Interpretation of Near-Infrared Spectroscopy (NIRS) Signals in Skeletal Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Experimental Procedures
2.4. Testing Protocol
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
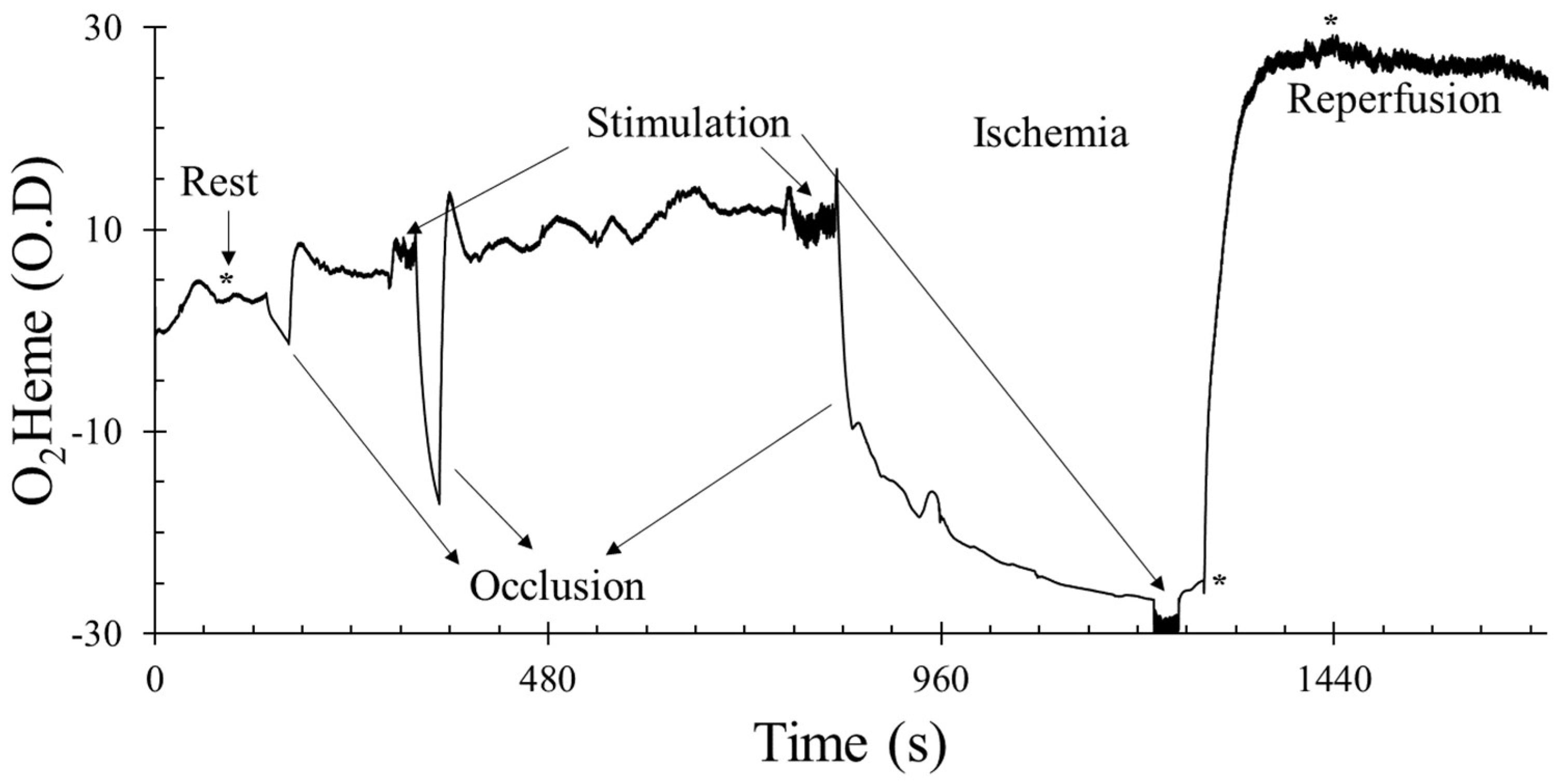
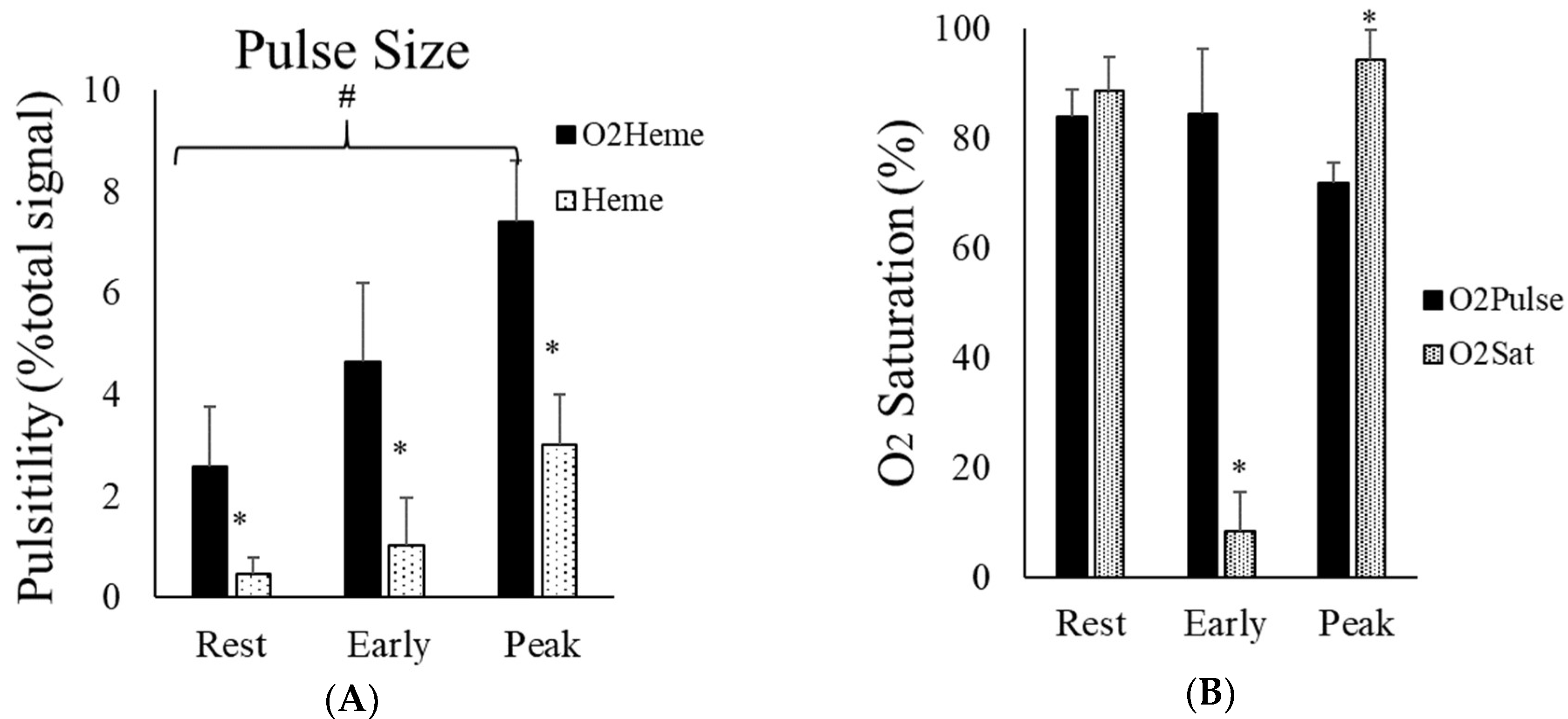
3.1. Oxygen Saturation
3.2. Physiological Calibration
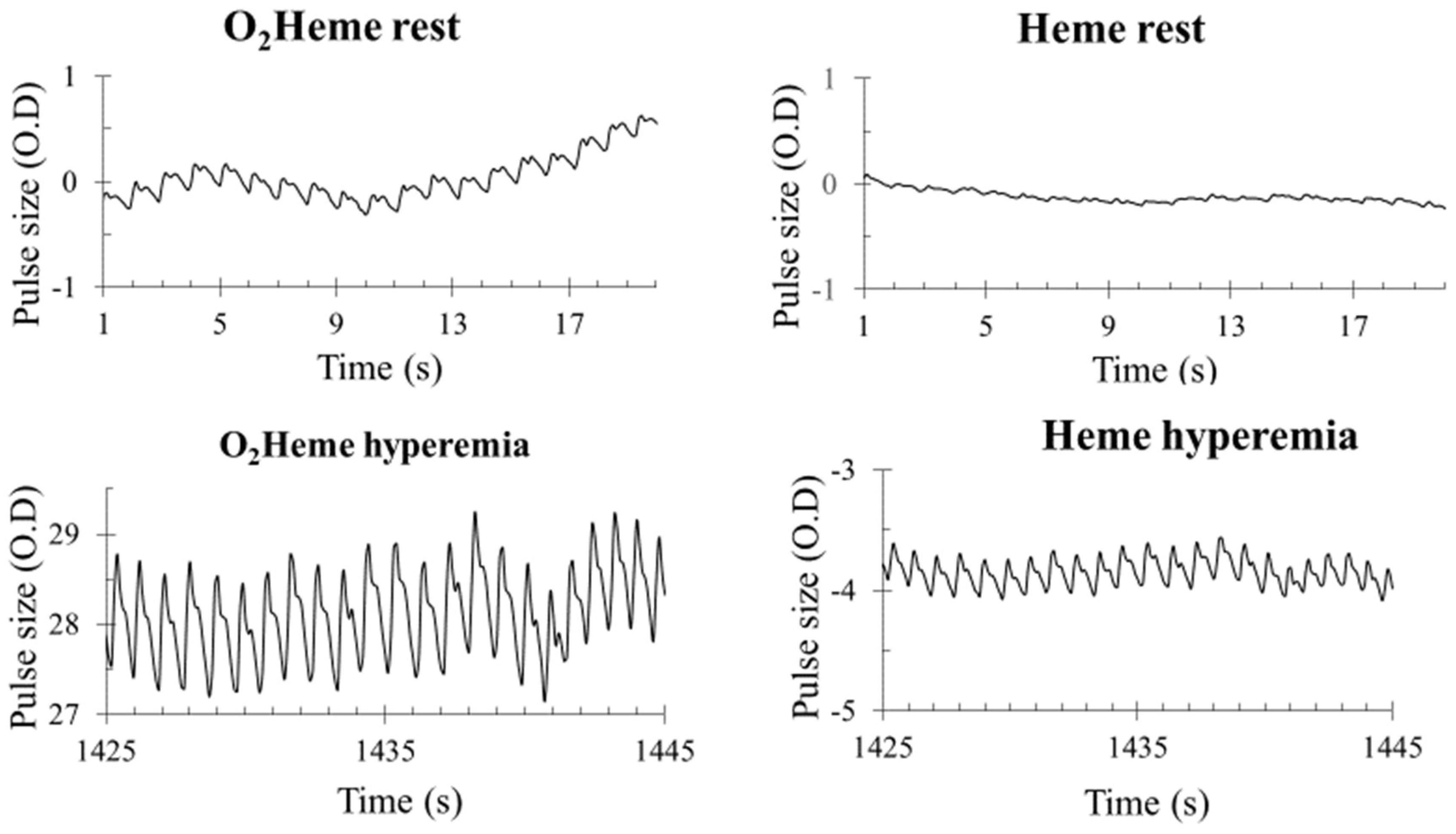
3.3. NIRS Signal Pulsation
4. Discussion
4.1. Appropriate NIRS Signal for Oxygen Saturation in the Muscle
4.2. Physiological Calibration Using 5 Min Ischemia with Prior Exercise
4.3. Interpretation of the Pulsatile O2heme and Heme Signals from Muscle
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trovato, F.M.; Imbesi, R.; Conway, N.; Castrogiovanni, P. Morphological and Functional Aspects of Human Skeletal Muscle. J. Func. Morphol. Kinesiol. 2016, 1, 289–302. [Google Scholar] [CrossRef]
- Addabbo, F.; Ratliff, B.; Park, H.C.; Kuo, M.C.; Ungvari, Z.; Ciszar, A.; Krasnikof, F.; Sodhi, K.; Zhang, F.; Nasjletti, A.; et al. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: Proteomic approach. Am. J. Pathol. 2009, 174, 34–43. [Google Scholar] [CrossRef][Green Version]
- Coen, P.M.; Jubrias, S.A.; Distefano, G.; Amati, F.; Mackey, D.C.; Glynn, N.W.; Manini, T.M.; Wohlgemuth, S.E.; Leeuwenburgh, C.; Cummings, S.R. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 447–455. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.K.; Hamaoka, T. Near-infrared spectroscopy: What can it tell us about oxygen saturation in skeletal muscle? Exerc. Sport Sci. Rev. 2000, 28, 123–127. [Google Scholar] [PubMed]
