Investigating the Calcination Temperature and Grinding Time of Calcined Clay on the Mechanical Properties and Durability of LC3 Concrete
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials Used and Mixture Properties
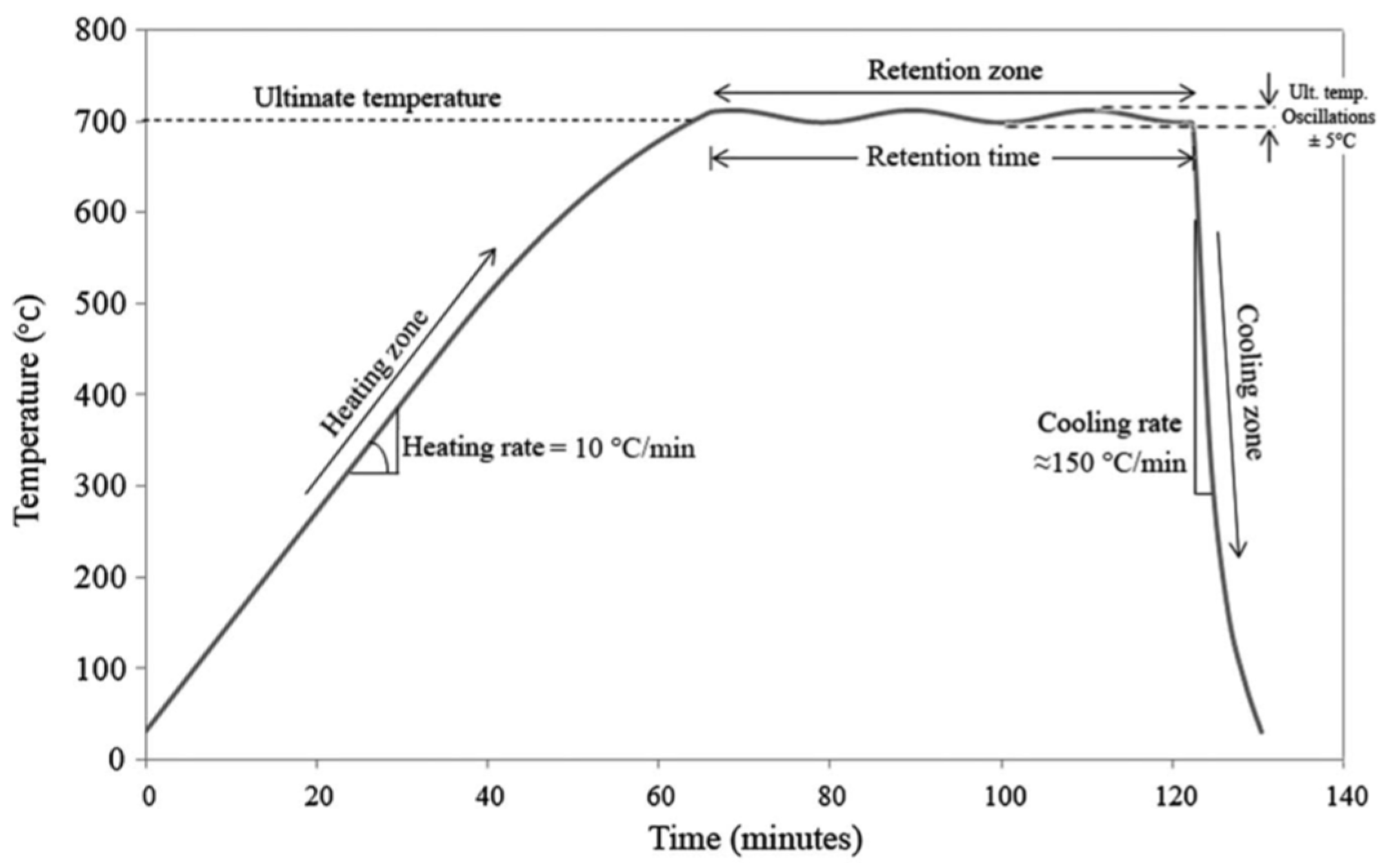
2.2. Tests Performed
- Dnssm = Non-steady-state diffusion coefficient (× 10−12 m2/s);
- U = Absolute value of the applied voltage (V);
- T = Average value of the initial and final temperatures in the anolyte solution (°C);
- L = Thickness of the specimen (mm);
- xd = Average value of the penetration depths (mm);
- t = Test duration (hours).
3. Results and Discussion
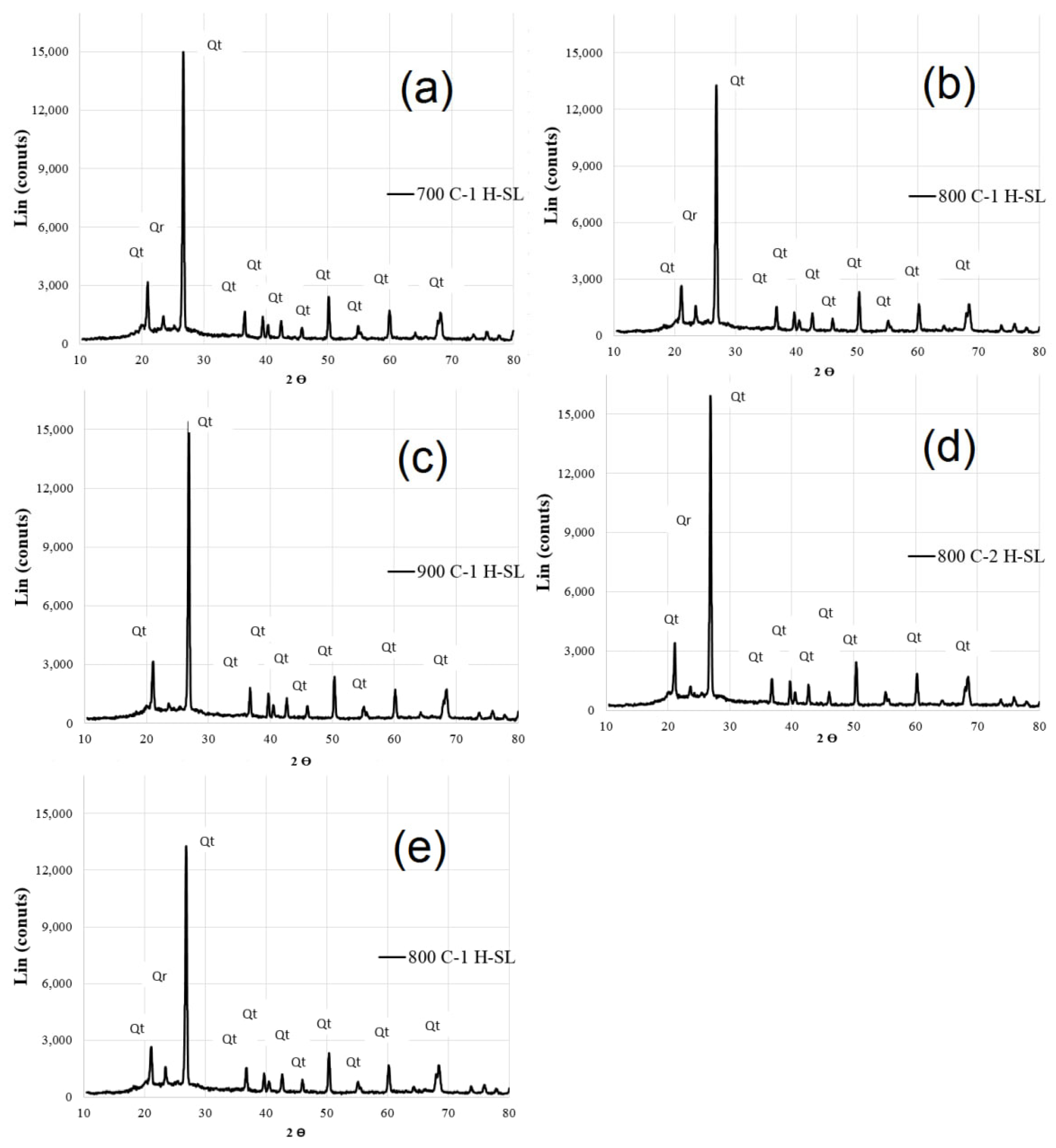
3.1. Properties of Calcinated Clay
3.2. Pozzolanic Activity Index
3.3. Workability of Concretes
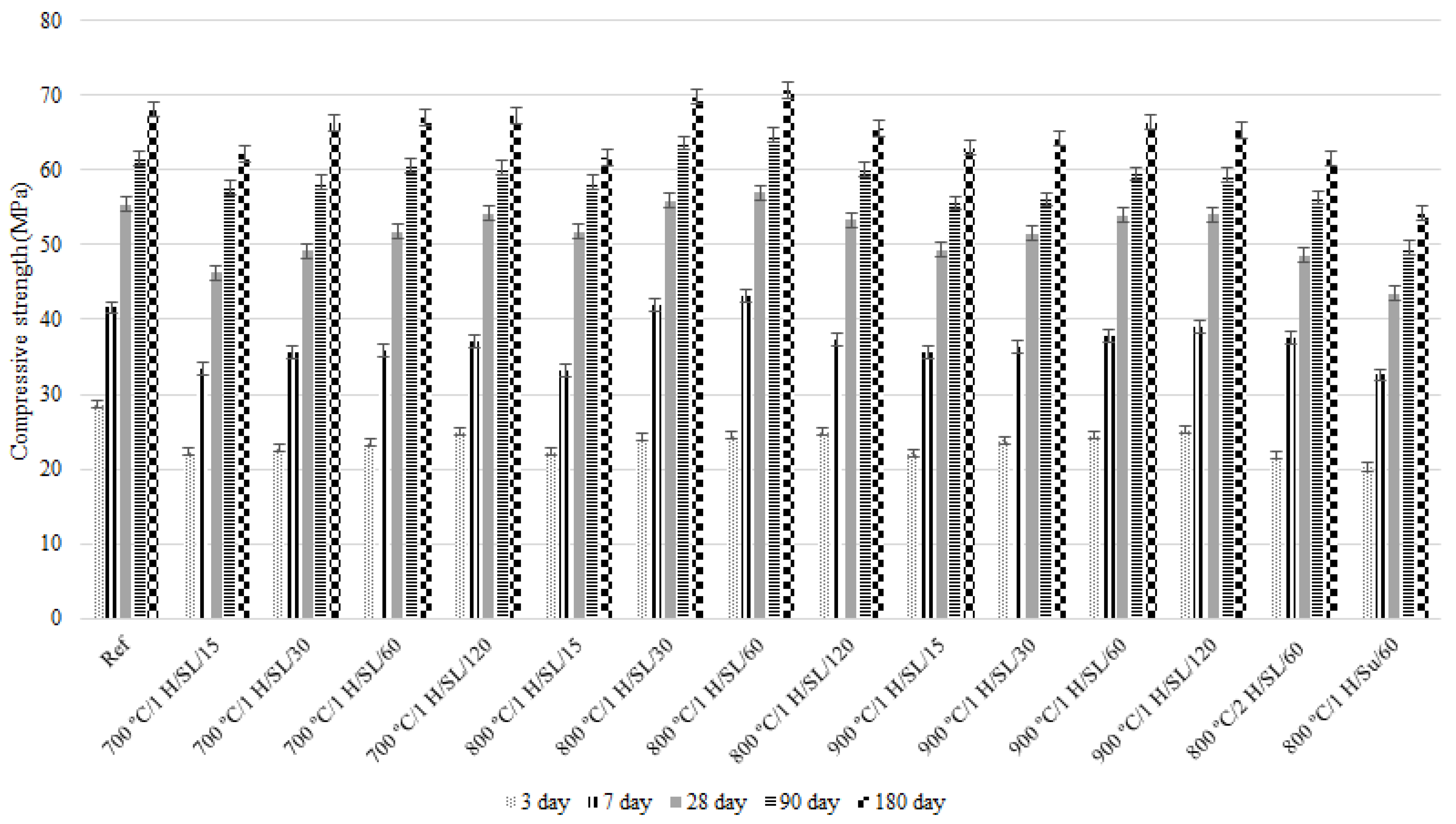
3.4. Compressive Strength
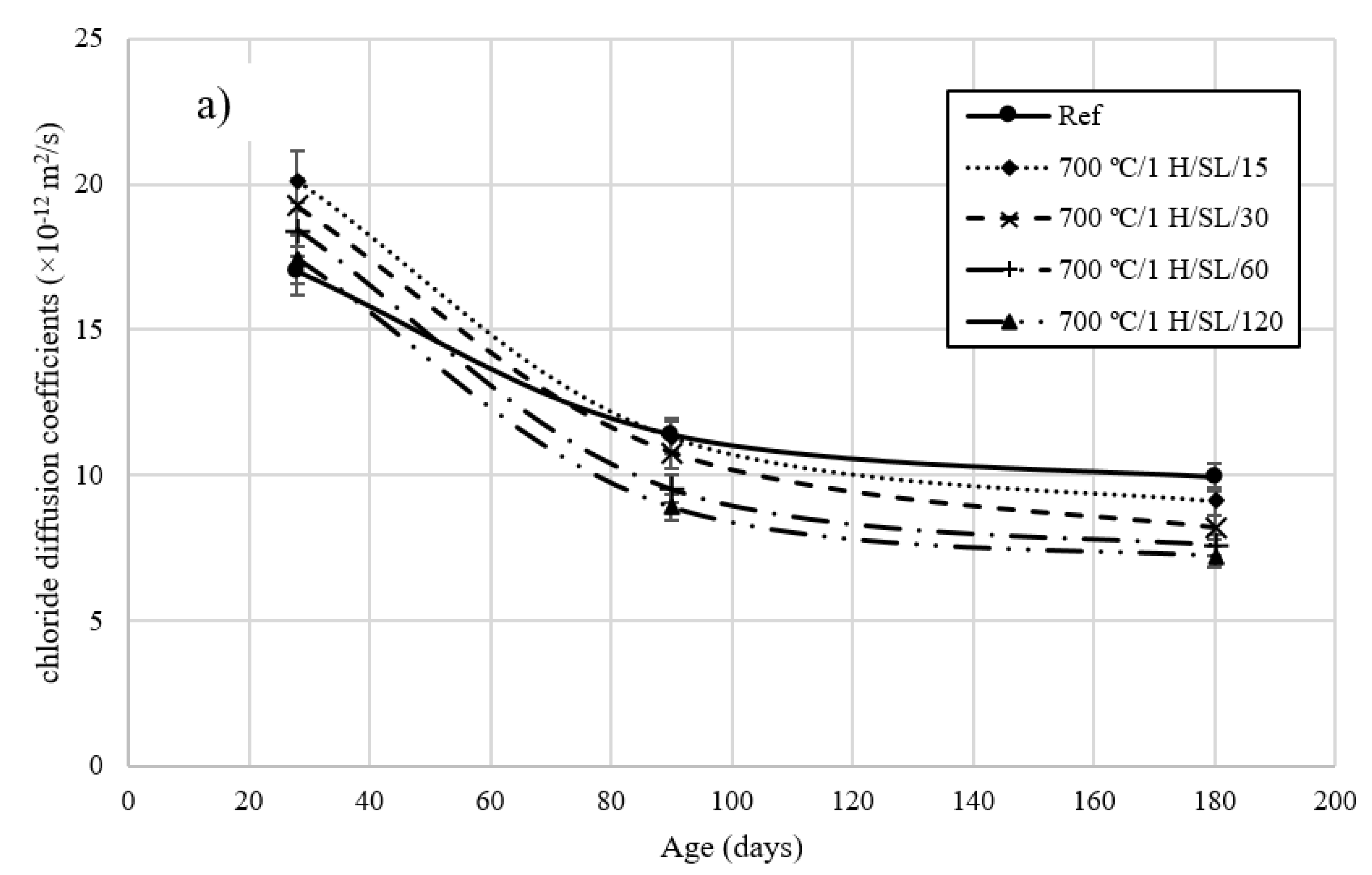
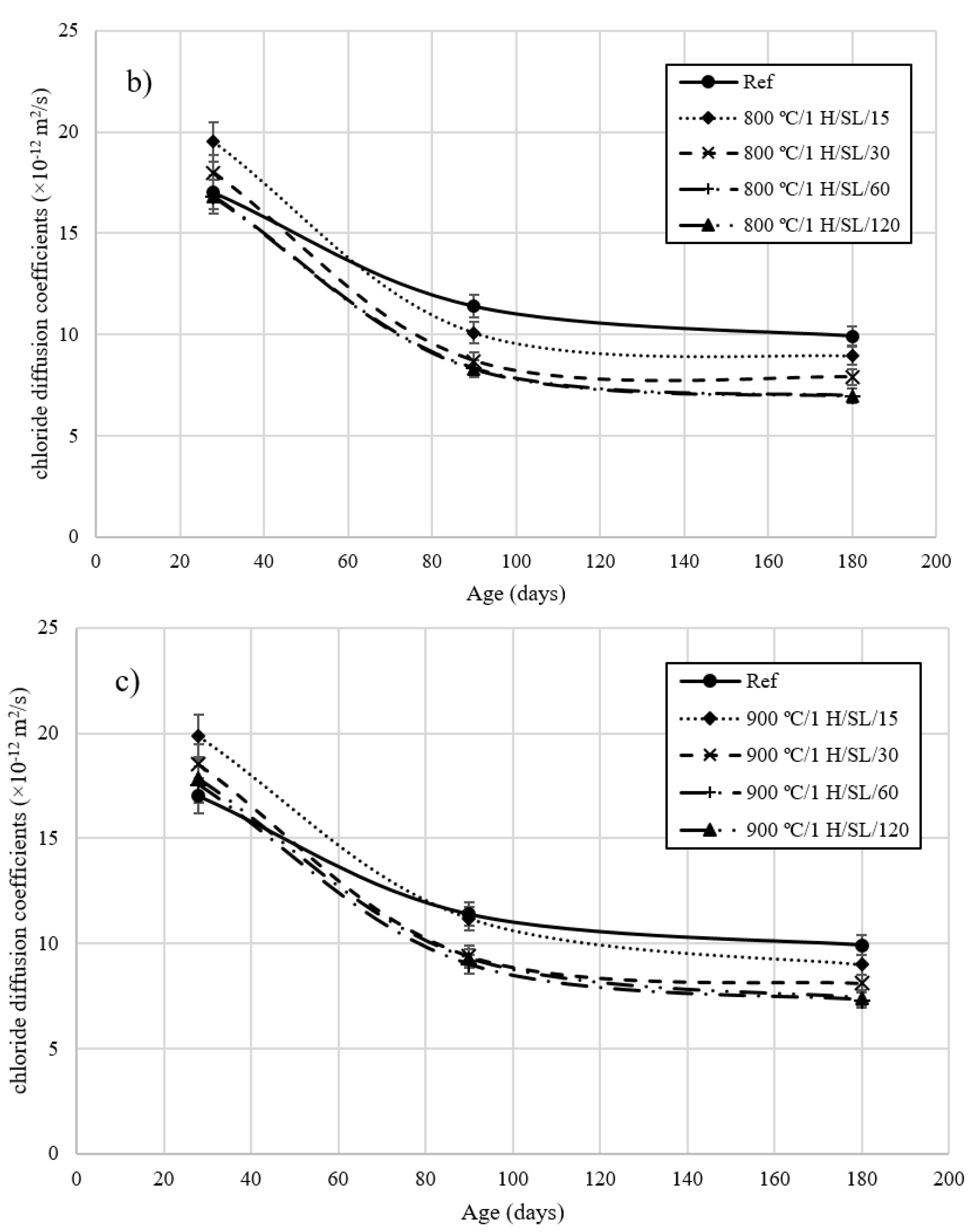
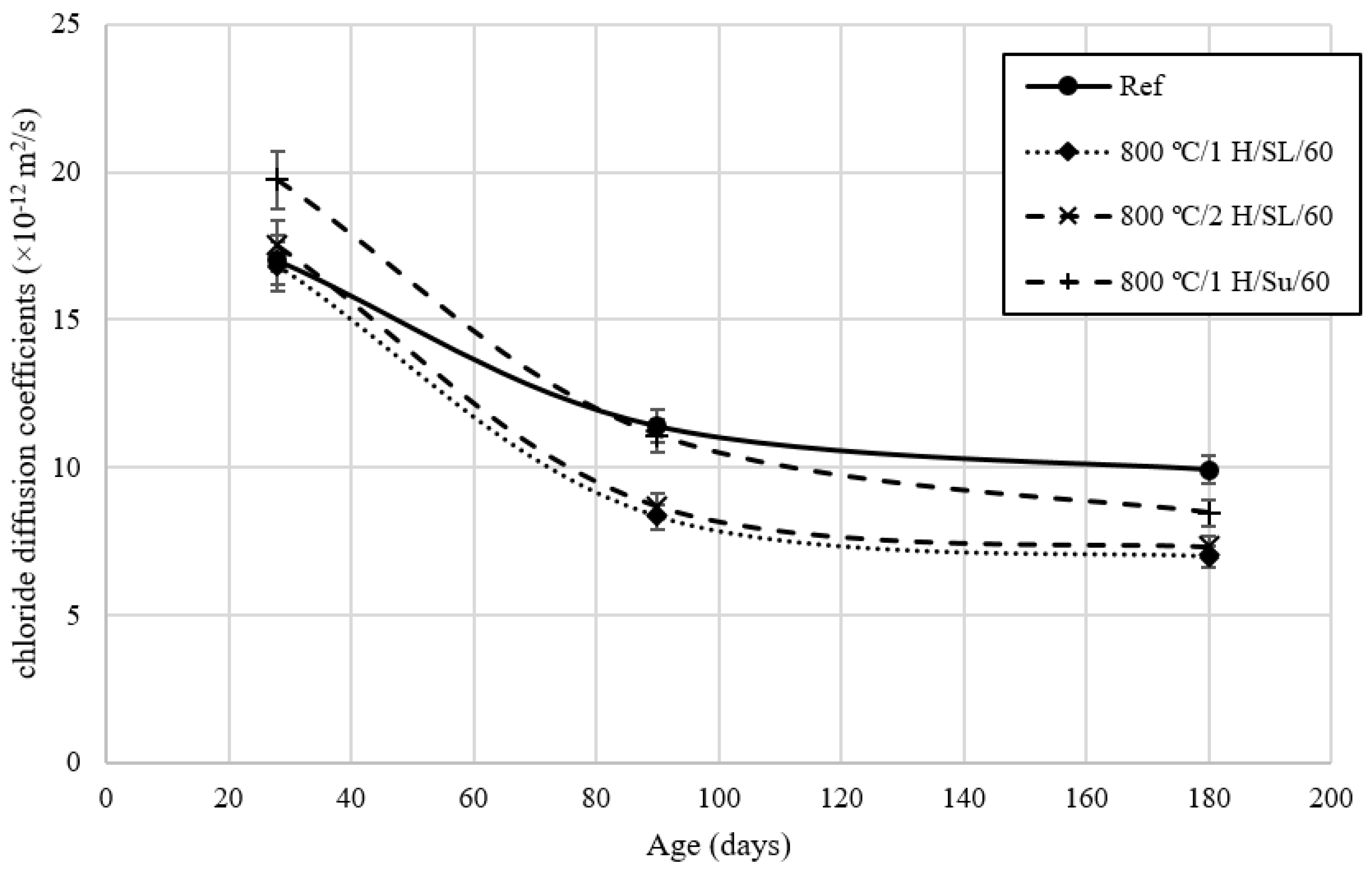
3.5. Rapid Chloride Migration Test
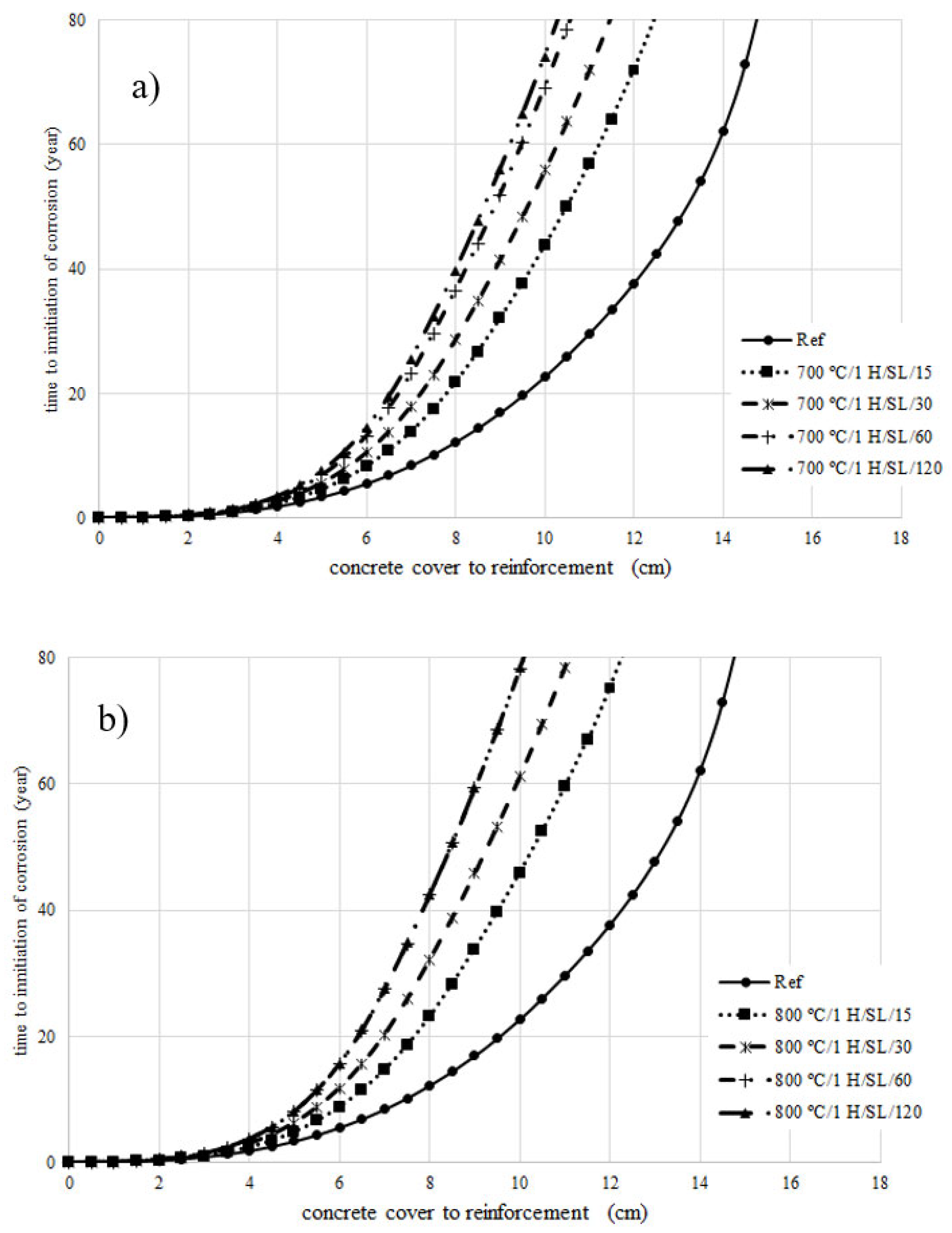
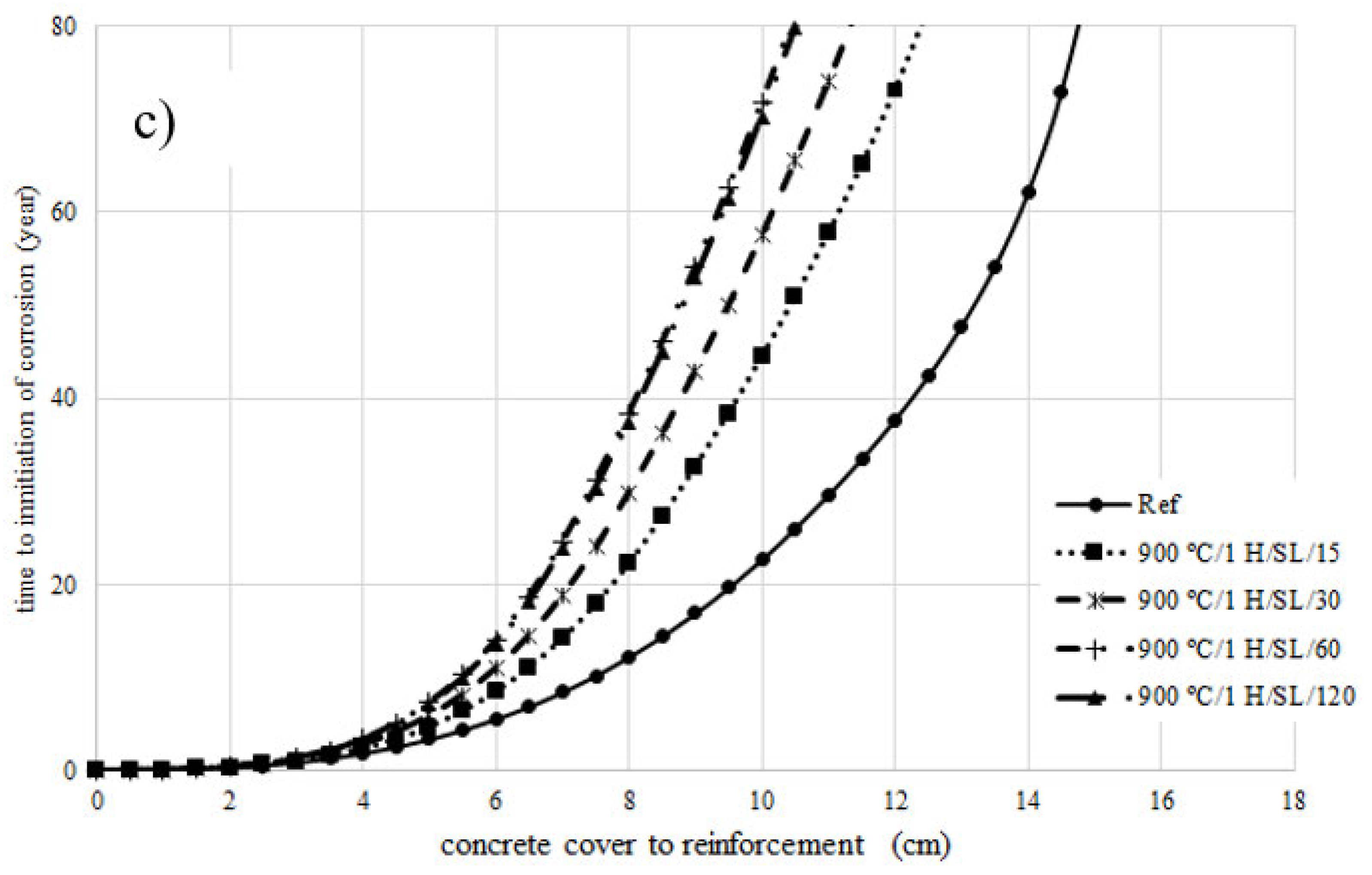
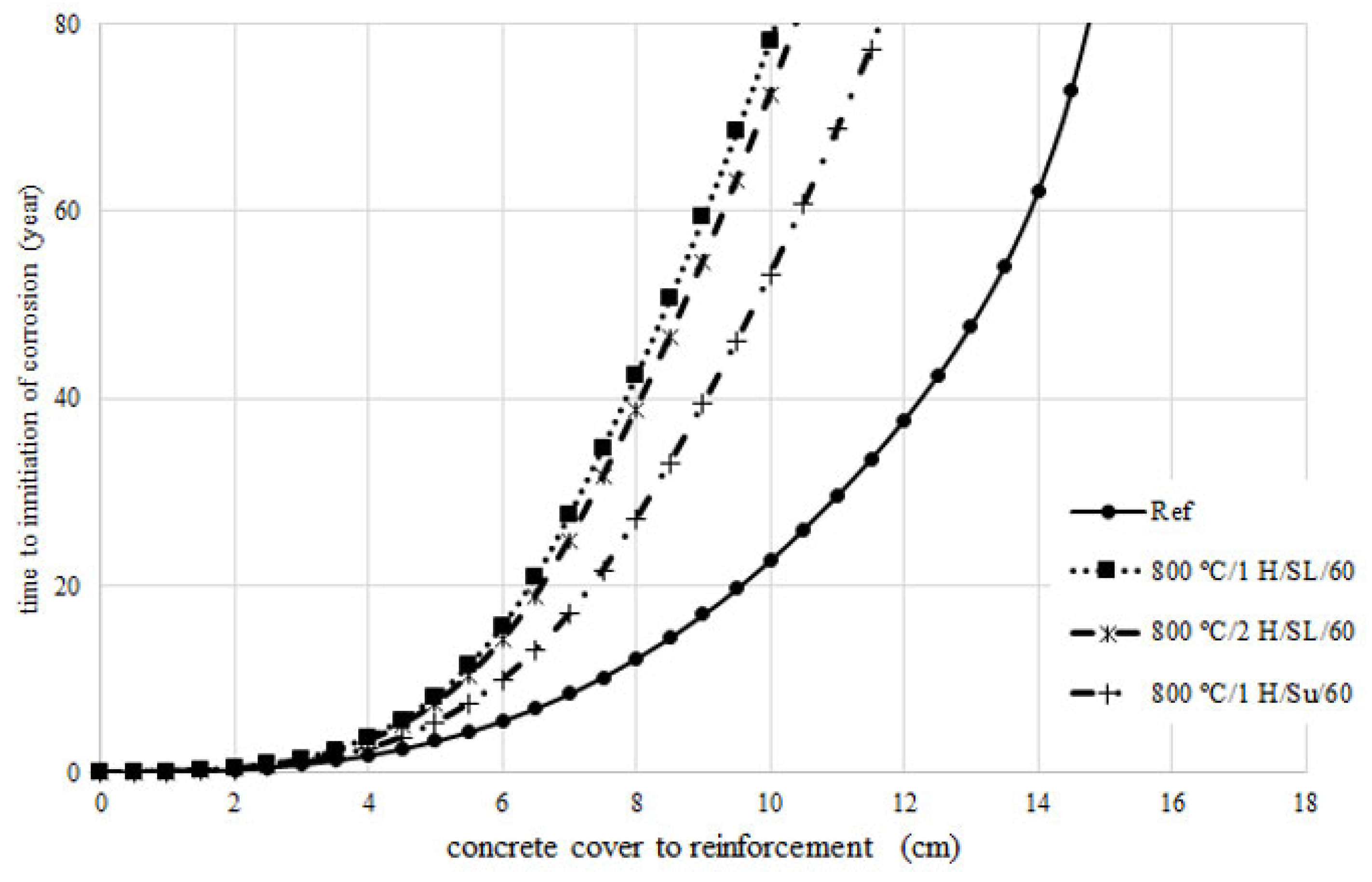
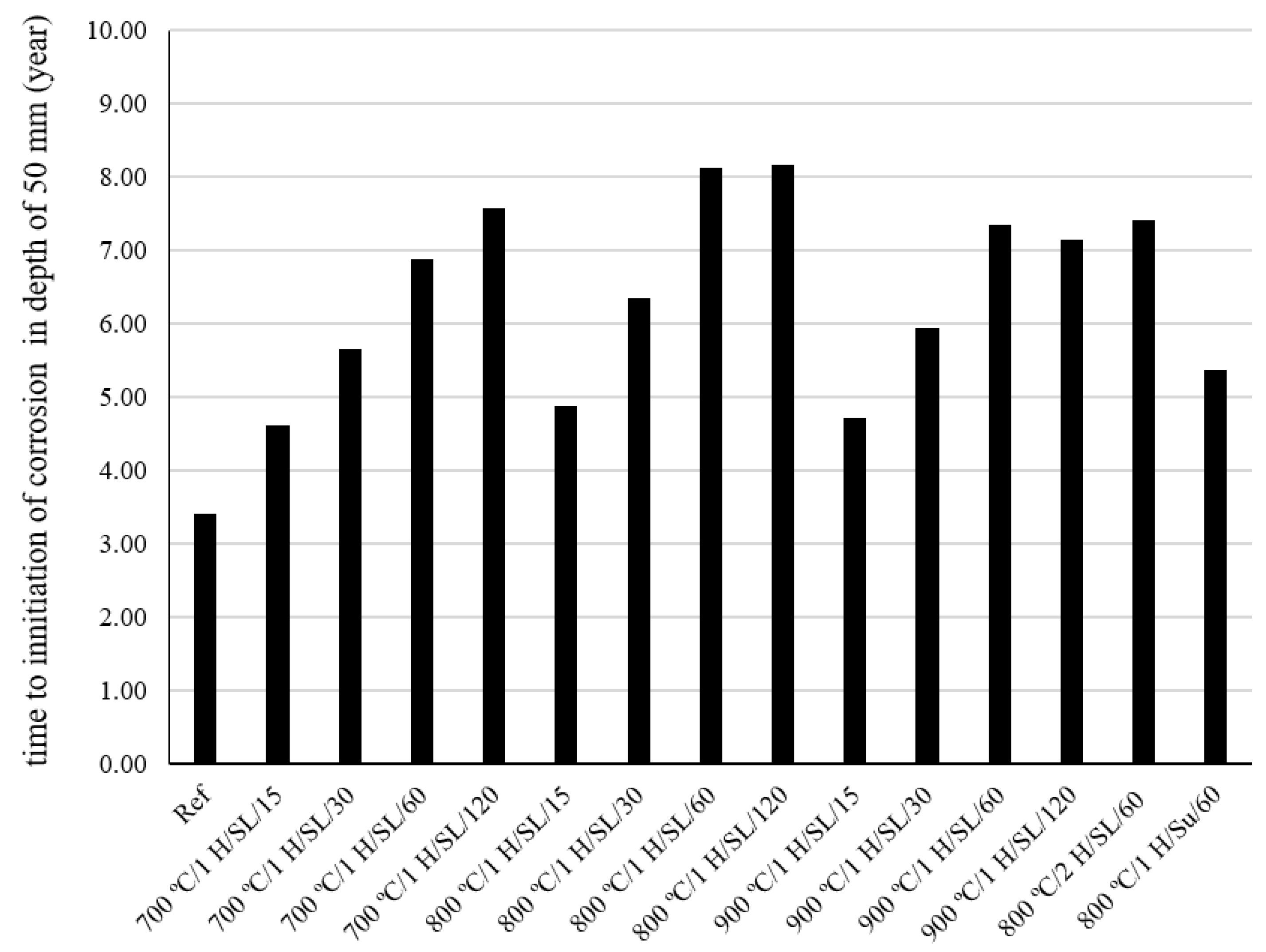
4. Prediction of Corrosion Initiation
5. Conclusions
- 1.
- By increasing the temperature from 800 °C to 900 °C, the pozzolanic index according to ASTM C1240 and ASTM C311 over 28 days decreased by approximately 4% and 7%, respectively. This indicates that at temperatures above 800 °C, the pozzolanic activity of calcined clay diminishes. The pozzolanic index of the calcined clay at 800 °C using the controlled combustion method, as per ASTM C1240 and ASTM C311 in 28 days, was 17% and 20% higher than the pozzolanic index of calcined clay at 800 °C using the instantaneous combustion method. This suggests that the instantaneous combustion method has led to a reduction in the pozzolanic activity of calcined clay.
