Chromium–Aluminum Coatings for Oxidation Protection of Titanium–Aluminum Intermetallic Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
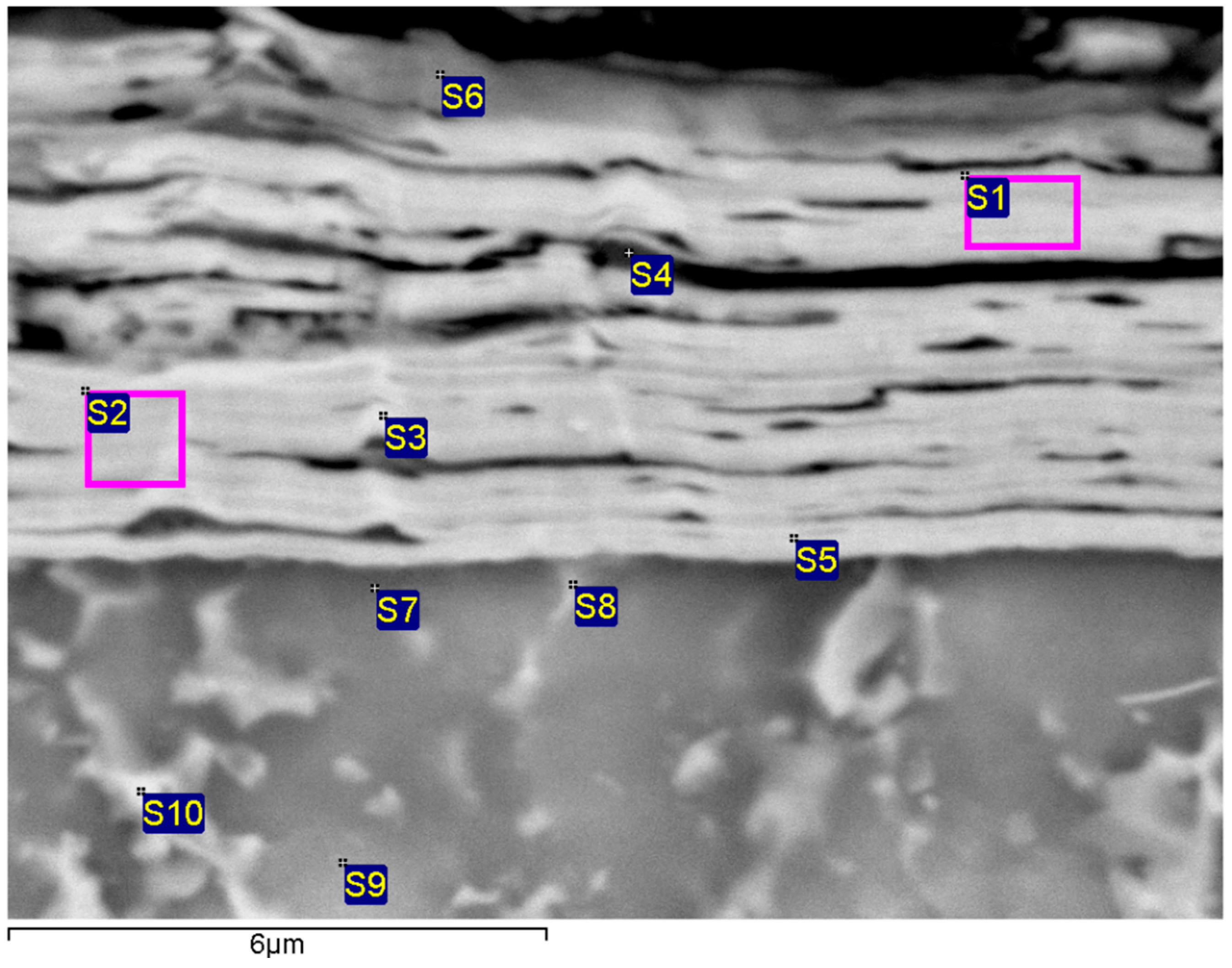
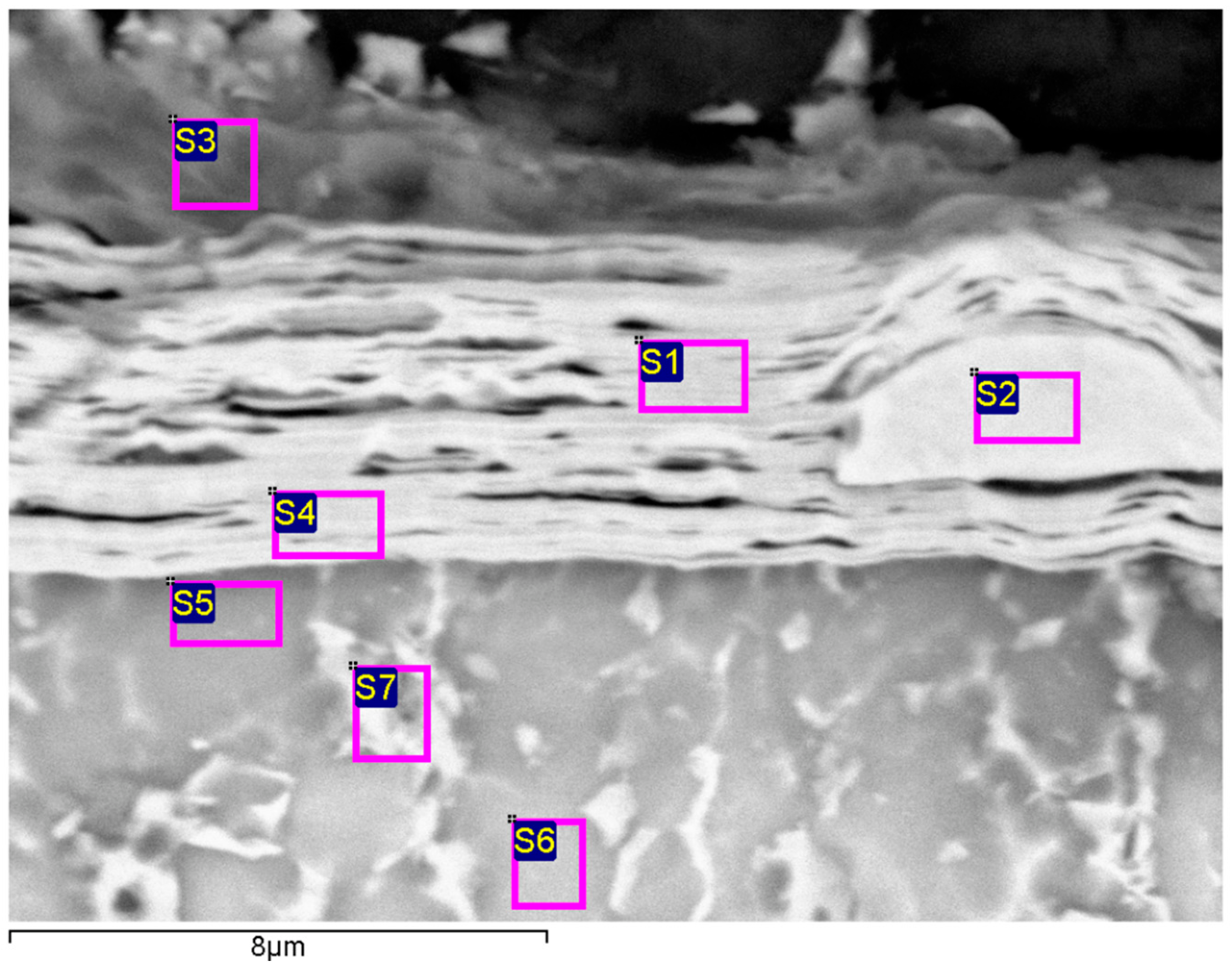
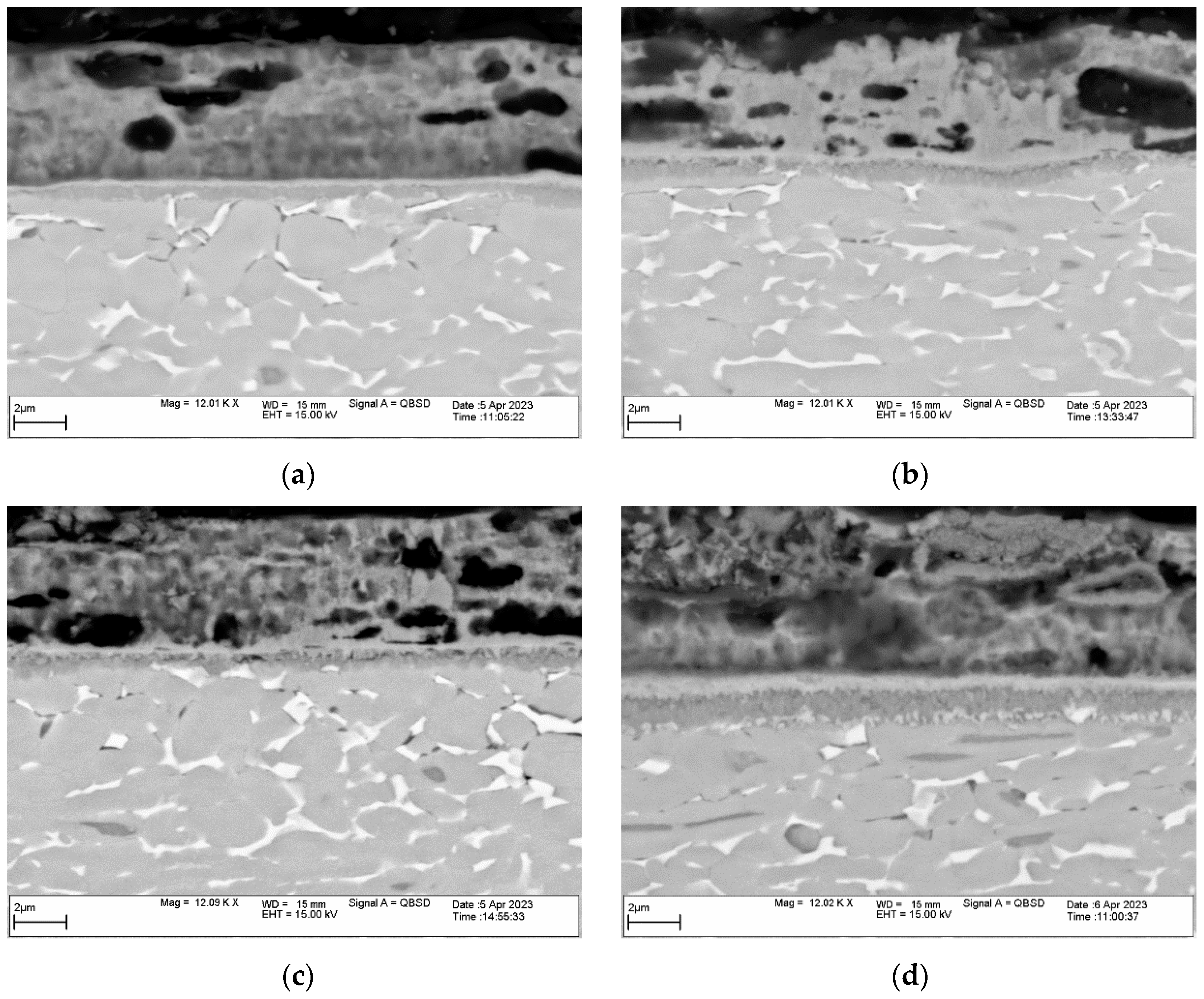
3.1. Microstructure
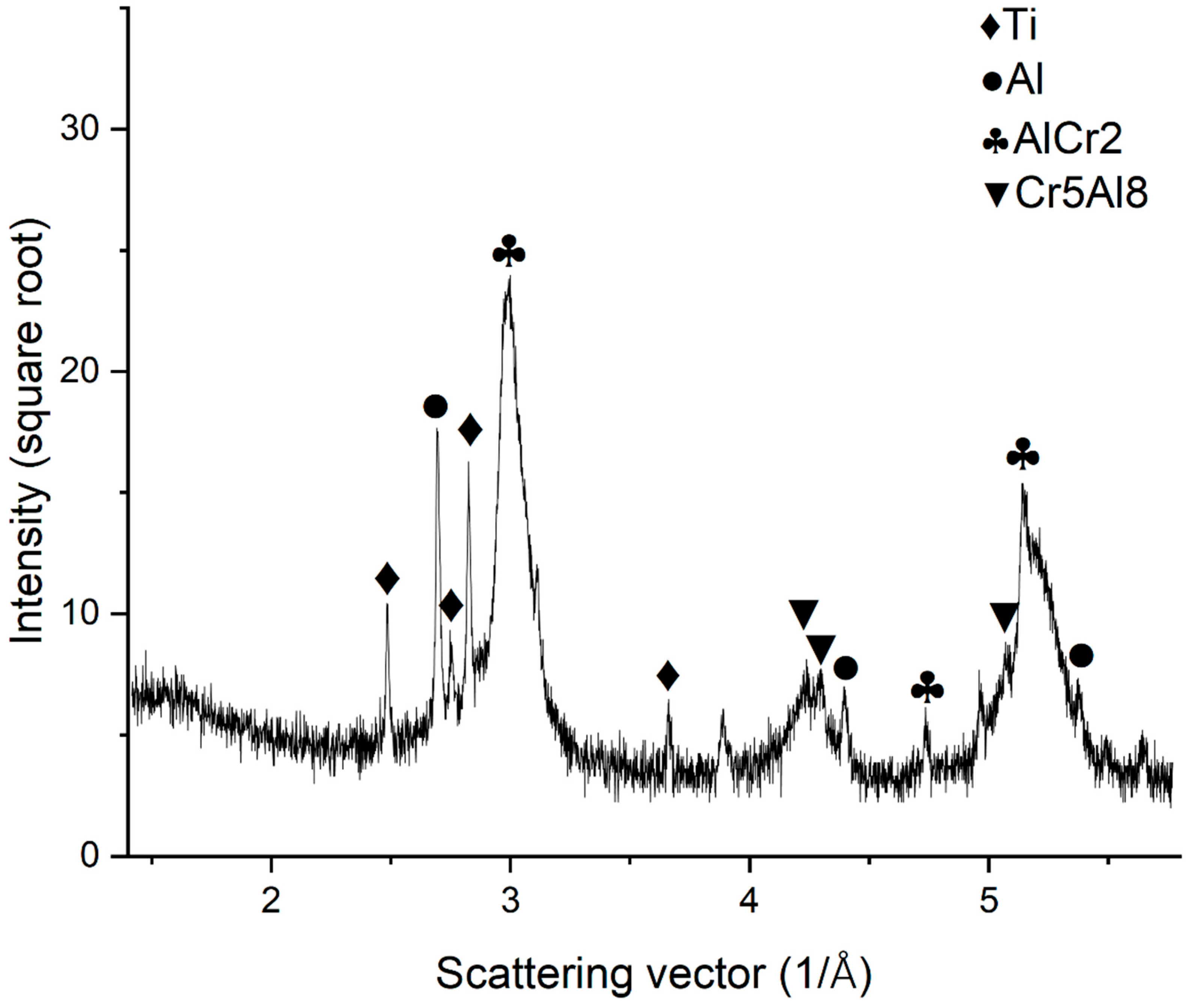
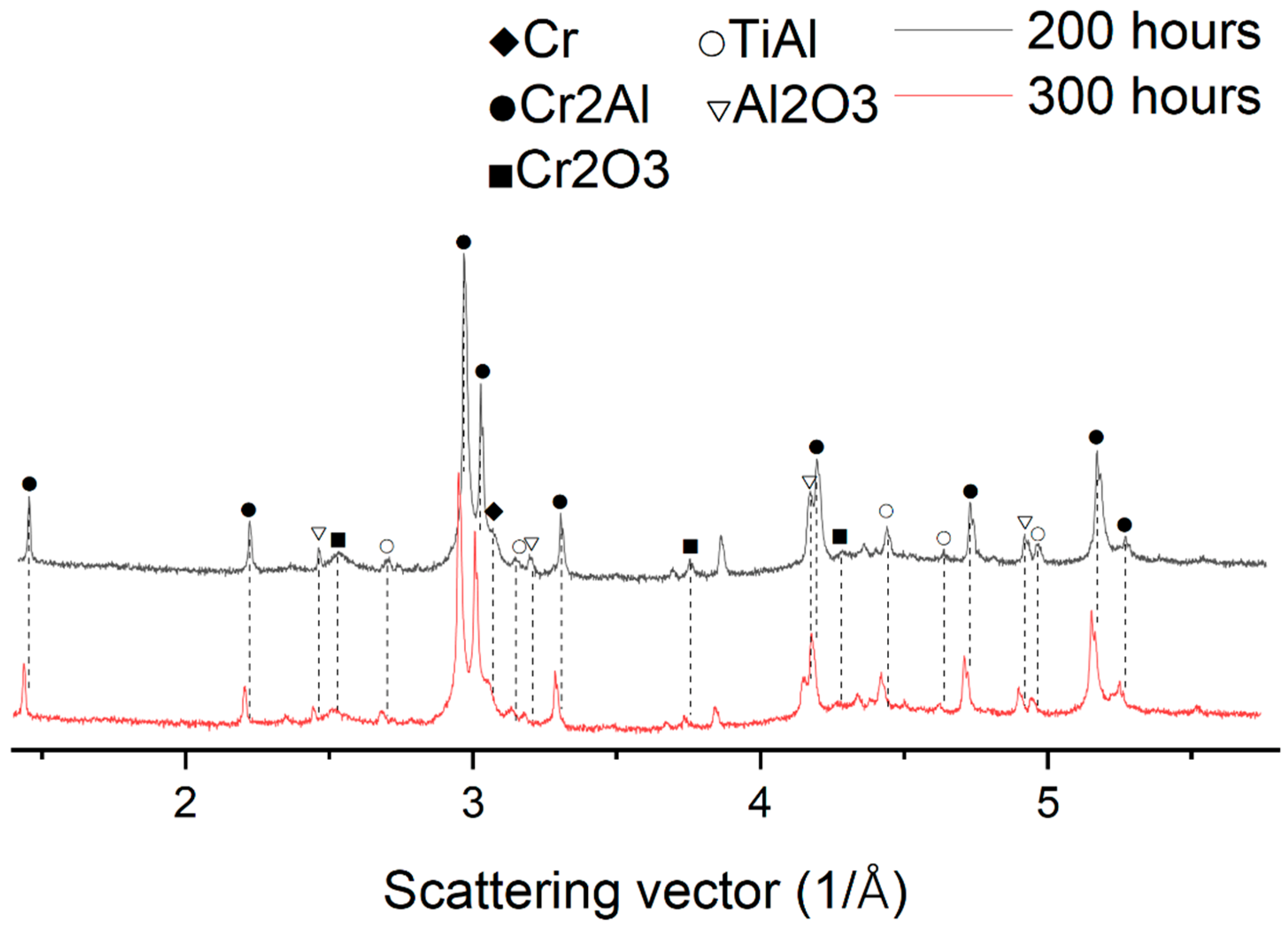
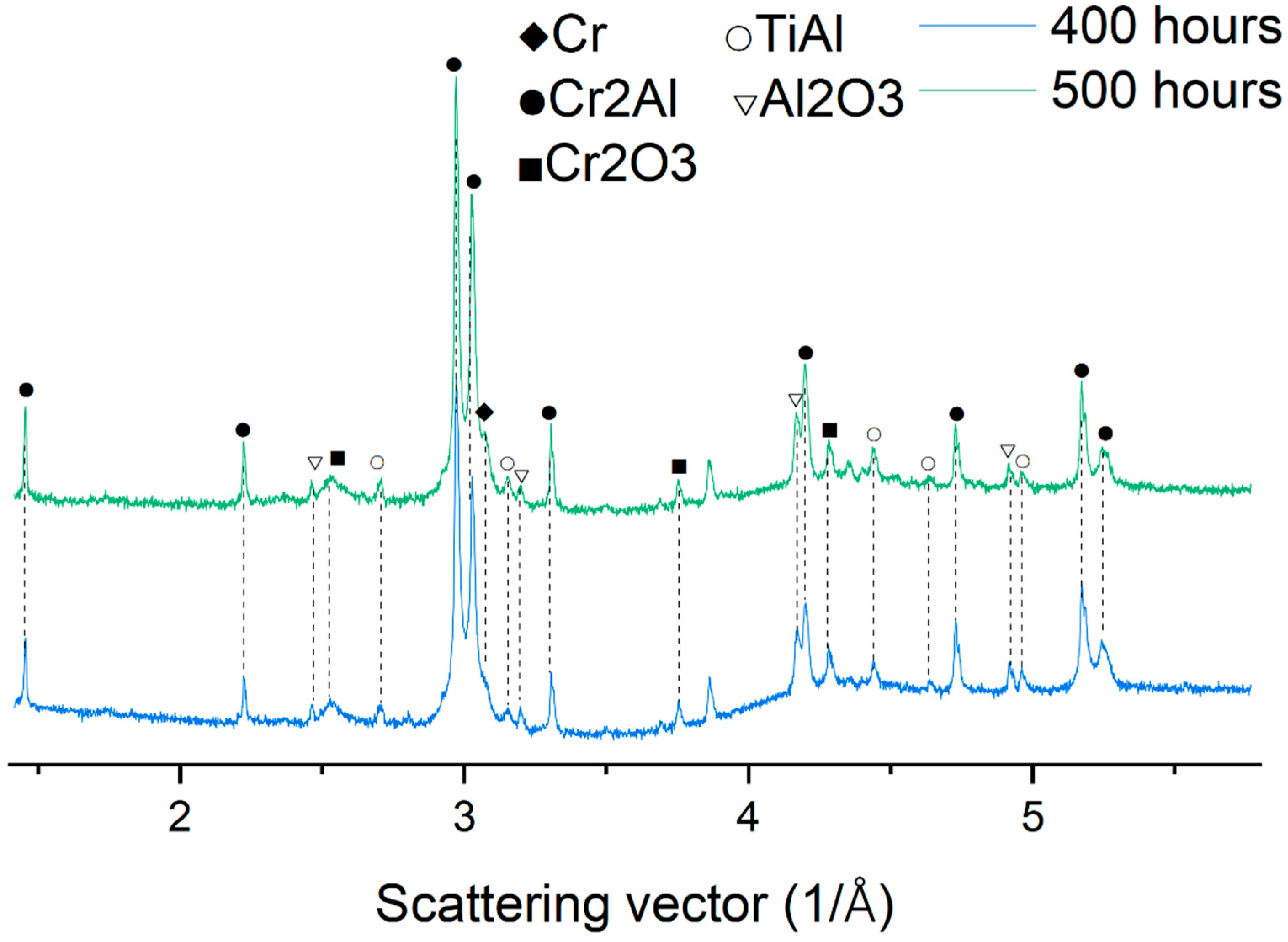
3.2. X-ray Diffraction Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, H.P.; Liu, W.Y.H.; Melvin, G.J.H.; Jiang, Z.T. A brief review of the phase structure, oxidation kinetics and mechanical properties of complex Ti-Al alloys. Materials 2021, 14, 1677. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, J.; Song, Y. Progress in research on the basic principles of studying the oxidation behavior of Ti -Al alloys and the effect of alloying. Metals 2021, 11, 985. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.H.; Ehring, M. Gamma Aluminide Titanium Alloys: Science and Technology; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Brady, M.P.; Brindley, V.J.; Smyalek, J.L.; Locci, I.E. Oxidation and protection of titanium gamma aluminides. JOM 1996, 48, 46–50. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Zhu, Y.; Lin, J.; Ren, Z. Oxidative behavior of γ-TiAl alloys at 900 °C in air. Mater. High Temp. 2016, 33, 234–240. [Google Scholar] [CrossRef]
