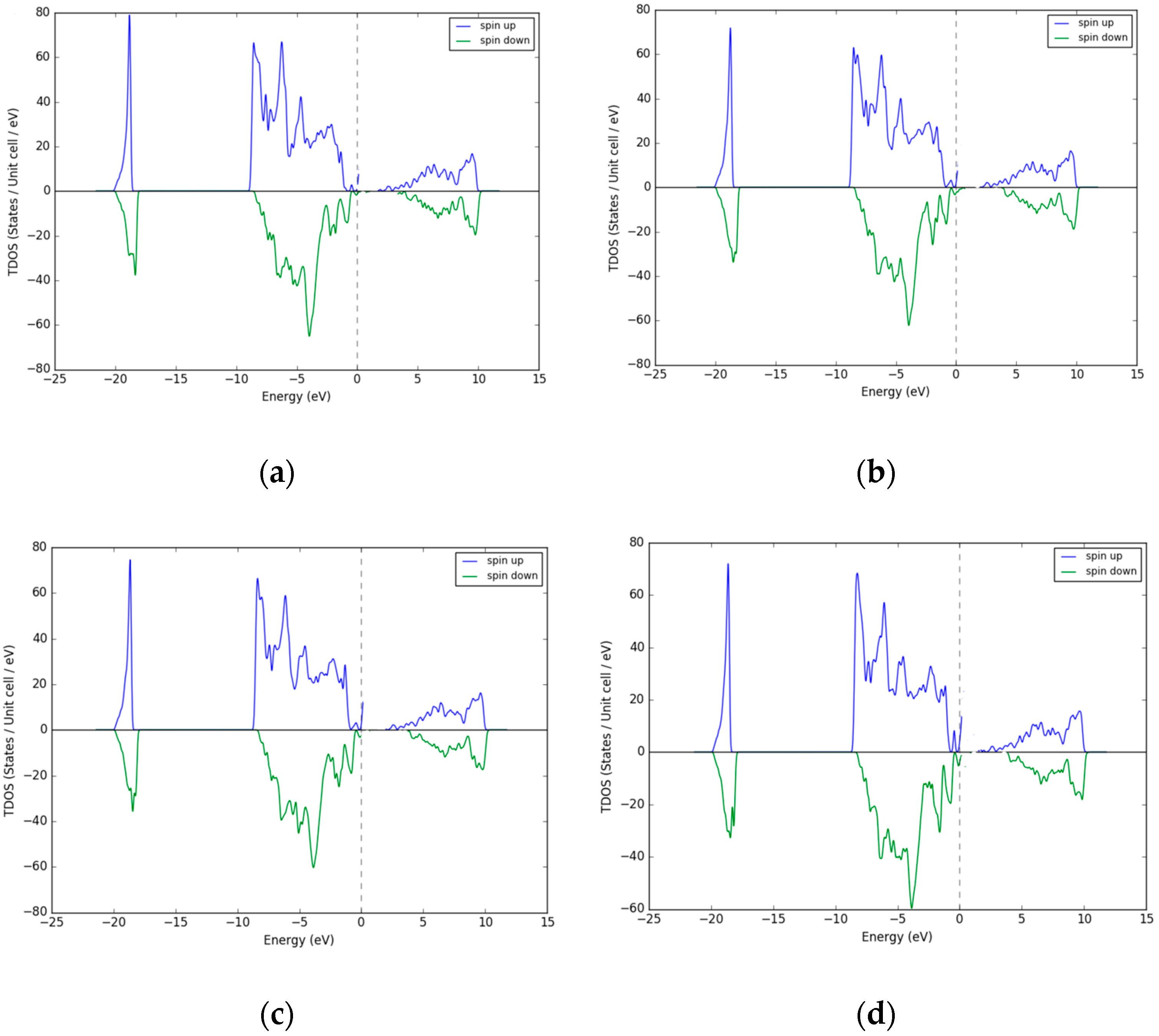
3.1. Density of States (DOS)–Excess Ni Occupying Interstitial Sites inNiO
3.2. Density of States (DOS)—Excess O Occupying Interstitial Sites in NiO
3.3. Formation energies of oxides of Pd and Pt
3.4. Density of States (DOS) - Pd as Dopant Occupying Interstitial Sites inNiO
3.5. Density of States (DOS)—Pt as Dopant Occupying Interstitial Sites inNiO
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide:An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Mott, N.F.; Peierls, R. Discussion on the paper by de Boer and Verwey. Proc. Phys. Soc. 1937, 49, 72–73. [Google Scholar] [CrossRef]
- Davoli, I.; Marcelli, A.; Bianconi, A.; Tomellini, M.; Fanfoni, M. Multi-electron configurations in the X-ray absorption near-edge structure of NiO at the oxygen K threshold. Phys. Rev. B 1986, 33, 2979–2982. [Google Scholar] [CrossRef]
- Tomellini, M.; Gozzi, D.; Bianconi, A.; Davoli, I. Local structure of nickel oxide grown at high temperatures in ceramic electrolyte cells. J. Chem. Soc. Faraday Trans. 1987, 83, 289–298. [Google Scholar] [CrossRef]
- Bengone, O.; Alouani, M.; Blöchl, P.; Hugel, J. Implementation of the projector augmented-wave LDA+U method: Application to the electronic structure of NiO. Phys. Rev. B 2000, 62, 16392–16401. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Ferrero, M.; Pisani, C. An ab Initio Periodic Study of NiO Supported at the Pd(100) Surface. Part 2: The Nonstoichiometric Ni3O4 Phase. J. Phys. Chem. B 2006, 110, 7918–7927. [Google Scholar] [CrossRef]
- Yi, J.B.; Ding, J.; Feng, Y.P.; Peng, G.W.; Chow, G.M.; Kawazoe, Y.; Liu, B.H.; Yin, J.H.; Thongmee, S. Size-dependent magnetism and spin-glass behavior of amorphous NiO bulk, clusters, and nanocrystals: Experiments and first-principles calculations. Phys. Rev. B 2007, 76, 224402. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Yu, N.; Yu, W.-Y.; Tang, B.-Y. Stability and magnetism of vacancy in NiO: A GGA+U study. Eur. Phys. J. B 2008, 64, 153–158. [Google Scholar] [CrossRef]
- Park, S.; Ahn, H.-S.; Lee, C.-K.; Kim, H.; Jin, H.; Lee, H.-S.; Seo, S.; Yu, J.; Han, S. Interaction and ordering of vacancy defects in NiO. Phys. Rev. B 2008, 77, 134103. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Kim, J.Y.; Pérez, M. Experimental and Theoretical Studies on the Reaction of H2 with NiO: Role of O Vacancies and Mechanism for Oxide Reduction. J. Am. Chem. Soc. 2001, 124, 346–354. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Pisani, C.; Cinquini, F.; Giordano, L.; Pacchioni, G. Cationic and anionic vacancies on the NiO(100) surface: DFT+U and hybrid functional density functional theory calculations. J. Chem. Phys. 2007, 127, 174711. [Google Scholar] [CrossRef] [PubMed]