- 2.
- The pozzolanic performance of calcined clay in the presence of a superplasticizer is slightly better than its performance in mixtures without a superplasticizer.
- 3.
- The 800 °C/1 H/SL/60 mixture exhibits the highest compressive strength among all investigated LC3 mixtures and the control mix. Its compressive strength development process is also desirable. Additionally, the 28-day compressive strength experienced by the 800 °C/1 H/SL/30 mixture was higher than that of the control mix and comparable to the compressive strength development of the 800 °C/1 H/SL/60 mixture.
- 4.
- The chloride diffusion coefficient of the 800 °C/1 H/SL/120 mixture was lower than that of the other investigated LC3 mixtures. Moreover, the time to corrosion initiation for the 800 °C/1 H/SL/120 mixture was higher than that of the other investigated mixtures.
- 5.
- Based on these results, the optimal temperature for clay calcination is about 800 °C, and the optimal grinding time is about 60 min.
- 6.
- The compressive strength and the onset of corrosion initiation time for the LC3 mixture containing calcined clay produced by the instantaneous calcination method, in comparison to the LC3 mixture containing calcined clay produced by the controlled calcination method, experienced a reduction of 24% and 34%, respectively. The instantaneous combustion of clay reduced the quality of calcined clay.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ijaz, N.; Ye, W.; Rehman, Z.U.; Ijaz, Z. Novel application of low carbon limestone calcined clay cement (LC3) in expansive soil stabilization: An eco-efficient approach. J. Clean. Prod. 2022, 371, 133492. [Google Scholar] [CrossRef]
- Sanjuán, M.A.; Argiz, C.; Mora, P.; Zaragoza, A. Carbon dioxide uptake in the roadmap 2050 of the Spanish cement industry. Energies 2020, 13, 3452. [Google Scholar] [CrossRef]