- Swadźba, R.; Laska, N.; Bauer, P.P.; Krztoń, H. Effect of pre-oxidation on cyclic oxidation stability of γ-TiAl at 900 °C. Corros. Sci. 2020, 177, 108985. [Google Scholar] [CrossRef]
- Kenel, K.; Lis, A.; Dawson, K.; Stiefel, M.; Pechnik, K.; Barras, J.; Wegener, K. Mechanical performance and oxidation resistance of an ODS γ-TiAl alloy processed by spark plasma sintering and laser additive manufacturing. Intermet 2017, 91, 169–180. [Google Scholar] [CrossRef]
- Kenel, K.; Dawson, K.; Barras, J.; Houser, K.; Dasargiri, G.; Bauer, T.; Wegener, K. Microstructure and stability of oxide particles in a novel γ-TiAl ODS alloy treated by spark plasma sintering and laser additive manufacturing. Intermet 2017, 90, 63–73. [Google Scholar] [CrossRef]
- Prokopets, A.D.; Bazhin, P.M.; Konstantinov, A.S.; Chizhikov, A.P.; Antipov, M.S.; Avdeeva, V.V. Structural Features of TiB2/ TiAl /Ti6Al4V Layered Composite Material Obtained by Unlimited SHS Compression. Lett. Mater. 2021, 300, 130165. [Google Scholar] [CrossRef]
- Bazhina, A.; Chizhikov, A.; Konstantinov, A.; Khomenko, N.; Bazhin, P.; Avdeeva, V.; Chernogorova, O.; Drozdova, E. Structure, phase composition and mechanical characteristics of layered composite materials based on TiB/xTi-Al/α-Ti (x = 1, 1.5, 3) obtained by combustion and high-temperature shear deformation. Mater. Sci. Eng. A 2022, 858, 144161. [Google Scholar] [CrossRef]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation Protection of γ-TiAl Based