Experimental Study on the Physical and Mechanical Characteristics of Refractory Concrete Using Heat-Treated Steel Slag Coarse Aggregates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cement
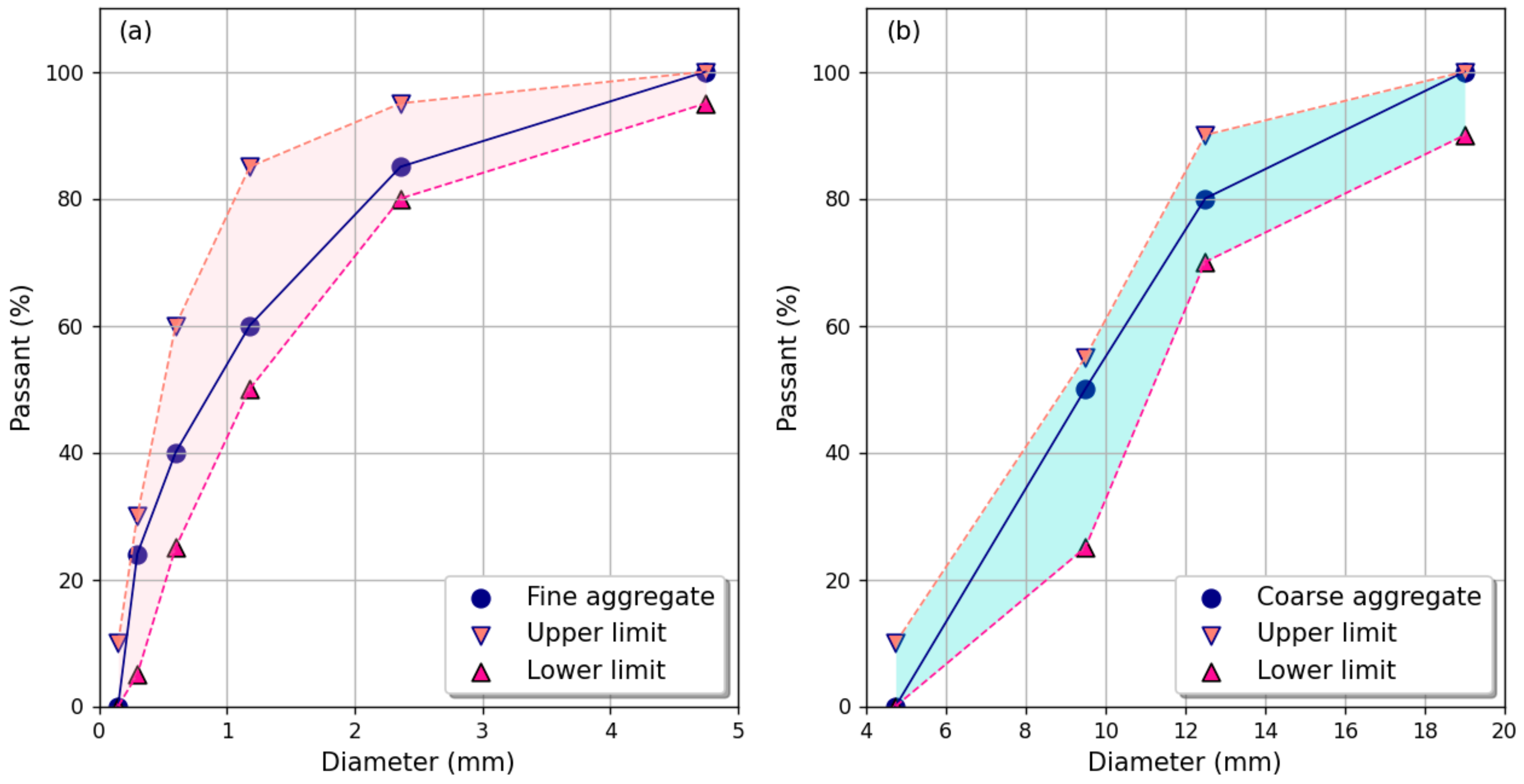
2.1.2. Aggregates
2.1.3. Steel Slag
2.2. Concrete Mixture Design
2.3. Tests Methods