- Maraghechi, H.; Avet, F.; Wong, H.; Kamyab, H.; Scrivener, K. Performance of Limestone Calcined Clay Cement (LC3) with various kaolinite contents with respect to chloride transport. Mater. Struct. Constr. 2018, 51, 125. [Google Scholar] [CrossRef]
- Lothenbach, B.; Saout, G.L.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Shi, Z.G.; Geiker, M.R.; DeWeerdt, K.; Ostnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of calcium on chloride binding in hydrated Portland cement-metakaolin-limestone blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Nicolas, R.S.; Cyr, M.; Escadeillas, G. Performance-based approach to durability of concrete containing flash-calcined metakaolin as cement replacement. Constr. Build. Mater. 2014, 55, 313–322. [Google Scholar] [CrossRef]
- Mishra, G.; Emmanuel, A.C.; Bishnoi, S. Influence of temperature on hydration and microstructure properties of limestone-calcined clay blended cement. Mater. Struct. 2019, 52, 91. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Khan, M.S.H.; Castel, A. Engineering Properties of Limestone Calcined Clay Concrete. J. Adv. Concr. Technol. 2018, 16, 343–357. [Google Scholar] [CrossRef]
- Tironi, A.; Scian, A.N.; Irassar, E.F. Blended Cements with Limestone Filler and Kaolinitic Calcined Clay: Filler and Pozzolanic effects. J. Mater. Civ. Eng. 2017, 29, 04017116. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical properties and durability performance of concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Sommariva, Z.; Alberto, F. Limestone Calcined Clay Cements (LC3): Raw Material Processing, Sulfate Balance and Hydration Kinetics. Ph.D. Thesis, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2020. [Google Scholar] [CrossRef]