- Hamaoka, T.; McCully, K.K.; Niwayama, M.; Chance, B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: Recent developments. Philos. Trans. R. Soc. A 2011, 369, 4591–4604. [Google Scholar] [CrossRef]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4577–4590. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.; Iotti, S.; Kendrick, K.; Wang, Z.; Posner, J.D.; Leigh, J., Jr.; Chance, B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J. Appl. Physiol. 1994, 77, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.L.; Barstow, T.J. Estimated contribution of hemoglobin and myoglobin to near infrared spectroscopy. Respir. Physiol. Neurobiol. 2013, 186, 180–187. [Google Scholar] [CrossRef]
- Hesford, C.M.; Laing, S.J.; Cardinale, M.; Cooper, C.E. Asymmetry of quadriceps muscle oxygenation during elite short-track speed skating. Med. Sci. Sports Exerc. 2012, 44, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Cooper, C.E. Near Infrared Spectroscopy (NIRS) Observation of Vastus Lateralis (Muscle) and Prefrontal Cortex (Brain) Tissue Oxygenation During Synchronised Swimming Routines in Elite Athletes. In Oxygen Transport to Tissue XL; Thews, O., LaManna, J.C., Harrison, D.K., Eds.; Springer: New York, NY, USA, 2018; pp. 111–117. [Google Scholar]
- Bendahan, D.; Chatel, B.; Jue, T. Comparative NMR and NIRS analysis of oxygen-dependent metabolism in exercising finger flexor muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R740–R753. [Google Scholar] [CrossRef] [PubMed]
- Seiyama, A.; Hazeki, O.; Tamura, M. Noninvasive quantitative analysis of blood oxygenation in rat skeletal muscle. J. Biochem. 1988, 103, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Bolinger, L.; Li, H.; Kendrick, K.; Chance, B.; Wilson, J.R. Validation of near-infrared spectroscopy in humans. J. Appl. Physiol. 1994, 77, 2740–2747. [Google Scholar] [CrossRef]