2.3.1. Tests on Fresh Concrete
2.3.2. Tests on Hardened Concrete
- Compressive Strength
- Splitting Strength
3. Results and Discussion
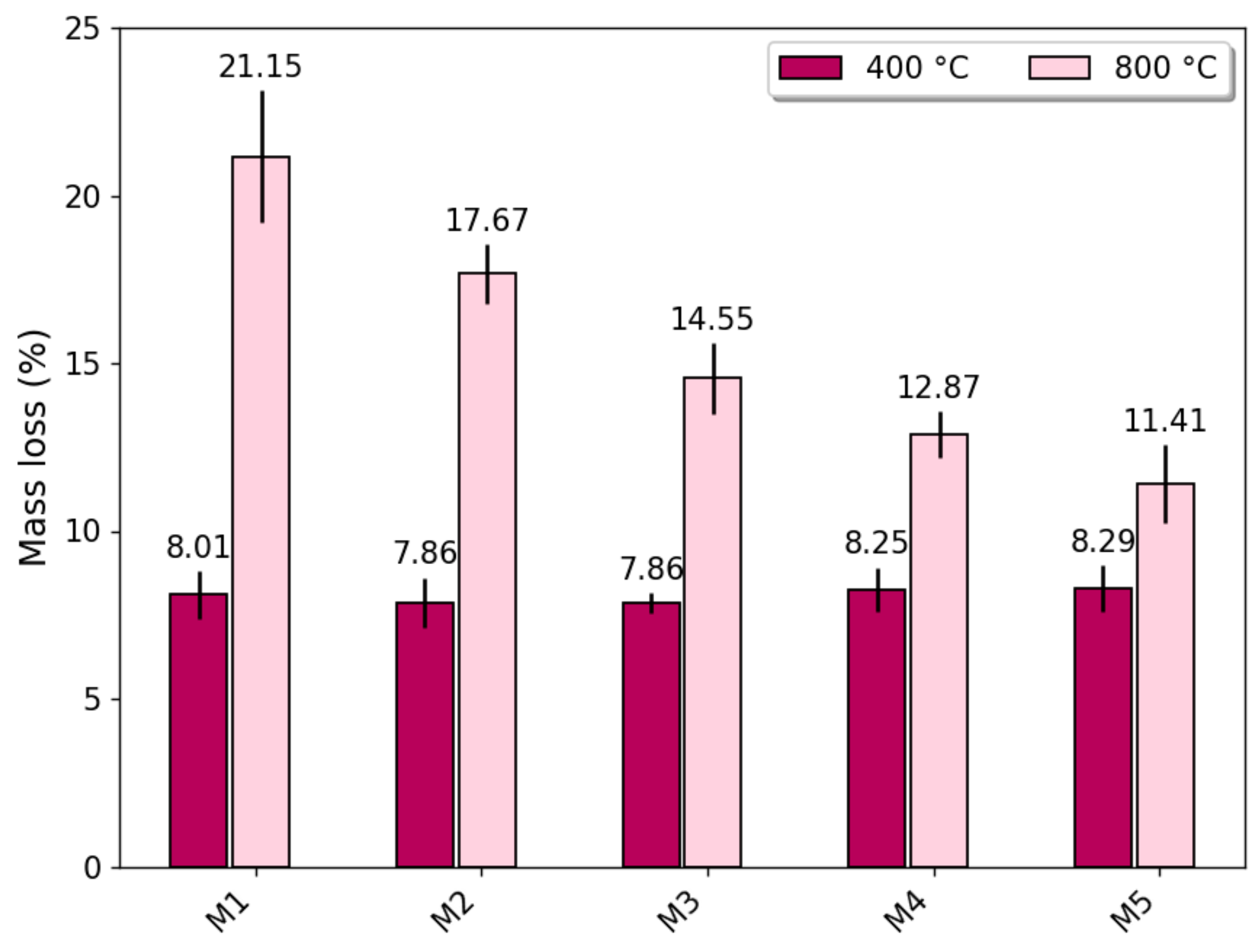
3.1. Mass Loss
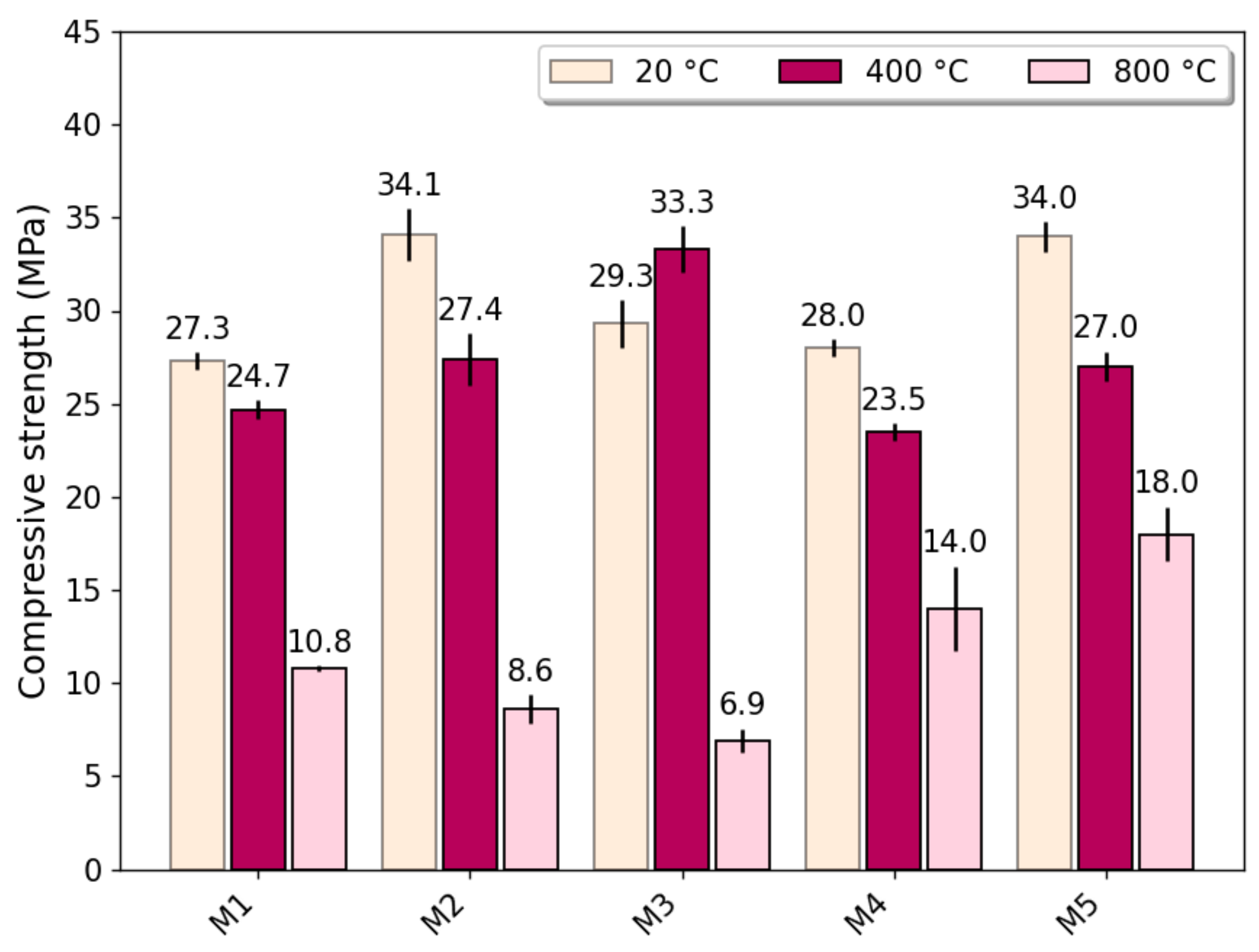
3.2. Compressive Strength
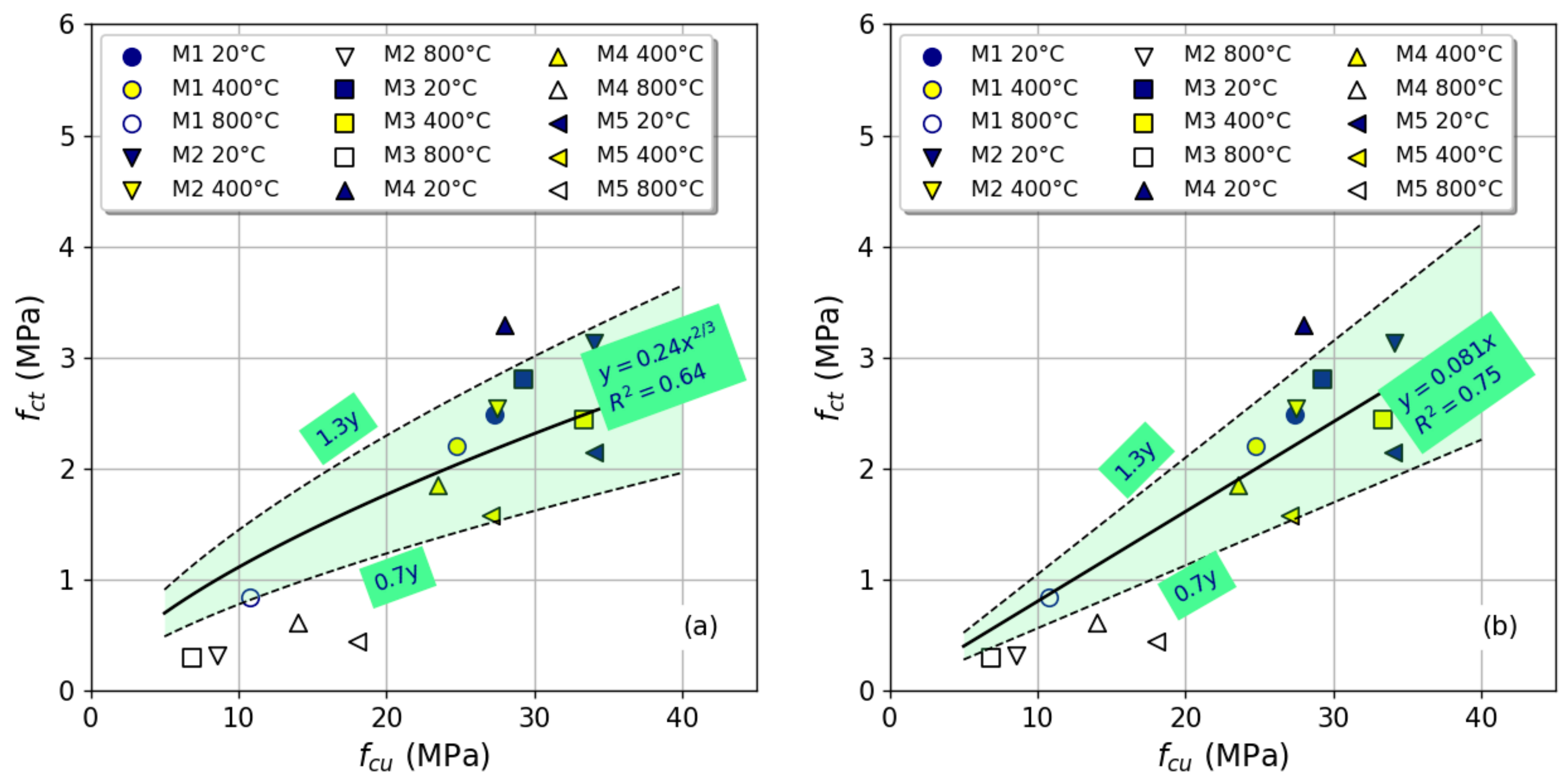
3.3. Splitting Strength