- Kenne Diffo, B.B.; Elimbi, A.; Cyr, M.; Dika Manga, J.; Tchakoute Kouamo, H. Effect of the rate of calcination of kaolin on the properties of metakaolin-based geopolymers. J. Asian Ceram. Soc. 2015, 3, 130–138. [Google Scholar] [CrossRef]
- Ez-zaki, H.; Marangu, J.M.; Bellotto, M.; Dalconi, M.C.; Artioli, G.; Valentini, L. A Fresh View on Limestone Calcined Clay Cement (LC3) Pastes. Materials 2021, 14, 3037. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, M.A.; Rivera, R.A.; Martín, D.A.; Estévez, E. Chloride Diffusion in Concrete Made with Coal Fly Ash Ternary and Ground Granulated Blast-Furnace Slag Portland Cements. Materials 2023, 15, 8914. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Zanganeh, H.; Alizadeh, H.; Shakerinia, M.; Marian, M.A.S. Comparing the performance of fine fly ash and silica fume in enhancing the properties of concretes containing fly ash. Constr. Build. Mater. 2013, 47, 1402–1408. [Google Scholar] [CrossRef]
- Marangu, J.M. Physico-chemical properties of Kenyan made calcined Clay-Limestone cement (LC3). Case Stud. Constr. Mater. 2020, 12, e00333. [Google Scholar] [CrossRef]
- Lin, R.S.; Oh, S.; Du, W.; Wang, X.Y. Strengthening the performance of limestone-calcined clay cement (LC3) using nano silica. Constr. Build. Mater. 2022, 340, 127723. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Afroz, S.; Castel, A. Influence of Calcined Clay Reactivity on the Mechanical Properties and Chloride Diffusion Resistance of Limestone Calcined Clay Cement (LC3) Concrete. J. Mar. Sci. Eng. 2020, 8, 301. [Google Scholar] [CrossRef]
- Bahman-Zadeh, F.; Ramezanianpour, A.A.; Zolfagharnasab, A. Effect of carbonation on chloride binding capacity of limestone calcined clay cement (LC3) and binary pastes. J. Build. Eng. 2022, 52, 104447. [Google Scholar] [CrossRef]
- Avet, F.; Sofia, L.; Scrivener, K. Concrete Performance of Limestone Calcined Clay Cement (LC3) Compared with Conventional Cements. Adv. Civ. Eng. Mater. 2019, 8, 20190052. [Google Scholar] [CrossRef]
