Optical Coherence Tomography Reveals Sigmoidal Crystalline Lens Changes during Accommodation
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
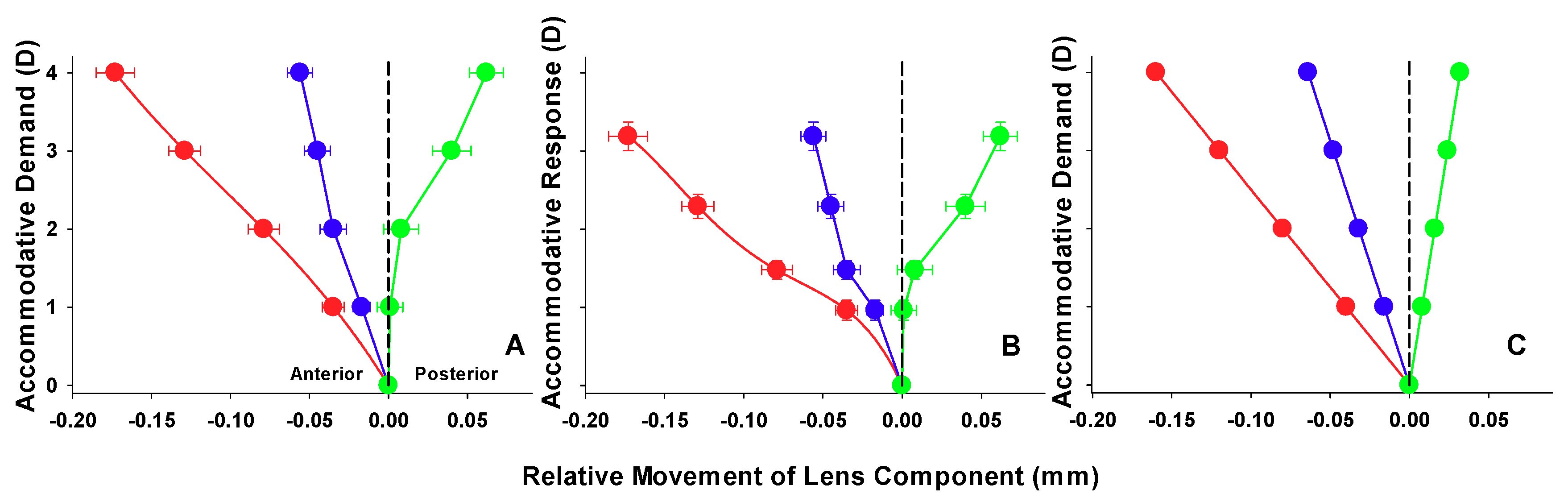
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koretz, J.F.; Cook, C.A.; Kaufman, P.L. Accommodation and presbyopia in the human eye. Changes in the anterior segment and crystalline lens with focus. Investig. Ophthalmol. Vis. Sci. 1997, 38, 569–578. [Google Scholar]
- Koretz, J.F.; Cook, C.A.; Kaufman, P.L. Aging of the human lens: Changes in lens shape upon accommodation and with accommodative loss. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2002, 19, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Rosales, P.; Dubbelman, M.; Marcos, S.; van der Heijde, R. Crystalline lens radii of curvature from Purkinje and Scheimpflug imaging. J. Vis. 2006, 6, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Garner, L.F.; Yap, M.K. Changes in ocular dimensions and refraction with accommodation. Ophthalmic Physiol. Opt. 1997, 17, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Kirschkamp, T.; Dunne, M.; Barry, J.C. Phakometric measurement of ocular surface radii of curvature, axial separations and alignment in relaxed and accommodated human eyes. Ophthalmic Physiol. Opt. 2004, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, K.J. The Glenn, A. Fry invited lecture. Accommodation to gratings and more naturalistic stimuli. Optom. Vis. Sci. 1991, 68, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Glasser, A.; Kaufman, P.L. The mechanism of accommodation in primates. Ophthalmology 1999, 106, 863–872. [Google Scholar] [CrossRef]
- Strenk, S.A.; Semmlow, J.L.; Strenk, L.M.; Munoz, P.; Gronlund-Jacob, J.; DeMarco, J.K. Age-related changes in human ciliary muscle and lens: A Magnetic Resonance Imaging study. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1162–1169. [Google Scholar]
- Richdale, K.; Sinnott, L.T.; Bullimore, M.A.; Wassenaar, P.A.; Schmalbrock, P.; Kao, C.Y.; Patz, S.; Mutti, D.O.; Glasser, A.; Zadnik, K. Quantification of age-related and per diopter accommodative changes of the lens and ciliary muscle in the emmetropic human eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Richdale, K.; Bullimore, M.A.; Sinnott, L.T.; Zadnik, K. The effect of age, accommodation, and refractive error on the adult human eye. Optom. Vis. Sci. 2016, 93, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kasthurirangan, S.; Markwell, E.L.; Atchison, D.A.; Pope, J.M. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J. Vis. 2011, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.E.; Atchison, D.A.; Pope, J.M. Changes in lens dimensions and refractive index with age and accommodation. Optom. Vis. Sci. 2007, 84, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Pope, J.M.; Verkicharla, P.K.; Suheimat, M.; Atchison, D.A. Change in human lens dimensions, lens refractive index distribution and ciliary body ring diameter with accommodation. Biomed. Opt. Express 2018, 9, 1272–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheppard, A.L.; Evans, C.J.; Singh, K.D.; Wolffsohn, J.S.; Dunne, M.C.; Davies, L.N. Three-dimensional Magnetic Resonance Imaging of the phakic crystalline lens during accommodation. Investig. Ophthalmol. Vis. 2011, 52, 3689–3697. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Enriquez, E.; Perez-Merino, P.; Velasco-Ocana, M.; Marcos, S. OCT-based full crystalline lens shape change during accommodation in vivo. Biomed. Opt. Express 2017, 8, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Shum, P.J.; Ko, L.S.; Ng, C.L.; Lin, S.L. A biometric study of ocular changes during accommodation. Am. J. Ophthalmol. 1993, 115, 76–81. [Google Scholar] [CrossRef]