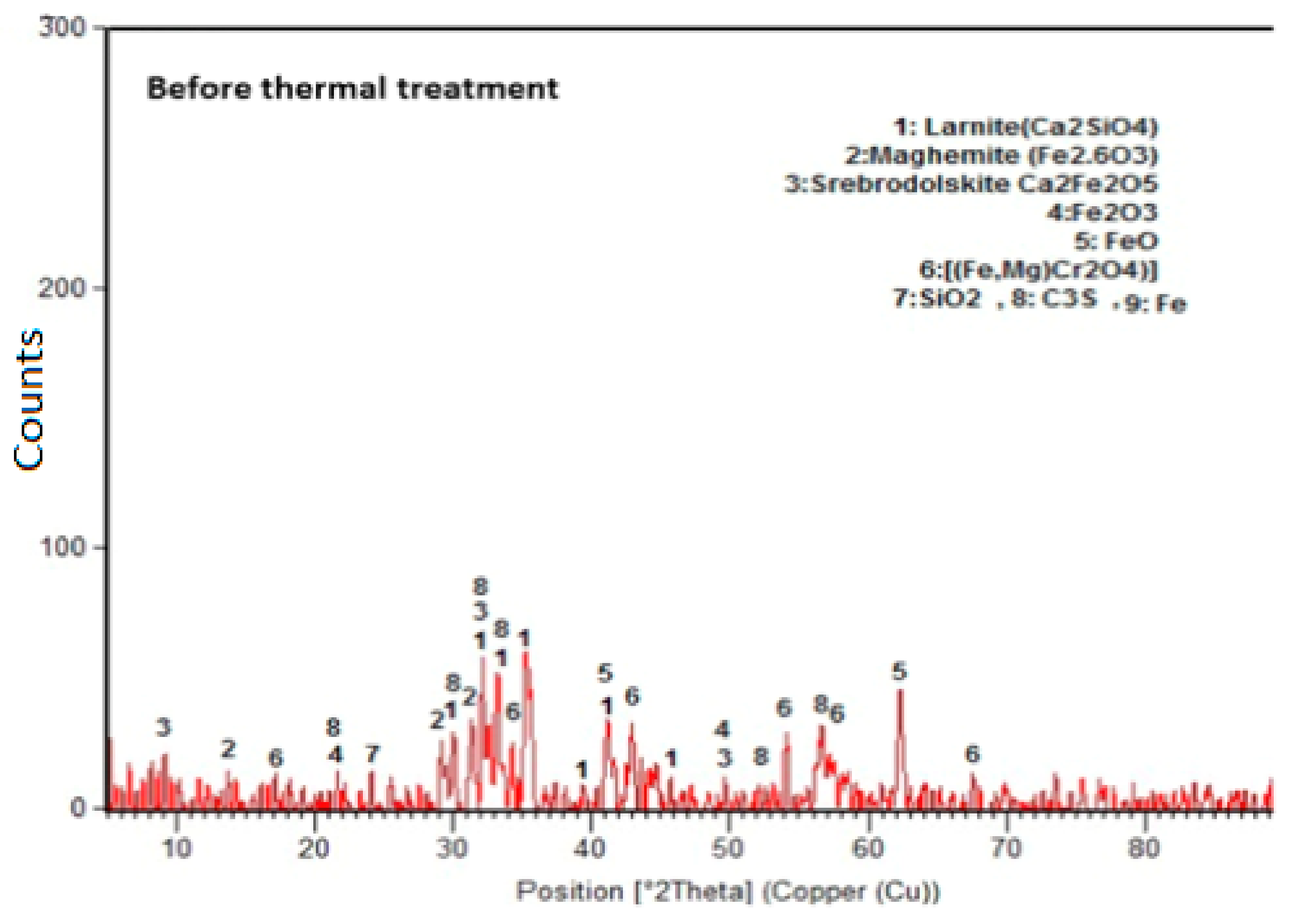
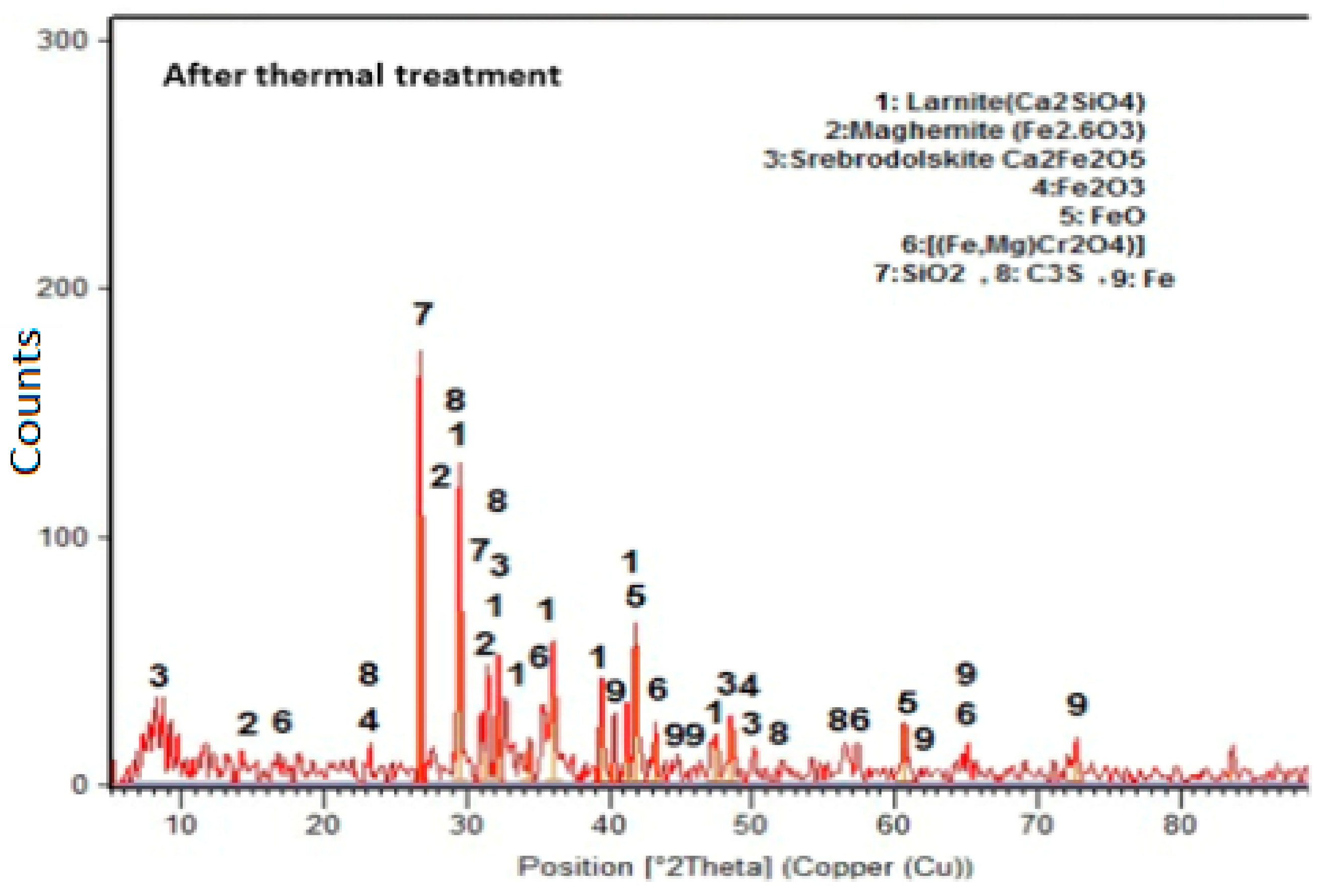
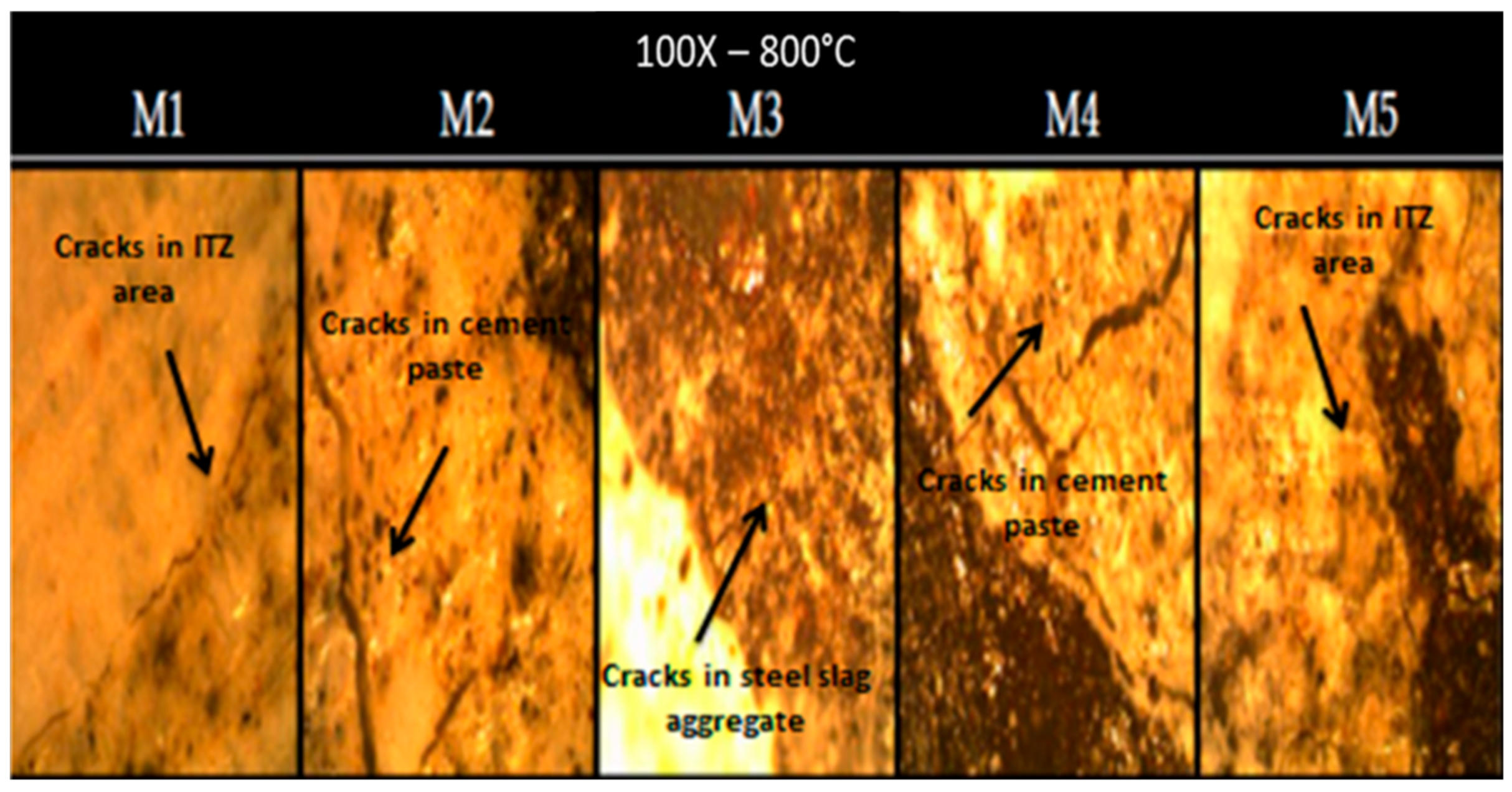
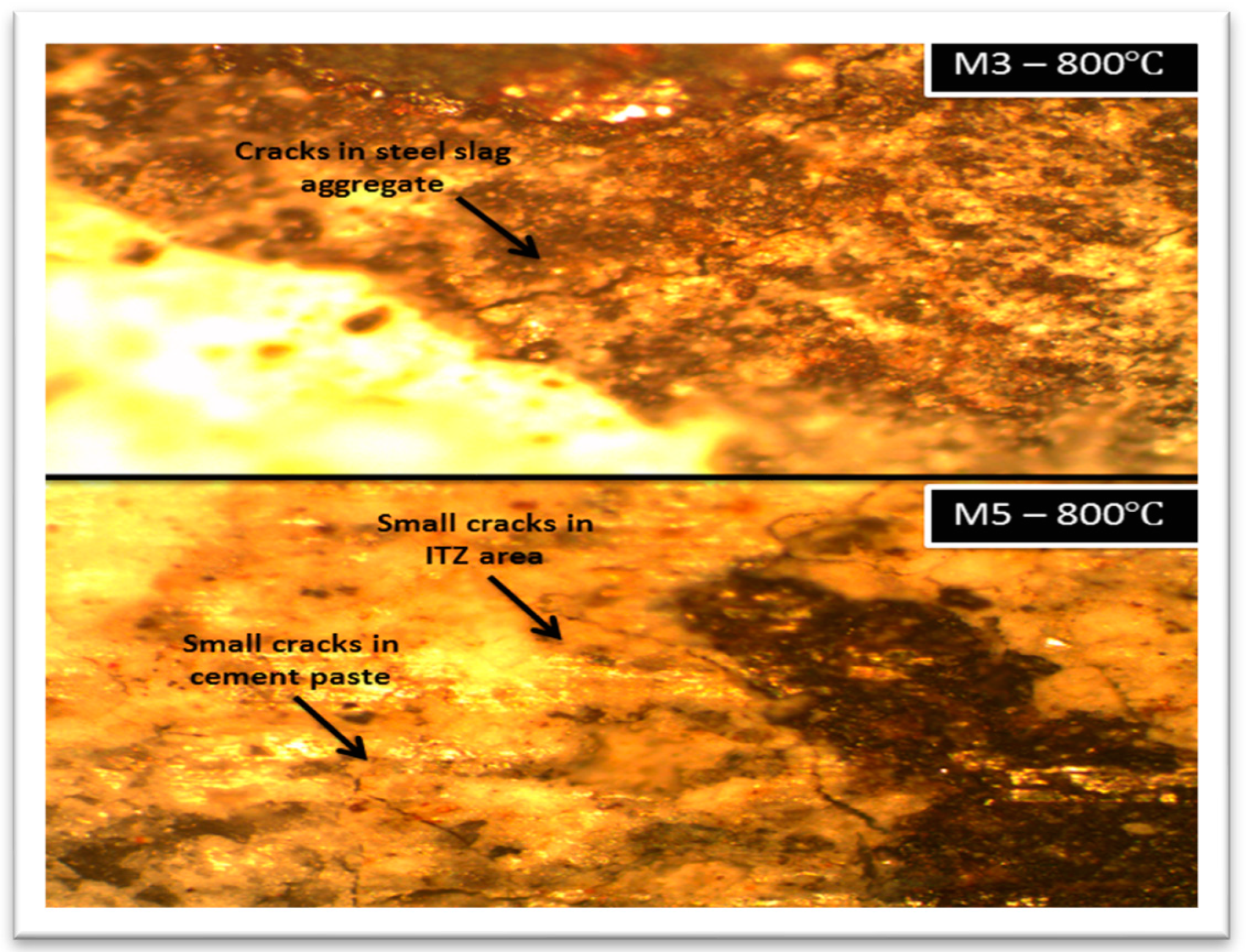
3.4. Microstructure of the Studied Concretes
4. Conclusions
- The density of fresh concrete mixtures containing slag increased with increasing slag percentage compared to the reference mixture. Conversely, there was no significant difference in air content and slump between the reference mixture and others containing steel slag.
- In comparison to mixtures containing natural aggregate, mixtures including steel slag are denser.
- Thermally treated steel slag has a significant effect on the compressive strength of concrete exposed to high temperatures of 800 °C when used to replace 100% of natural coarse aggregate.
- A mixture containing fully thermally treated steel slag as a coarse aggregate after being exposed to a high temperature of 800 °C demonstrated the effect of thermal treatment on the stability of the weight of concrete.
- At 800 °C, the thermally treated steel slag-containing mixtures’ cracks were smaller than those of the other mixtures.
- The mixtures that included steel slag to replace part of the natural coarse aggregate had the highest splitting tensile strength.