- Yaraghi, A.H.Y.; Ramezanianpour, A.M.; Ramezanianpour, A.A.; Bahman-Zadeh, F.; Zolfagharnasab, A. Evaluation of test procedures for durability and permeability assessment of concretes containing calcined clay. J. Build. Eng. 2022, 58, 105016. [Google Scholar] [CrossRef]
- Alghamdi, H.; Shoukry, H.; Abadel, A.A.; Khawaji, M. Performance assessment of limestone calcined clay cement (LC3)-Based lightweight green mortars incorporating recycled waste aggregate. J. Mater. Res. Technol. 2023, 23, 2065–2074. [Google Scholar] [CrossRef]
- Sui, S.; Georget, F.; Maraghechi, H.; Sun, W.; Scrivener, K. Towards a generic approach to durability: Factors affecting chloride transport in binary and ternary cementitious materials. Cem. Concr. Res. 2019, 124, 105783. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Influence of pH on the chloride binding capacity of Limestone Calcined Clay Cements (LC3). Cem. Concr. Res. 2020, 131, 106031. [Google Scholar] [CrossRef]
- Yang, P.; Dhandapani, Y.; Santhanam, M.; Neithalath, N. Simulation of chloride diffusion in fly ash and limestone-calcined clay cement (LC3) concretes and the influence of damage on service-life. Cem. Concr. Res. 2020, 130, 106010. [Google Scholar] [CrossRef]
- Tironi, A.; Castellano, C.C.; Bonavetti, V.L.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Kaolinitic calcined clays–Portland cement system: Hydration and properties. Constr. Build. Mater. 2014, 64, 215–221. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Gbozee, M.; Zheng, K.; He, F.; Zeng, X. The influence of aluminum from metakaolin on chemical binding of chloride ions in hydrated cement pastes. Appl. Clay Sci. 2018, 158, 186–194. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Ferreiro, S.; Canut, M.M.C.; Lund, J.; Herfort, D. Influence of fineness of raw clay and calcination temperature on the performance of calcined clay-limestone blended cements. Appl. Clay Sci. 2019, 169, 81–90. [Google Scholar] [CrossRef]