- Marcinek, D.J.; Amara, C.E.; Matz, K.; Conley, K.E.; Schenkman, K.A. Wavelength shift analysis: A simple method to determine the contribution of hemoglobin and myoglobin to in vivo optical spectra. Appl. Spectrosc. 2007, 61, 665–669. [Google Scholar] [CrossRef]
- Bossie, H.M.; Willingham, T.B.; Schoick, R.A.V.; O’Connor, P.J.; McCully, K.K. Mitochondrial capacity, muscle endurance, and low energy in friedreich ataxia. Muscle Nerve 2017, 56, 773–779. [Google Scholar] [CrossRef]
- Ryan, T.E.; Erickson, M.L.; Brizendine, J.T.; Young, H.J.; McCully, K.K. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: Correcting for blood volume changes. J. Appl. Physiol. 2012, 113, 175–183. [Google Scholar] [CrossRef]
- Niemeijer, V.M.; Spee, R.F.; Jansen, J.P.; Buskermolen, A.B.; van Dijk, T.; Wijn, P.F.; Kemps, H.M. Test-retest reliability of skeletal muscle oxygenation measurements during submaximal cycling exercise in patients with chronic heart failure. Clin. Physiol. Funct. Imaging 2017, 37, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Barstow, T.J. CORP: Understanding near infrared spectroscopy (NIRS) and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
- McCully, K.K. The influence of passive stretch on muscle oxygen saturation. Adv. Exp. Med. Biol. 2010, 662, 317–322. [Google Scholar]
- Quaresima, V.; Colier, W.N.; Van Der Sluijs, M.; Ferrari, M. Nonuniform quadriceps O2 consumption revealed by near infrared multipoint measurements. Biochem. Biophys. Res. Commun. 2001, 285, 1034–1039. [Google Scholar] [CrossRef]