Alloys—Review. Intermetallides 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Schütze, M. The Role of Surface Protection for the High Temperature Performance of TiAl Alloys. JOM 2017, 69, 2602–2609. [Google Scholar] [CrossRef]
- Wang, J.; Kong, L.; Li, T.; Xiong, T. New TiAl3 /Al2 O3 composite coating on γ-TiAl alloy and evaluation of oxidation performance. Appl. Surf. Sci. 2016, 361, 90–94. [Google Scholar] [CrossRef]
- Simova, V.; Knittel, S.; Cavarroque, M.; Martinou, L.; Klemberg-Sapega, J.E. Amorphous Si–B–C–N coatings for protection against high-temperature oxidation of the γ-TiAl alloy. Surf. Coat. Technol. 2022, 442, 128544. [Google Scholar] [CrossRef]
- Lin, H.; Liang, W.; Miao, Q.; Li, S.; Ding, Z.; Cui, S.; Yu, H. Creation of a self-supplied Al2O3-Y2O3 coating for γ-TiAl alloy with enhanced oxidation protection. Appl. Surf. Sci. 2020, 522, 146439. [Google Scholar] [CrossRef]
- Wang, J.; Kong, L.; Li, T.; Xiong, T. Oxidation performance of TiAl3 bonded thermal barrier coatings on γ-TiAl alloy. J. Therm. Spray Technol. 2015, 24, 467–475. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, W.-Y.; Jiang, M.-Y.; Cao, F.; Wu, H.-X.; Sun, D.-B.; Yu, H.-Y.; Wu, L.-K. Improved TiAl Alloy Oxidation Performance with New Al-Si Composite Coating. Technol. Surf. Coat. 2020, 381, 125126. [Google Scholar] [CrossRef]