- Balykov, A.S.; Nizina, A.A.; Volodin, V.V.; Kyashkin, V.M. Effects of calcination temperature and time on the physical-chemical efficiency of thermally activated clays in cement systems. Mater. Sci. Forum 2021, 1017, 61–70. [Google Scholar] [CrossRef]
- Nguyen, H.N.; De-Steene, L.V.; Le, T.T.H.; Le, D.D.; Ha-Duong, M. Rice Husk Gasification: From Industry to Laboratory. IOP Conf. Ser. Earth Environ. Sci. 2018, 159, 012033. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N.; Majeed, J. Effect of calcination temperature and heating rate on the optical properties and reactivity of rice husk ash. J. Mater. Sci. 2006, 41, 7926–7933. [Google Scholar] [CrossRef]
- Krishnarao, R.V.; Subrahmanyam, J.; Kumar, T.J. Studies on the formation of black particles in rice husk silica ash. J. Eur. Ceram. Soc. 2001, 21, 99–104. [Google Scholar] [CrossRef]
- ASTM C150; Standard Specification for Portland Cement. ASTM International: Philadelphia, PA, USA, 2017.
- ASTM C33; Standard Specification for Concrete Aggregates. ASTM International: Philadelphia, PA, USA, 2017.
- ASTM C311; Standard Test Methods for Sampling and Testing Fly Ash or Natural Pozzolans for Use in Portland-Cement Concrete. ASTM International: Philadelphia, PA, USA, 2017.
- ASTM C1240; Standard Specification for Silica Fume Used in Cementitious Mixtures. ASTM International: Philadelphia, PA, USA, 2017.
- BS EN 12390-part 3; Testing Hardened Concrete Part 3: Compressive Strength of Test Specimens. BSI-British Standard Institute: London, UK, 2009.
- NT Build 492; Chloride Migration Coefficient from Non-Steady Sate Migration Experiments. NORDTEST: Espoo, Finland, 1999.
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: Philadelphia, PA, USA, 2017.