- Richardson, R.S.; Grassi, B.; Gavin, T.P.; Haseler, L.J.; Tagore, K.; Roca, J.A.; Wagner, P.D. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J. Appl. Physiol. 1999, 86, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.N.; McLay, K.M.; George, M.A.; Murias, J.M. Differences in oxidative metabolism modulation induced by ischemia/reperfusion between trained and untrained individuals assessed by NIRS. Physiol. Rep. 2017, 5, e13384. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.; Tarnopolsky, M.A.; Green, H.J.; Hughson, R.L. Comparison of femoral blood gases and muscle near-infrared spectroscopy at exercise onset in humans. J. Appl. Physiol. 1999, 86, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Southern, W.M.; Ryan, T.E.; Reynolds, M.A.; McCully, K. Reproducibility of near-infrared spectroscopy measurements of oxidative function and postexercise recovery kinetics in the medial gastrocnemius muscle. Appl. Physiol. Nutr. Metab. 2014, 39, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; McCully, K.K.; Quaresima, V.; Yamamoto, K.; Chance, B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J. Biomed. Opt. 2007, 12, 062105. [Google Scholar] [CrossRef]
- Stoner, L.; McCully, K.K. Peak and time-integrated shear rates independently predict flow-mediated dilation. J. Clin. Ultrasound 2012, 40, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; Iwane, H.; Shimomitsu, T.; Katsumura, T.; Murase, N.; Nishio, S.; Osada, T.; Kurosawa, Y.; Chance, B. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J. Appl. Physiol. 1996, 81, 1410–1417. [Google Scholar] [CrossRef]
- Folkow, B.; Haglund, U.; Jodal, M.; Lundgren, O. Blood flow in the calf muscle of man during heavy rhythmic exercise. Acta Physiol. Scand. 1971, 81, 157–163. [Google Scholar] [CrossRef]