- Results showed that thermally treating slag could increase the fire resistance of concrete exposed to high temperatures up to 800 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sanguino, R.; Barroso, A.; Fernández-Rodríguez, S.; Sánchez-Hernández, M.I. Current trends in economy, sustainable development, and energy: A circular economy view. Environ. Sci. Pollut. Res. 2020, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kheirbek, A.; Ibrahim, A.; Asaad, M.; Wardeh, G. Experimental Study on the Physical and Mechanical Characteristics of Roller Compacted Concrete Made with Recycled Aggregates. Infrastructures 2022, 7, 54. [Google Scholar] [CrossRef]
- Ghorbel, E.; Wardeh, G.; González-Fonteboa, B. Effect of 10% recycled sands from various storage sites on the mortar’s properties. Eur. J. Environ. Civ. Eng. 2023, 1–19. [Google Scholar] [CrossRef]
- Ferreiro-Cabello, J.; Fraile-Garcia, E.; Pernia-Espinoza, A.; Martinez-de-Pison, F.J. Strength Performance of Different Mortars Doped Using Olive Stones as Lightweight Aggregate. Buildings 2022, 12, 1668. [Google Scholar] [CrossRef]
- Los Santos-Ortega, J.; Fraile-García, E.; Ferreiro-Cabello, J. Methodology for the environmental analysis of mortar doped with crumb rubber from end-of-life tires, Constr. Build. Mater. 2023, 399, 132519. [Google Scholar] [CrossRef]
- Liu, L. Stability Processing Technology and Application Prospect of Steel Slag. 2009. Available online: https://api.semanticscholar.org/CorpusID:137893129 (accessed on 25 August 2023).
- World Steel Association (WSA). December 2021 Crude Steel Production and 2021 Global Crude Steel Production Totals. 2021. Available online: https://worldsteel.org/media-centre/press-releases/2022/december-2021-crude-steel-production-and-2021-global-totals/ (accessed on 25 August 2023).
- World Steel Association (WSA). World Steel in Figures 2022. 2022. Available online: https://worldsteel.org/steel-topics/statistics/world-steel-in-figures-2022/ (accessed on 25 August 2023).
- M.C.S.; U.S. Geological Survey. Iron and Steel Slag. 2022. Available online: https://www.usgs.gov/centers/national-minerals-information-center/iron-and-steel-slag-statistics-and-information (accessed on 25 August 2023).
- EUROSLAG. In The European Association, Metallurgical Slag Producers and Processors; The European Slag Association: Duisburg, Germany, 2018.
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Du, H.L.; Zhang, H.; Ma, W.L. Systematic Research on the Application of Steel Slag Resources under the Background of Big Data. Complexity 2018, 2018, 6703908. [Google Scholar] [CrossRef]
- Ma, L.; Xu, D.; Wang, S.; Gu, X. Expansion inhibition of steel slag in asphalt mixture by a surface water isolation structure. Road Mater. Pavement Des. 2020, 21, 2215–2229. [Google Scholar] [CrossRef]
- Ahmedzade, P.; Sengoz, B. Evaluation of steel slag coarse aggregate in hot mix asphalt concrete. J. Hazard. Mater. 2009, 165, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Guo, H.; Yan, F. Thermal transfer characteristics of asphalt mixtures containing hot poured steel slag through microwave heating. J. Clean. Prod. 2021, 308, 127225. [Google Scholar] [CrossRef]
- Netinger, I.; Rukavina, M.J.; Serdar, M.; Bjegović, D. Steel slag as a valuable material for concrete production. Teh. Vjesn. 2014, 21, 1081–1088. [Google Scholar]
- Subathra Devi, V.; Gnanavel, B.K.; Murthi, P. Experimental Investigation on the mechanical properties of steel slag ceramic concrete. Int. J. ChemTech Res. 2015, 8, 152–160. [Google Scholar]
- Netinger, I.; Rukavina, M.J.; Mladenovič, A. Improvement of Post-fire Properties of Concrete with Steel Slag Aggregate. Procedia Eng. 2013, 62, 745–753. [Google Scholar] [CrossRef]
- Maruthachalam, V.; Murthi, P. High performance concrete with steel slag aggregate. Gradjevinar 2014, 66, 605–612. [Google Scholar]
- Murmu, M.; Mohanta, N.R.; Bapure, N. Study on the fresh and hardened properties of concrete with steel slag as partial replacement for natural aggregates. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Akhil, N. Singh, Microstructural characteristics of iron-steel slag concrete: A brief review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Sharma, R.D.; Singh, N. Optimizing the compressive strength behavior of iron slag and recycled aggregate concretes. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Mostazid, M.I.; Sakai, Y. Effect of heat treatment on mechanical behavior and scaling resistance of slag impregnated low strength recycled compacted concrete. J. Build. Eng. 2023, 68, 106084. [Google Scholar] [CrossRef]
- Yu, Q.; Zhu, B.; Li, X.; Meng, L.; Cai, J.; Zhang, Y.; Pan, J. Investigation of the rheological and mechanical properties of 3D printed eco-friendly concrete with steel slag. J. Build. Eng. 2023, 72, 106621. [Google Scholar] [CrossRef]
- Hager, I. Behaviour of cement concrete at high temperature. Bull. Pol. Acad. Sci. Tech. Sci. 2013, 61, 145–154. [Google Scholar] [CrossRef]
- BS EN 197-1; Cement Composition, Specifications and Conformity Criteria for Common Cements. British Standards Institution: London, UK, 2011.