- Shvarzman, A.; Konstantin, K.; Grader, G.S.; Shter, G.E. The effect of dehydroxylation/amorphization degree on pozzolanic activity of kaolinite. Cem. Concr. Res. 2003, 33, 405–416. [Google Scholar] [CrossRef]
- Guatame-García, A.; Buxton, M.; Deon, F.; Lievens, C. Toward an on-line characterization of kaolin calcination process using shortwave infrared spectroscopy. Miner. Process. Extr. Metall. Rev. 2018, 39, 420–431. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Mackinnon, I.D.R.; Allen, C.; Gu, Y.; Xi, Y. Thermal behaviors of clay minerals as key components and additives for fired brick properties: A review. J. Build. Eng. 2022, 66, 105802. [Google Scholar] [CrossRef]
- Wangb, H.; Liu, X.; Zhang, Z. Pozzolanic activity evaluation methods of solid waste: A review. J. Clean. Prod. 2023, 402, 136783. [Google Scholar] [CrossRef]
- Mohammed, S. Processing, effect and reactivity assessment of artificial pozzolans obtained from clays and clay wastes: A review. Constr. Build. Mater. 2017, 140, 10–19. [Google Scholar] [CrossRef]
- Bagheri, A.; Ajam, A.; Zanganeh, H. Investigation of chloride ingress into concrete under very early age exposure conditions, Constr. Build. Mater. 2019, 225, 801–811. [Google Scholar] [CrossRef]
- Bagheri, A.; Ajam, A.; Zanganeh, H. Effect of very early age exposure on chloride ingress and service life performance of binary and ternary concretes, Constr. Build. Mater. 2021, 289, 123137. [Google Scholar] [CrossRef]
- Ehlen, M.A. Life-365 Service Life Prediction Model and Computer Program for Predicting the Service Life and Life-Cycle Cost of Reinforced Concrete Exposed to Chlorides, User’s Manual, Produced by the Life-365™ Consortium III, Version 2.2.3. 2020. Available online: http://www.life-365.org/download/Life-365_v2.2.3_Users_Manual.pdf (accessed on 9 August 2023).