- Drexler, W.; Baumgartner, A.; Findl, O.; Hitzenberger, C.K.; Fercher, A.F. Biometric investigation of changes in the anterior eye segment during accommodation. Vis. Res. 1997, 37, 2789–2800. [Google Scholar] [CrossRef]
- Dubbelman, M.; van der Heijde, G.L.; Weeber, H.A.; Vrensen, G.F. Changes in the internal structure of the human crystalline lens with age and accommodation. Vis. Res. 2003, 43, 2363–2375. [Google Scholar] [CrossRef]
- Dubbelman, M.; van der Heijde, G.L.; Weeber, H.A. Change in shape of the aging human crystalline lens with accommodation. Vis. Res. 2005, 45, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Ostrin, L.; Kasthurirangan, S.; Win-Hall, D.; Glasser, A. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom. Vis. Sci. 2006, 83, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Tsorbatzoglou, A.; Nemeth, G.; Szell, N.; Biro, Z.; Berta, A. Anterior segment changes with age and during accommodation measured with Partial Coherence Interferometry. J. Cataract. Refract. Surg. 2007, 33, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Collins, M.J.; Woodman, E.C.; Cheong, S.H. Axial length changes during accommodation in myopes and emmetropes. Optom. Vis. Sci. 2010, 87, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Strenk, S.A.; Strenk, L.M.; Semmlow, J.L.; DeMarco, J.K. Magnetic Resonance Imaging study of the effects of age and accommodation on the human lens cross-sectional area. Investig. Ophthalmol. Vis. Sci. 2004, 45, 539–545. [Google Scholar] [CrossRef]
- Strenk, S.A.; Strenk, L.M.; Koretz, J.F. The mechanism of presbyopia. Prog. Retin. Eye. Res. 2005, 24, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.; Dubbelman, M.; van der Heijde, R.; Heethaar, R. The shape of the human lens nucleus with accommodation. J. Vis. 2007, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Richdale, K.; Bullimore, M.A.; Zadnik, K. Lens thickness with age and accommodation by Optical Coherence Tomography. Ophthalmic Physiol. Opt. 2008, 28, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Little, J.A.; Saunders, K.J. Repeatability of OCT lens thickness measures with age and accommodation. Optom. Vis. Sci. 2013, 90, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.N.; Dunne, M.C.; Gibson, G.A.; Wolffsohn, J.S. Vergence analysis reveals the influence of axial distances on accommodation with age and axial ametropia. Ophthalmic Physiol. Opt. 2010, 30, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Vilupuru, A.S.; Glasser, A. The relationship between refractive and biometric changes during Edinger-Westphal stimulated accommodation in Rhesus monkeys. Exp. Eye Res. 2005, 80, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.K.; Rabie, E.P. Ultrasound-A research tool in the study of accommodation. Ophthalmic Physiol. Opt. 1983, 3, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.; Mitchell, B. Ultrasound measures of vitreous chamber depth during ocular accommodation. Am. J. Optom. Physiol. Opt. 1985, 62, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Koretz, J.F.; Bertasso, A.M.; Neider, M.W.; True-Gabelt, B.A.; Kaufman, P.L. Slit-lamp studies of the Rhesus monkey eye: II. Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp. Eye Res. 1987, 45, 317–326. [Google Scholar] [CrossRef]