- Bauer, P.P.; Laska, N.; Svadzba, R. Improving the oxidation resistance of γ-TiAl by magnetron sputtering based on aluminum and silicon. Intermetallides 2021, 133, 107177. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, P.; Qiu, J.; Wu, A.; Cui, S.; Li, Z.; Tao, X. Oxidation and self-healing characteristics of MoSiAlY coating on γ-TiAl substrate by surface fusion method. Vacuum 2019, 165, 148–156. [Google Scholar] [CrossRef]
- Fröhlich, M.; Braun, R.; Leyens, S. Oxidation resistant coatings combined with thermal barrier coatings on γ-TiAl alloys for high temperature applications. Surf. Coat. Technol. 2006, 201, 3911–3917. [Google Scholar] [CrossRef]
- Bazhina, A.D.; Bazhin, P.M.; Chizhikov, A.P.; Konstantinov, A.S.; Stolin, A.M. Influence of high-temperature annealing on the structure of titanium aluminide materials obtained by combustion and high-temperature shear deformation. Intermetallics 2021, 5, 107313. [Google Scholar] [CrossRef]
- Chen, Z.; Geng, L.; Qu, W.; Wang, J.; Chen, M.; Li, C.; Wang, F. Characteristics and hot corrosion resistance of co-deposition layers with different activators and Al-Cr ratios. Corros. Sci. 2022, 202, 110320. [Google Scholar] [CrossRef]
- Laska, N.; Brown, R.; Knittel, S. Oxidation performance of Ti -Al-Cr- based protective coatings deposited on γ-TiAl Ti-48-2-2 and TNM-B1 alloys. Technol. Surf. Coat. 2018, 349, 347–356. [Google Scholar] [CrossRef]