- Manders, J.R.; Tsang, S.-W.; Hartel, M.J.; Lai, T.-H.; Chen, S.; Amb, C.M.; Reynolds, J.R.; So, F. Solution-Processed Nickel Oxide Hole Transport Layers in High Efficiency Polymer Photovoltaic Cells. Adv. Funct. Mater. 2013, 23, 2993–3001. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, T.Y.; Sun, T.H. Microstructures, electrical and optical properties of non-stoichiometric p-type nickel oxide films by radio frequency reactive sputtering. Surf. Coat. Technol. 2010, 205, S236–S240. [Google Scholar] [CrossRef]
- Sato, H.; Minami, T.; Takata, S.; Yamada, T. Transparent conducting p-type NiO thin films prepared by magnetron sputtering. Thin Solid Films 1993, 236, 27–31. [Google Scholar] [CrossRef]
- Chen, H.-L.; Lu, Y.-M.; Hwang, W.-S. Characterization of sputtered NiO thin films. Surf. Coat. Technol. 2005, 198, 138–142. [Google Scholar] [CrossRef]
- Reddy, Y.A.K. Influence of Growth Temperature on the Properties of DC Reactive Magnetron Sputtered NiO Thin Films. Int. J. Curr. Eng. Technol. 2013, 2, 351–357. [Google Scholar] [CrossRef]
- Subramanian, B.; Ibrahim, M.M.; Murali, K.R.; Vidhya, V.S.; Sanjeeviraja, C.; Jayachandran, M. Structural, optoelectronic and electrochemical properties of nickel oxide films. J. Mater. Sci. Mater. Electron. 2009, 20, 953–957. [Google Scholar] [CrossRef]
- Agrawal, A.; Habibi, H.R.; Agrawal, R.K.; Cronin, J.P.; Roberts, D.M.; Caron-Popowich, R.; Lampert, C.M. Effect of deposition pressure on the microstructure and electrochromic properties of electron-beam-evaporated nickel oxide films. Thin Solid Films 1992, 221, 239–253. [Google Scholar] [CrossRef]
- Yeh, W.; Matsumura, M. Chemical Vapor Deposition of Nickel Oxide Films from Bis-π-Cyclopentadienyl-Nickel. Jpn. J. Appl. Phys. 1997, 36, 6884–6887. [Google Scholar] [CrossRef]
- Raut, B.T.; Pawar, S.G.; Chougule, M.A.; Sen, S.; Patil, V.B. New process for synthesis of nickel oxide thin films and their characterization. J. Alloys Compd. 2011, 509, 9065–9070. [Google Scholar] [CrossRef]
- Guo, W.; Hui, K.N.; Hui, K.S. High conductivity nickel oxide thin films by a facile sol–gel method. Mater. Lett. 2013, 92, 291–295. [Google Scholar] [CrossRef]
- Tanaka, M.; Mukai, M.; Fujimori, Y.; Kondoh, M.; Tasaka, Y.; Baba, H.; Usami, S. Transition metal oxide films prepared by pulsed laser deposition for atomic beam detection. Thin Solid Films 1996, 281, 453–456. [Google Scholar] [CrossRef]
- Reguig, B.A.; Khelil, A.; Cattin, L.; Morsli, M.; Bernède, J.C. Properties of NiO thin films deposited by intermittent spray pyrolysis process. Appl. Surf. Sci. 2007, 253, 4330–4334. [Google Scholar] [CrossRef]
- Kang, J.-K.; Rhee, S.-W. Chemical vapor deposition of nickel oxide films from Ni(C5H5)2/O2. Thin Solid Films 2001, 391, 57–61. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, W.; Yan, X.; Feng, B. Studies on electrochromic properties of nickel oxide thin films prepared by reactive sputtering. J. Alloys Compd. 2008, 462, 356–361. [Google Scholar] [CrossRef]