- Wendt, M.; Croft, M.A.; McDonald, J.; Kaufman, P.L.; Glasser, A. Lens diameter and thickness as a function of age and pharmacologically stimulated accommodation in rhesus monkeys. Exp. Eye Res. 2008, 86, 746–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Heijde, G.L.; Weber, J. Accommodation used to determine ultrasound velocity in the human lens. Optom. Vis. Sci. 1989, 66, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijde, G.L.; Beers, A.P.; Dubbelman, M. Microfluctuations of steady-state accommodation measured with Ultrasonography. Ophthalmic Physiol. Opt. 1996, 16, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Beers, A.P.; van der Heijde, G.L. In vivo determination of the biomechanical properties of the component elements of the accommodation mechanism. Vis. Res. 1994, 34, 2897–2905. [Google Scholar] [CrossRef]
- Beers, A.P.; van der Heijde, G.L. Presbyopia and velocity of sound in the lens. Optom. Vis. Sci. 1994, 71, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Beers, A.P.; van der Heijde, G.L. Age-related changes in the accommodation mechanism. Optom. Vis. Sci. 1996, 73, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.A.; McDonald, J.P.; Nadkarni, N.V.; Lin, T.L.; Kaufman, P.L. Age-related changes in centripetal ciliary body movement relative to centripetal lens movement in monkeys. Exp. Eye Res. 2009, 89, 824–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croft, M.A.; Nork, T.M.; McDonald, J.P.; Katz, A.; Lutjen-Drecoll, E.; Kaufman, P.L. Accommodative movements of the vitreous membrane, choroid, and sclera in young and presbyopic human and nonhuman primate eyes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5049–5058. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.A.; Heatley, G.; McDonald, J.P.; Katz, A.; Kaufman, P.L. Accommodative movements of the lens/capsule and the strand that extends between the posterior vitreous zonule insertion zone & the lens equator, in relation to the vitreous face and aging. Ophthalmic Physiol. Opt. 2016, 36, 21–32. [Google Scholar] [PubMed]
- He, L.; Wendt, M.; Glasser, A. Pharmacologically and Edinger-Westphal stimulated accommodation in Rhesus monkeys does not rely on changes in anterior chamber pressure. Exp. Eye Res. 2014, 125, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Lutjen-Drecoll, E.; Kaufman, P.L.; Wasielewski, R.; Ting-Li, l.; Croft, M.A. Morphology and accommodative function of the vitreous zonule in human and monkey eyes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Brown, N. The change in shape and internal form of the lens of the eye on accommodation. Exp. Eye Res. 1973, 15, 441–459. [Google Scholar] [CrossRef]
- Dubbelman, M.; van der Heijde, G.L. The shape of the aging human lens: Curvature, equivalent refractive index and the lens paradox. Vis. Res. 2001, 41, 1867–1877. [Google Scholar] [CrossRef]
- Dubbelman, M.; van der Heijde, G.L.; Weeber, H.A. The thickness of the aging human lens obtained from corrected Scheimpflug images. Optom. Vis. Sci. 2001, 78, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dubbelman, M.; Weeber, H.A.; van der Heijde, R.G.; Volker-Dieben, H.J. Radius and asphericity of the posterior corneal surface determined by corrected Scheimpflug photography. Acta Ophthalmol. Scand. 2002, 80, 379–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallen, E.A.; Kashyap, P.; Hampson, K.M. Transient axial length change during the accommodation response in young adults. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Baikoff, G.; Lutun, E.; Ferraz, C.; Wei, J. Static and dynamic analysis of the anterior segment with Optical Coherence Tomography. J. Cataract. Refract. Surg. 2004, 30, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Baikoff, G.; Lutun, E.; Ferraz, C.; Wei, J. Analysis of the eye’s anterior segment with Optical Coherence Tomography. Static and dynamic study. J. Fr. Ophtalmol. 2005, 28, 343–352. [Google Scholar] [CrossRef]