- He, J.; Liao, X.; Lan, X.; Qiu, W.; Yu, H.; Zhang, J.; Liu, Z. Annealed Al-Cr Coating: A hard anti-corrosion coating with grain boundary modification effect for Nd -Fe-B magnets. J. Alloys Compd. 2021, 870, 159229. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, W.; Miao, K.; Chen, B.; Ding, Z.; Roy, N. Oxidative behavior of Al/Crcoating on Ti2AlNb alloy at 900 °C. Mater. Res. Express 2018, 5, 046408. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Lin, C.-Y.; Chen, J.-X. Wear and Corrosion Performance of Ti-6Al-4V Alloy Arc-Coated TiN/CrN Nano-Multilayer Film. Metals 2023, 13, 907. [Google Scholar] [CrossRef]
- Wang, F.; Wu, J. Applications of hard coatings. In Modern Ion Plating Technology: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 411–429. [Google Scholar]
- Akhter, R.; Bendavid, A.; Munroe, P. Microstructure, mechanical properties and optical reflectance of TiNiN films deposited on silicon substrates using cathodic arc evaporation. Thin Solid Film. 2023, 777, 139896. [Google Scholar] [CrossRef]
- Li, K.Q.; Chen, L.; Hu, C.; Zhang, J.; Du, J.W. Structure, mechanical and thermal properties of CrAlBSiN coatings prepared by cathodic arc evaporation. Surf. Coat. Technol. 2023, 452, 129094. [Google Scholar] [CrossRef]
- Sysyn, M.; Nabochenko, O.; Kovalchuk, V.; Przybyłowicz, M.; Fischer, S. Investigation of interlocking effect of crushed stone ballast during dynamic loading. Rep. Mech. Eng. 2021, 2, 65–76. [Google Scholar] [CrossRef]