- Brückner, W.; Kaltofen, R.; Thomas, J.; Hecker, M.; Uhlemann, M.; Oswald, S.; Elefant, D.; Schneider, C.M. Stress development in sputtered NiO thin films during heat treatment. J. Appl. Phys. 2003, 94, 4853. [Google Scholar] [CrossRef]
- Kuzmin, A.; Purans, J.; Rodionov, A. X-ray absorption spectroscopy study of the Ni K edge in magnetron-sputtered nickel oxide thin films. J. Phys. Condens. Matter 1997, 9, 6979–6993. [Google Scholar] [CrossRef]
- Itapu, S.; Khan, K.; Georgiev, D.G. Effect of UV Laser Irradiation on the properties of NiO films and ZnO/NiO Heterostructures. MRS Adv. 2016, 1, 293–298. [Google Scholar] [CrossRef]
- Itapu, S.; Georgiev, D.G.; Uprety, P.; Podraza, N.J. Modification of reactively sputtered NiOx thin films by pulsed UV laser irradiation. Phys. Status Solidi 2017, 214, 1600414. [Google Scholar] [CrossRef]
- He, J.H.; Yuan, S.L.; Yin, Y.S.; Tian, Z.M.; Li, P.; Wang, Y.Q.; Liu, K.L.; Wang, C.H. Exchange bias and the origin of room-temperature ferromagnetism in Fe-doped NiO bulk samples. J. Appl. Phys. 2008, 103, 23906. [Google Scholar] [CrossRef]
- Han, D.; Jing, X.; Wang, J.; Yang, P.; Song, D.; Liu, J. Porous lanthanum doped NiO microspheres for supercapacitor application. J. Electroanal. Chem. 2012, 682, 37–44. [Google Scholar] [CrossRef]
- Li, J.-C.; Hou, X.-Y.; Cao, Q. Effect of Cu doping on the resistive switching of NiO thin films. J. Appl. Phys. 2014, 115, 164507. [Google Scholar] [CrossRef]
- Dutta, T.; Gupta, P.; Gupta, A.; Narayan, J. Effect of Li doping in NiO thin films on its transparent and conducting properties and its application in heteroepitaxial p-n junctions. J. Appl. Phys. 2010, 108, 83715. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Jiang, D.; Shi, J. Design of a meso-structured Pd/NiO catalyst for highly efficient low temperature CO oxidation under ambient conditions. RSC Adv. 2015, 5, 40352–40357. [Google Scholar] [CrossRef]
- Sambi, M.; Sensolo, R.; Rizzi, G.A.; Petukhov, M.; Granozzi, G. Growth of NiO ultrathin films on Pd(100) by post-oxidation of Ni films: The effect of pre-adsorbed oxygen. Surf. Sci. 2003, 537, 36–54. [Google Scholar] [CrossRef]
- Zou, X.; Rui, Z.; Ji, H. Core–Shell NiO@PdO Nanoparticles Supported on Alumina as an Advanced Catalyst for Methane Oxidation. ACS Catal. 2017, 7, 1615–1625. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Cheng, B.; Yu, J.; Jiang, C. Flexible nickel foam decorated with Pt/NiO nanoflakes with oxygen vacancies for enhanced catalytic formaldehyde oxidation at room temperature. Environ. Sci. Nano 2017, 4, 2215–2224. [Google Scholar] [CrossRef]
- Nie, L.; Meng, A.; Teng, F.; Cheng, B. Hierarchically macro-mesoporous flowerlike Pt/NiO composite microspheres for efficient formaldehyde oxidation at room temperature. RSC Adv. 2015, 5, 83997–84003. [Google Scholar] [CrossRef]