- Baikoff, G.; Lutun, E.; Wei, J.; Ferraz, C. An in vivo OCT study of human natural accommodation in a 19-year-old albino. J. Fr. Ophtalmol. 2005, 28, 514–519. [Google Scholar] [CrossRef]
- Goldsmith, J.A.; Li, Y.; Chalita, M.R.; Westphal, V.; Patil, C.A.; Rollins, A.M.; Izatt, J.A.; Huang, D. Anterior chamber width measurement by high-speed Optical Coherence Tomography. Ophthalmology 2005, 112, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, R.; Teo, L.; Friedman, D.S.; Aung, H.T.; Baskaran, M.; Gao, H.; Alfred, T.; Seah, S.K.; Kashiwagi, K.; Foster, P.J.; et al. Comparison of anterior chamber depth measurements using the IOLMaster, scanning peripheral anterior chamber depth analyser, and anterior segment Optical Coherence Tomography. Br. J. Ophthalmol. 2007, 91, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, G.; Vajas, A.; Tsorbatzoglou, A.; Kolozsvari, B.; Modis, L., Jr.; Berta, A. Assessment and reproducibility of anterior chamber depth measurement with anterior segment Optical Coherence Tomography compared with immersion Ultrasonography. J. Cataract. Refract. Surg. 2007, 33, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.K.; Li, H.; Weinreb, R.N.; Liu, J.; Cheung, C.Y.; Lai, R.Y.; Pang, C.P.; Lam, D.S. Anterior chamber angle measurement with anterior segment Optical Coherence Tomography: A comparison between slit lamp OCT and Visante OCT. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3469–3474. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, J.; Koenigsdoerffer, E.; Augsten, R.; Strobel, J. Anterior segment Optical Coherence Tomography for evaluation of changes in anterior chamber angle and depth after intraocular lens implantation in eyes with glaucoma. Eur. J. Ophthalmol. 2007, 17, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.P.; Cottet, L.; Dosso, A.A. Evaluation of the anterior chamber depth after cataract surgery with OCT Visante. Klin. Monbl. Augenheilkd. 2008, 225, 438–440. [Google Scholar]
- Baikoff, G. Anterior segment OCT and phakic intraocular lenses: A perspective. J. Cataract. Refract. Surg. 2006, 32, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Koivula, A.; Kugelberg, M. Optical Coherence Tomography of the anterior segment in eyes with phakic refractive lenses. Ophthalmology 2007, 114, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Guell, J.L.; Morral, M.; Gris, O.; Gaytan, J.; Sisquella, M.; Manero, F. Evaluation of Verisyse and Artiflex phakic intraocular lenses during accommodation using Visante optical coherence tomography. J. Cataract. Refract. Surg. 2007, 33, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Kaiserman, I.; Bahar, I.; Rootman, D.S. Corneal wound malapposition after penetrating keratoplasty: An optical coherence tomography study. Br. J. Ophthalmol. 2008, 92, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, A.; Hossain, P.; Anderson, D.F. Recent advances in ophthalmic anterior segment imaging: A new era for ophthalmic diagnosis? Br. J. Ophthalmol. 2007, 91, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Rao, S.K.; Lau, S.; Leung, C.K.; Lam, D.S. Central corneal thickness measurements by Ultrasound, Orbscan II, and Visante OCT after LASIK for myopia. J. Refract. Surg. 2008, 24, 361–365. [Google Scholar] [PubMed]