- Korznikova, E.; Schafler, E.; Steiner, G.; Zehetbauer, M.J. Measurements of vacancy type defects in SPD deformed Ni. In Proceedings of the TMS Annual Meeting, San Antonio, TX, USA, 12–16 March 2006; pp. 97–102. [Google Scholar]
- Moradi Marjaneh, A.; Saadatmand, D.; Evazzade, I.; Babicheva, R.I.; Soboleva, E.G.; Srikanth, N.; Zhou, K.; Korznikova, E.A.; Dmitriev, S.V. Mass transfer in the Frenkel-Kontorova chain initiated by molecule impact. Phys. Rev. E 2018, 98, 023003. [Google Scholar] [CrossRef]
- Babicheva, R.I.; Evazzade, I.; Korznikova, E.A.; Shepelev, I.A.; Zhou, K.; Dmitriev, S.V. Low-energy channel for mass transfer in Pt crystal initiated by molecule impact. Comput. Mater. Sci. 2019, 163, 248–255. [Google Scholar] [CrossRef]
- Korznikova, G.; Korznikova, E.; Nazarov, K.; Shayakhmetov, R.; Khisamov, R.; Khalikova, G.; Mulyukov, R. Structure and Mechanical Behavior of Al–Nb Hybrids Obtained by High-Pressure-Torsion-Induced Diffusion Bonding and Subsequent Annealing. Adv. Eng. Mater. 2021, 23, 2000757. [Google Scholar] [CrossRef]
- Shlyakhova, G.V.; Bochkareva, A.V.; Nadezhkin, M.V. Effect of Heat Treatment on Microstructure and Mechanical Properties of the Precipitation Hardening Elinvar Alloy. Russ. Phys. J. 2021, 64, 838–843. [Google Scholar] [CrossRef]
- Astafurova, E.G.; Astafurov, S.V.; Reunova, K.A.; Melnikov, E.V.; Moskvina, V.A.; Panchenko, M.Y.; Maier, G.G.; Rubtsov, V.E.; Kolubaev, E.A. Structure Formation in Vanadium-Alloyed Chromium-Manganese Steel with a High Concentration of Interstitial Atoms C + N = 1.9 wt % during Electron-Beam Additive Manufacturing. Phys. Mesomech. 2022, 25, 1–11. [Google Scholar] [CrossRef]