| Composition % | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | L.O.I. | IR |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 21.8 | 4.1 | 3.58 | 62.7 | 1.9 | 1.51 | 1.2 | 1.46 | 1.61 | 0.75 |
| Limestone (LS) | 3.28 | 0.75 | 0.22 | 49.3 | 2.82 | 0.09 | - | 0.03 | 41.6 | - |
| Raw clay | 60.61 | 29.72 | 1.38 | 1.08 | 0.41 | - | 0.09 | 0.34 | 9.31 | - |
| Properties | Cement | ASTM C150 Requirements |
|---|---|---|
| Specific surface (cm2/gr)-min | 3060 | 2600 |
| Time of setting (minuts) | ||
| Initial min | 190 | 45 |
| Final-max | 235 | 375 |
| Compressive strength (MPa) | ||
| 3 days-min | 14.1 | 10.0 |
| 7 days-min | 23.5 | 17.0 |
| Mix Designation | W/C | Cement (kg/m3) | LS (kg/m3) | Calcined clay (kg/m3) | Water (kg/m3) | SP (% cement) | Coarse agg. SSD (kg/m3) | Fine agg. SSD (kg/m3) |
|---|---|---|---|---|---|---|---|---|
| Ref | 0.40 | 400.0 | - | - | 160.0 | 0.47 | 867 | 867 |
| Grinding Time (min) | Specific Surface Area | Specific Gravity(g/cm3) | Percent Retained on 45 μm Sieve | L.O.I. (%) | ||
|---|---|---|---|---|---|---|
| BET (m2/g) | Blaine (cm2/g) | |||||
| LS | - | - | 4640 | 2.61 | 11.1 | 41.6 |
| 700 °C/1 H/SL | 15 | 20.8 | 4130 | 2.57 | 15.6 | 0.94 |
| 30 | 21.1 | 4660 | 2.57 | 14.3 | ||
| 60 | 21.3 | 4970 | 2.57 | 12.3 | ||
| 120 | 21.4 | 5090 | 2.57 | 12.3 | ||
| 800 °C/1 H/SL | 15 | 35.2 | 5020 | 2.57 | 15.6 | 0.83 |
| 30 | 35.4 | 5560 | 2.57 | 14.3 | ||
| 60 | 35.5 | 5840 | 2.57 | 12.3 | ||
| 120 | 35.6 | 5980 | 2.57 | 12.3 | ||
| 900 °C/1 H/SL | 15 | 30.7 | 4760 | 2.57 | 15.6 | 0.64 |
| 30 | 30.9 | 5250 | 2.57 | 14.3 | ||
| 60 | 31.0 | 5530 | 2.57 | 12.3 | ||
| 120 | 31.0 | 5670 | 2.57 | 12.3 | ||
| 800 °C/2 H/SL | 60 | 23.8 | 5030 | 2.57 | 14.6 | 0.78 |
| 800 °C/1 H/Su | 60 | 19.2 | 4600 | 2.57 | 17.2 | 0.91 |
| Mix Designation | Grinding Time (min) | Accordance to ASTM C311 | Accordance to ASTM C1240 | ||
|---|---|---|---|---|---|
| Pozzolanic Activity Index with Portland Cement (%) | Water Requirement (%) | Accelerated Pozzolanic Strength Activity Indexin (%) | |||
| 7 Days | 28 Days | ||||
| 700 °C/1 H/SL | 15 | 60 | 80 | 110 | 115 |
| 30 | 63 | 82 | 115 | 119 | |
| 60 | 68 | 83 | 120 | 130 | |
| 120 | 65 | 81 | 126 | 130 | |
| 800 °C/1 H/SL | 15 | 63 | 84 | 111 | 118 |
| 30 | 65 | 85 | 116 | 121 | |
| 60 | 69 | 90 | 121 | 136 | |
| 120 | 64 | 84 | 126 | 134 | |
| 900 °C/1 H/SL | 15 | 61 | 79 | 112 | 116 |
| 30 | 63 | 81 | 117 | 117 | |
| 60 | 65 | 81 | 122 | 128 | |
| 120 | 63 | 79 | 130 | 129 | |
| 800 °C/2 H/SL | 60 | 66 | 76 | 132 | 120 |
| 800 °C/1 H/Su | 60 | 60 | 75 | 138 | 116 |
| Mix Designation | SP (% Cement) |
|---|---|
| Ref | 0.47 |
| 700 °C/1 H/SL/15 | 0.67 |
| 700 °C/1 H/SL/30 | 0.71 |
| 700 °C/1 H/SL/60 | 0.76 |
| 700 °C/1 H/SL/120 | 0.83 |
| 800 °C/1 H/SL/15 | 0.72 |
| 800 °C/1 H/SL/30 | 0.75 |
| 800 °C/1 H/SL/60 | 0.79 |
| 800 °C/1 H/SL/120 | 0.87 |
| 900 °C/1 H-SL/15 | 0.73 |
| 900 °C/1 H/SL/30 | 0.78 |
| 900 °C/1 H/SL/60 | 0.82 |
| 900 °C/1 H/SL/120 | 0.94 |
| 800 °C/2 H/SL/60 | 0.73 |
| 800 °C/1 H/Su/60 | 0.72 |
| Mix ID | D0 (×10−12 m2/s) | m |
|---|---|---|
| Ref | 17.02 | 0.295 |
| 700 °C/1 H/SL/15 | 20.12 | 0.433 |
| 700 °C/1 H/SL/30 | 19.24 | 0.461 |
| 700 °C/1 H/SL/60 | 18.43 | 0.485 |
| 700 °C/1 H/SL/120 | 17.46 | 0.485 |
| 800 °C/1 H/SL/15 | 19.51 | 0.434 |
| 800 °C/1 H/SL/30 | 17.98 | 0.461 |
| 800 °C/1 H/SL/60 | 16.82 | 0.486 |
| 800 °C/1 H/SL/120 | 16.79 | 0.486 |
| 900 °C/1 H/SL/15 | 19.87 | 0.433 |
| 900 °C/1 H/SL/30 | 18.53 | 0.458 |
| 900 °C/1 H/SL/60 | 17.55 | 0.481 |
| 900 °C/1 H/SL/120 | 17.84 | 0.481 |
| 800 °C/2 H/SL/60 | 17.51 | 0.482 |
| 800 °C/1 H/Su/60 | 19.74 | 0.459 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiri, S.; Madandoust, R.; Ranjbar, M.M. Investigating the Calcination Temperature and Grinding Time of Calcined Clay on the Mechanical Properties and Durability of LC3 Concrete. Infrastructures 2023, 8, 139. https://doi.org/10.3390/infrastructures8100139
Nasiri S, Madandoust R, Ranjbar MM. Investigating the Calcination Temperature and Grinding Time of Calcined Clay on the Mechanical Properties and Durability of LC3 Concrete. Infrastructures. 2023; 8(10):139. https://doi.org/10.3390/infrastructures8100139
Chicago/Turabian StyleNasiri, Sina, Rahmat Madandoust, and Malek Mohammad Ranjbar. 2023. "Investigating the Calcination Temperature and Grinding Time of Calcined Clay on the Mechanical Properties and Durability of LC3 Concrete" Infrastructures 8, no. 10: 139. https://doi.org/10.3390/infrastructures8100139