- Lee, J.J.; Tyml, K.; Menkis, A.H.; Novick, R.J.; McKenzie, F.N. Evaluation of pulsatile and nonpulsatile flow in capillaries of goat skeletal muscle using intravital microscopy. Microvasc. Res. 1994, 48, 316–327. [Google Scholar] [CrossRef]
- Huo, Y.; Kassab, G.S. Pulsatile blood flow in the entire coronary arterial tree: Theory and experiment. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1074–H1087. [Google Scholar] [CrossRef]


| Male | Female | |
|---|---|---|
| N | 5 | 5 |
| Age (years) | 23.6 (4.3) | 20.2 (0.25) |
| Height (cm) | 170 (0.2) | 1.66 (0.04) |
| Weight (kg) | 73.4 (12.3) | 64.22 (10.5) |
| Body Mass Index (kg/m2) | 25.2 (3.4) | 23.51 (4.9) |
| Adipose Tissue Thickness (cm) | 0.34 (0.09) | 0.40 (0.22) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanni, A.A.; McCully, K.K. Interpretation of Near-Infrared Spectroscopy (NIRS) Signals in Skeletal Muscle. J. Funct. Morphol. Kinesiol. 2019, 4, 28. https://doi.org/10.3390/jfmk4020028
Sanni AA, McCully KK. Interpretation of Near-Infrared Spectroscopy (NIRS) Signals in Skeletal Muscle. Journal of Functional Morphology and Kinesiology. 2019; 4(2):28. https://doi.org/10.3390/jfmk4020028
Chicago/Turabian StyleSanni, Adeola A., and Kevin K. McCully. 2019. "Interpretation of Near-Infrared Spectroscopy (NIRS) Signals in Skeletal Muscle" Journal of Functional Morphology and Kinesiology 4, no. 2: 28. https://doi.org/10.3390/jfmk4020028
APA StyleSanni, A. A., & McCully, K. K. (2019). Interpretation of Near-Infrared Spectroscopy (NIRS) Signals in Skeletal Muscle. Journal of Functional Morphology and Kinesiology, 4(2), 28. https://doi.org/10.3390/jfmk4020028