- Mulyukov, K.Y.; Korznikova, G.F.; Sagdatkireyeva, M.B.; Timofeyev, V.N.; Valiev, R.Z. The study of domain structure of submicron grained cobalt and its changes during heating. J. Magn. Magn. Mater. 1992, 110, 73–79. [Google Scholar] [CrossRef]
- Mulyukov, K.Y.; Korznikova, G.F.; Nikitin, S.A. Magnetization of nanocrystalline dysprosium: Annealing effects. J. Appl. Phys. 1996, 79, 8584–8587. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Wei, X.; Pan, B. Effects of annealing on the structure and microhardness of nanocrystalline Ni–Mn electrodeposits. J. Alloys Compd. 2023, 960, 170732. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Aluminum evaporator arc current | 50 to 65 A (stepped) |
| Chromium evaporator arc current | From 100 to 80 A (estimated) |
| Bias | 50 V |
| Vacuum chamber pressure | 0.36 Pa |
| Range | Al | Ti | Cr | Nb |
|---|---|---|---|---|
| S1 | 20.44 | 1.21 | 78.35 | |
| S2 | 18.03 | 1.98 | 79.99 | |
| S3 | 18.40 | 2.00 | 79.60 | |
| S4 | 37.54 | 1.25 | 61.21 | |
| S5 | 13.38 | 13.11 | 73.51 | |
| S6 | 25.83 | 74.17 | ||
| S7 | 13.41 | 46.75 | 39.84 | |
| S8 | 6.24 | 39.89 | 53.87 | |
| S9 | 11.18 | 46.90 | 41.92 | |
| S10 | 7.24 | 33.47 | 59.29 |
| Spectrum | Al | Ti | Cr | Nb |
|---|---|---|---|---|
| S1 | 18.75 | 1.17 | 80.08 | |