- Bolz, M.; Prinz, A.; Drexler, W.; Findl, O. Linear relationship of refractive and biometric lenticular changes during accommodation in emmetropic and myopic eyes. Br. J. Ophthalmol. 2007, 91, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S. The Dubbelman Eye Model analysed by ray tracing through aspheric surfaces. Ophthalmic Physiol. Opt. 2005, 25, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.N.; Mallen, E.A.; Wolffsohn, J.S.; Gilmartin, B. Clinical evaluation of the Shin-Nippon Nvision-K 5001/Grand Seiko WR-5100k autorefractor. Optom. Vis. Sci. 2003, 80, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Stark, L.R.; Atchison, D.A. Subject instructions and methods of target presentation in accommodation research. Investig. Ophthalmol. Vis. Sci. 1994, 35, 528–537. [Google Scholar]
- Francis, E.L.; Jiang, B.C.; Owens, D.A.; Tyrrell, R.A. Accommodation and vergence require effort-to-see. Optom. Vis. Sci. 2003, 80, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.D.; Sinnott, L.T.; Mutti, D.O. Ciliary body thickness and refractive error in children. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4353–4360. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R. A.; Eperjesi, F.; Gilmartin, B. The application of analysis of variance (ANOVA) to different experimental designs in optometry. Ophthalmic Physiol. Opt. 2002, 22, 248–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.C.; Davies, L. N.; Wolffsohn, J.S. Accuracy of cornea and lens biometry using anterior segment optical coherence tomography. J. Biomed. Opt. 2007, 12. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J. Anatomy and pathology of the vitreo-retinal interface. Eye 1992, 6, 541–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, S.A.; Buehren, T.; Collins, M.J. Influence of accommodation on the anterior and posterior cornea. J. Cataract. Refract. Surg. 2007, 33, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Bayramlar, H.; Sadigov, F.; Yildirim, A. Effect of accommodation on corneal topography. Cornea 2013, 32, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.; Pérez-Merino, P.; Gambra, E.; de Castro, A.; Marcos, S. In vivo human crystalline lens topography. Biomed. Opt. Express 2012, 3, 2471–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, L.F.; Smith, G. Changes in equivalent and gradient refractive index of the crystalline lens with accommodation. Optom. Vis. Sci. 1997, 74, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Glasser, A.; Campbell, M.C. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vis. Res. 1999, 39, 1991–2015. [Google Scholar] [CrossRef]
- Borja, D.; Siedlecki, D.; de Castro, A.; Uhlhorn, S.; Ortiz, S.; Arrieta, E.; Parel, J.M.; Marcos, S.; Manns, F. Distortions of the posterior surface in optical coherence tomography images of the isolated crystalline lens: Effect of the lens index gradient. Biomed. Opt. Express 2010, 1, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siedlecki, D.; de Castro, A.; Gambra, E.; Ortiz, S.; Borja, D.; Uhlhorn, S.; Manns, F.; Marcos, S.; Parel, J.M. Distortion correction of OCT images of the crystalline lens: Gradient index approach. Optom. Vis. Sci. 2012, 89, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Uhlhorn, S.R.; Borja, D.; Manns, F.; Parel, J.M. Refractive index measurement of the isolated crystalline lens using optical coherence tomography. Vis. Res. 2008, 27, 2732–2738. [Google Scholar] [CrossRef] [PubMed]

| Component | Parameter |
|---|---|
| Cornea | |