- BS EN 1097-6; Tests for Mechanical and Physical Properties of Aggregates. British Standards Institution: London, UK, 2022.
- BS EN 1097-9; Tests for Mechanical and Physical Properties of Aggregates Determination of the Resistance to Wear by Abrasion from Studded Tyres. Nordic Test. British Standards Institution: London, UK, 2014.
- BS EN 933-1; Tests for Geometrical Properties of Aggregates Determination of Particle Size Distribution. Sieving Method. British Standards Institution: London, UK, 2012.
- BS EN 933-2; Tests for Geometrical Properties of Aggregates Determination of Particle Size Distribution. Test Sieves, Nominal Size of Apertures. British Standards Institution: London, UK, 2020.
- ASTM C33/C33M-18; Standard Specification for Concrete Aggregates. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM C136/C136M-19; Standard Test Method for Sieve Analysis of Fine and Coarse Aggregates. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C127-15; Standard Test Method for Relative Density (Specific Gravity) and Absorption of Coarse Aggregate. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C128-22; Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate. ASTM International: West Conshohocken, PA, USA, 2023.
- ACI 211.1-91; Standard Practice for Selecting Proportions for Normal, Heavyweight, and Mass Concrete. ACI Committee: Farmington Hills, MI, USA, 2002.
- BS EN 12350-6; Testing Fresh Concrete Density. British Standards Institution: London, UK, 2019.
- BS EN 12350-7; Testing Fresh Concrete Air Content. Pressure Methods. British Standards Institution: London, UK, 2019.
- BS EN 12390-3; Testing Hardened Concrete Compressive Strength of Test Specimens. British Standards Institution: London, UK, 2019.
- BS EN 12390-6; Testing Hardened Concrete Tensile Splitting Strength of Test Specimens. British Standards Institution: London, UK, 2009.







| Properties of Portland Cement | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chemical composition | Constituent | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | CaOfree | SO3 | L.O.I |
| Content (% of mass) | 59.08 | 19.12 | 5.03 | 4.21 | 2.47 | 1.56 | 3.22 | 1.62 | |
| Physical properties | Specific gravity | 3.15 | |||||||
| Fineness (Blaine) m2/kg | 302 | ||||||||
| Initial setting time (min) | 135 | ||||||||
| Mechanical properties | Compressive strength MPa at (7 days) | 24.3 | |||||||
| Compressive strength MPa at (28 days) | 35.6 | ||||||||
| Property | Thermally Untreated Steel Slag | Thermally Treated Steel Slag | Natural Coarse Aggregate | Natural Fine Aggregate |
|---|---|---|---|---|
| Specific gravity | 3.592 | 3.493 | 2.802 | 2.794 |
| WA (%) | 2.564 | 3.498 | 1.854 | 1.6 |
| LA coefficient (%) | 21.98 | 17.64 | 18.94 | - |
| Sand equivalent (%) | - | - | - | 75 |
| Constituent | CaO | Fe2O3 | SiO2 | CuO | AL2O3 | MgO | MnO | ZnO | BrO |
|---|---|---|---|---|---|---|---|---|---|
| content (% of mass) | 27.82 | 44.15 | 13.63 | 0.04 | 7.18 | 2.75 | 2.68 | 0.66 | 0.58 |
| Element | Fe | Ca | Si | Al | Mn | Mg | Zn | Cr | Ti | S | K | Pb | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| content % | 37.98 | 35.36 | 9.97 | 5.29 | 3.04 | 2.54 | 1.88 | 1.36 | 0.90 | 0.34 | 0.26 | 0.23 | 0.17 |
| Component (kg for 1 m3) | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|
| Cement | 380 | 380 | 380 | 380 | 380 |
| Water | 218 | 223 | 228 | 223 | 228 |
| Natural fine Aggregate | 820 | 896 | 966 | 896 | 966 |
| Natural coarse aggregate | 960 | 480 | - | 480 | - |
| Thermally untreated steel slag coarse aggregate | - | 507 | 1014 | - | - |
| Thermally treated steel slag coarse aggregate | - | - | - | 507 | 1014 |
| Properties | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|
| Density (kg/m3) | 2390 | 2493 | 2579 | 2488 | 2588 |
| Slump (mm) | 49 | 48 | 48 | 47 | 48 |
| Air content (%) | 1.8 | 1.9 | 1.7 | 1.9 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhedr, M.; Asaad, M.; Ismail, M.; Wardeh, G. Experimental Study on the Physical and Mechanical Characteristics of Refractory Concrete Using Heat-Treated Steel Slag Coarse Aggregates. Infrastructures 2023, 8, 151. https://doi.org/10.3390/infrastructures8100151
Alkhedr M, Asaad M, Ismail M, Wardeh G. Experimental Study on the Physical and Mechanical Characteristics of Refractory Concrete Using Heat-Treated Steel Slag Coarse Aggregates. Infrastructures. 2023; 8(10):151. https://doi.org/10.3390/infrastructures8100151
Chicago/Turabian StyleAlkhedr, Munaf, Majed Asaad, Mahmoud Ismail, and George Wardeh. 2023. "Experimental Study on the Physical and Mechanical Characteristics of Refractory Concrete Using Heat-Treated Steel Slag Coarse Aggregates" Infrastructures 8, no. 10: 151. https://doi.org/10.3390/infrastructures8100151