| S2 | 1.68 | 98.32 | ||
| S3 | 96.18 | 3.82 | ||
| S4 | 15.81 | 4.18 | 80.01 | |
| S5 | 16.00 | 44.26 | 39.74 | |
| S6 | 11.96 | 46.80 | 41.24 | |
| S7 | 9.50 | 38.33 | 52.17 |
| Range | O | Al | Ti | Cr | Nb |
|---|---|---|---|---|---|
| S1 | 24.55 | 65.05 | |||
| S2 | 15.36 | 20.83 | 53.07 | ||
| S3 | 8.95 | 14.96 | 61.54 | ||
| S4 | 22.34 | 21.30 | 45.38 | ||
| S5 | 7.00 | 7.41 | 60.60 | ||
| S6 | 11.59 | 14.63 | 7.37 | 53.99 | |
| S7 | 6.28 | 19.12 | 12.89 | 52.88 | 8.85 |
| S8 | 23.27 | 21.27 | 23.71 | 31.76 | |
| S9 | 28.75 | 37.72 | 1.94 | 31.60 | |
| S10 | 20.68 | 36.46 | 42.86 | ||
| S11 | 13.39 | 46.59 | 40.02 | ||
| S12 | 9.44 | 39.12 | 51.44 | ||
| S13 | 11.34 | 49.96 | 38.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarov, A.; Maslov, A.; Korznikova, E.; Ramazanov, K. Chromium–Aluminum Coatings for Oxidation Protection of Titanium–Aluminum Intermetallic Alloys. Quantum Beam Sci. 2023, 7, 36. https://doi.org/10.3390/qubs7040036
Nazarov A, Maslov A, Korznikova E, Ramazanov K. Chromium–Aluminum Coatings for Oxidation Protection of Titanium–Aluminum Intermetallic Alloys. Quantum Beam Science. 2023; 7(4):36. https://doi.org/10.3390/qubs7040036
Chicago/Turabian StyleNazarov, Almaz, Alexey Maslov, Elena Korznikova, and Kamil Ramazanov. 2023. "Chromium–Aluminum Coatings for Oxidation Protection of Titanium–Aluminum Intermetallic Alloys" Quantum Beam Science 7, no. 4: 36. https://doi.org/10.3390/qubs7040036