| Anterior radius (mm) | 7.87 |
| Thickness (mm) | 0.574 |
| Refractive index | 1.376 |
| Posterior radius (mm) | 6.40 |
| Anterior Chamber | |
| Depth (mm) | 3.87 − 0.010A − D (0.048 − 0.0004A) |
| Refractive index | 1.336 |
| Crystalline Lens | |
| Anterior radius (mm) | 1/[1/(12.7 − 0.058A) + 0.0077D] |
| Thickness (mm) | 2.93 + 0.0236A + D (0.058 − 0.0005A) |
| Refractive index | 1.441 − 0.00039A + 0.0013D |
| Posterior radius (mm) | 1/[1/(5.9 − 0.0013A) + 0.0043D] |
| Vitreous | |
| Depth (mm) | Variable (see text) |
| Refractive index | 1.376 |
| Accommodation Demand (D) | Dubbelman Model (mm) | Dubbelman Model (mm) Adjusted for CT | AS-OCT (mm ± SD) | AS-OCT (mm ± SD) Adjusted for CT |
|---|---|---|---|---|
| Corneal Thickness (CT) | ||||
| 0 | 0.574 | - | 0.551 ± 0.030 | - |
| 1 | 0.574 | - | 0.552 ± 0.033 | - |
| 2 | 0.574 | - | 0.553 ± 0.029 | - |
| 3 | 0.574 | - | 0.554 ± 0.029 | - |
| 4 | 0.574 | - | 0.552 ± 0.033 | - |
| Anterior Chamber Depth (ACD) | ||||
| 0 | 3.096 | 3.670 | 3.102 ± 0.280 | 3.653 ± 0.277 |
| 1 | 3.056 | 3.630 | 3.066 ± 0.287 | 3.618 ± 0.285 |
| 2 | 3.016 | 3.590 | 3.021 ± 0.287 | 3.574 ± 0.286 |
| 3 | 2.976 | 3.550 | 2.970 ± 0.283 | 3.524 ± 0.278 |
| 4 | 2.936 | 3.510 | 2.928 ± 0.282 | 3.480 ± 0.280 |
| Lens Thickness (LT) | ||||
| 0 | 3.402 | - | 3.632 ± 0.205 | - |
| 1 | 3.450 | - | 3.669 ± 0.200 | - |
| 2 | 3.498 | - | 3.719 ± 0.210 | - |
| 3 | 3.546 | - | 3.802 ± 0.226 | - |
| 4 | 3.594 | - | 3.867 ± 0.219 | - |
| Lens Centroid (ACD + LT/2) | ||||
| 0 | 4.797 | 5.371 | 4.918 ± 0.235 | 5.469 ± 0.232 |
| 1 | 4.781 | 5.355 | 4.900 ± 0.235 | 5.452 ± 0.233 |
| 2 | 4.765 | 5.339 | 4.881 ± 0.239 | 5.434 ± 0.237 |
| 3 | 4.749 | 5.323 | 4.871 ± 0.232 | 5.425 ± 0.229 |
| 4 | 4.733 | 5.307 | 4.861 ± 0.228 | 5.413 ± 0.226 |
| Anterior segment Length (ACD + LT) | ||||
| 0 | 6.498 | 7.072 | 6.734 ± 0.230 | 7.285 ± 0.229 |
| 1 | 6.506 | 7.080 | 6.735 ± 0.220 | 7.287 ± 0.218 |
| 2 | 6.514 | 7.088 | 6.740 ± 0.231 | 7.294 ± 0.230 |
| 3 | 6.522 | 7.096 | 6.772 ± 0.232 | 7.326 ± 0.231 |
| 4 | 6.530 | 7.104 | 6.795 ± 0.221 | 7.347 ± 0.218 |
| Accommodation Demand (D) | Dubbelman Model (mm) | AS-OCT (mm ± SD) |
|---|---|---|
| Anterior chamber depth (ACD) | ||
| 1 | −0.040 | −0.035 ± 0.038 |
| 2 | −0.080 | −0.079 ± 0.054 |
| 3 | −0.120 | −0.129 ± 0.055 |
| 4 | −0.160 | −0.173 ± 0.067 |
| Lens Thickness (LT) | ||
| 1 | 0.048 | 0.037 ± 0.059 |
| 2 | 0.096 | 0.087 ± 0.070 |
| 3 | 0.144 | 0.170 ± 0.083 |
| 4 | 0.192 | 0.235 ± 0.091 |
| Lens Centroid (ACD + LT/2) | ||
| 1 | −0.016 | −0.017 ± 0.029 |
| 2 | −0.032 | −0.035 ± 0.046 |
| 3 | −0.048 | −0.045 ± 0.045 |
| 4 | −0.064 | −0.056 ± 0.043 |
| Anterior segment Length (ACD + LT) | ||
| 1 | 0.008 | 0.001 ± 0.044 |
| 2 | 0.016 | 0.008 ± 0.061 |
| 3 | 0.024 | 0.040 ± 0.067 |
| 4 | 0.032 | 0.062 ± 0.059 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, G.A.; Cruickshank, F.E.; Wolffsohn, J.S.; Davies, L.N. Optical Coherence Tomography Reveals Sigmoidal Crystalline Lens Changes during Accommodation. Vision 2018, 2, 33. https://doi.org/10.3390/vision2030033
Gibson GA, Cruickshank FE, Wolffsohn JS, Davies LN. Optical Coherence Tomography Reveals Sigmoidal Crystalline Lens Changes during Accommodation. Vision. 2018; 2(3):33. https://doi.org/10.3390/vision2030033
Chicago/Turabian StyleGibson, George A., Fiona E. Cruickshank, James S. Wolffsohn, and Leon N. Davies. 2018. "Optical Coherence Tomography Reveals Sigmoidal Crystalline Lens Changes during Accommodation" Vision 2, no. 3: 33. https://doi.org/10.3390/vision2030033