- Qi, L.; Cheng, B.; Ho, W.; Liu, G.; Yu, J. Hierarchical Pt/NiO Hollow Microspheres with Enhanced Catalytic Performance. ChemNanoMat 2015, 1, 58–67. [Google Scholar] [CrossRef]
- Walker, M.; Parkinson, C.R.; Draxler, M.; Brown, M.G.; Mcconville, C.F. Initial growth of platinum on oxygen-covered Ni(1 1 0) surfaces. Surf. Sci. 2006, 600, 3327–3336. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Av, G.K.; Furthmiiller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter. 1994, 6, 8245–8257. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef]
- Hahn, T. (Ed.) International Tables for Crystallography Volume A: Space-Group Symmetry, 5th ed.; Springer: Amsterdam, The Netherlands, 2002; p. 688. [Google Scholar]
- Bruska, M.K.; Czekaj, I.; Delley, B.; Mantzaras, J.; Wokaun, A. Electronic structure and oxygen vacancies in PdO and ZnO: Validation of DFT models. Phys. Chem. Chem. Phys. 2011, 13, 15947–15954. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T. Theoretical investigations on the potential-induced formation of Pt-oxide surfaces. J. Electroanal. Anal. Chem. 2007, 607, 158–166. [Google Scholar] [CrossRef]





| % Excess Ni | 3 | 12 | 18 | 25 |
| Bandgap (eV) | 3.8 ± 0.003 | 3.83 ± 0.005 | 3.85 ± 0.006 | 3.86 ± 0.008 |
| % Excess O | 3 | 12 | 18 | 25 |
| Bandgap (eV) | 3.80 ± 0.02 | 3.85 ± 0.03 | 3.90 ± 0.03 | 4.00 ± 0.05 |
| Types of Defects | O-Rich Condition Eform(eV) | O-Poor Condition Eform(eV) |
|---|---|---|
| PdNi | −0.33 | 1.54 |
| PtNi | −0.05 | 2.18 |
| IPd | 2.55 | 2.55 |
| IPt | 3.18 | 3.18 |
| % Pd doping | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 25 |
| Bandgap (eV) | 3.80 ± 0.02 | 3.75 ± 0.08 | 3.50 ± 0.06 | 3.20 ± 0.04 | 2.95 ± 0.03 | 2.80 ± 0.04 | 2.70 ± 0.02 | 2.50 ± 0.02 |
| % Pt doping | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 25 |
| Bandgap (eV) | 2.50 ± 0.02 | 2.40 ± 0.04 | 2.40 ± 0.04 | 2.25 ± 0.05 | 2.15 ± 0.06 | 2.10 ± 0.03 | 2.0 ± 0.01 | 2.0 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itapu, S.; Borra, V.; Mossayebi, F. A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO. Condens. Matter 2018, 3, 46. https://doi.org/10.3390/condmat3040046
Itapu S, Borra V, Mossayebi F. A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO. Condensed Matter. 2018; 3(4):46. https://doi.org/10.3390/condmat3040046
Chicago/Turabian StyleItapu, Srikanth, Vamsi Borra, and Faramarz Mossayebi. 2018. "A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO" Condensed Matter 3, no. 4: 46. https://doi.org/10.3390/condmat3040046
APA StyleItapu, S., Borra, V., & Mossayebi, F. (2018). A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO. Condensed Matter, 3(4), 46. https://doi.org/10.3390/condmat3040046





