Assessment of Spatial Variations in Pesticide, Heavy Metal, and Selenium Residues in Honey Bee (Apis mellifera L.) Products
Abstract
:1. Introduction
2. Materials and Methods
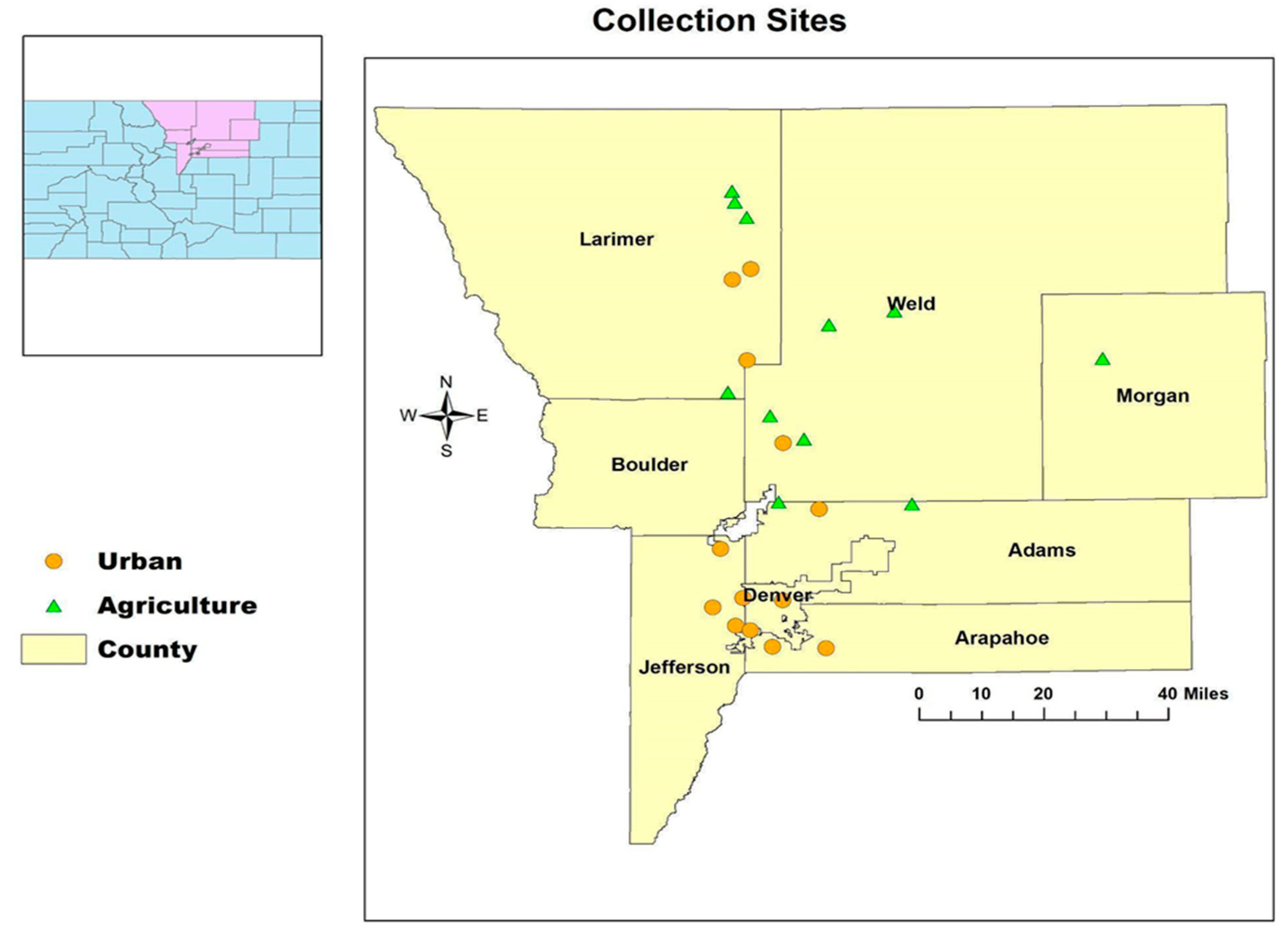
2.1. Study Sites and Site Selection
2.2. Sample Collection, Preparation, and Analysis
2.3. Selenium and Heavy Metals Analysis
2.3.1. Chemicals and Reagents
2.3.2. Analysis
2.4. Pesticide Analysis
2.4.1. Chemicals and Reagents
2.4.2. Pollen Samples
2.4.3. Honey Samples
2.4.4. Analysis
2.5. Statistical Analysis
3. Results
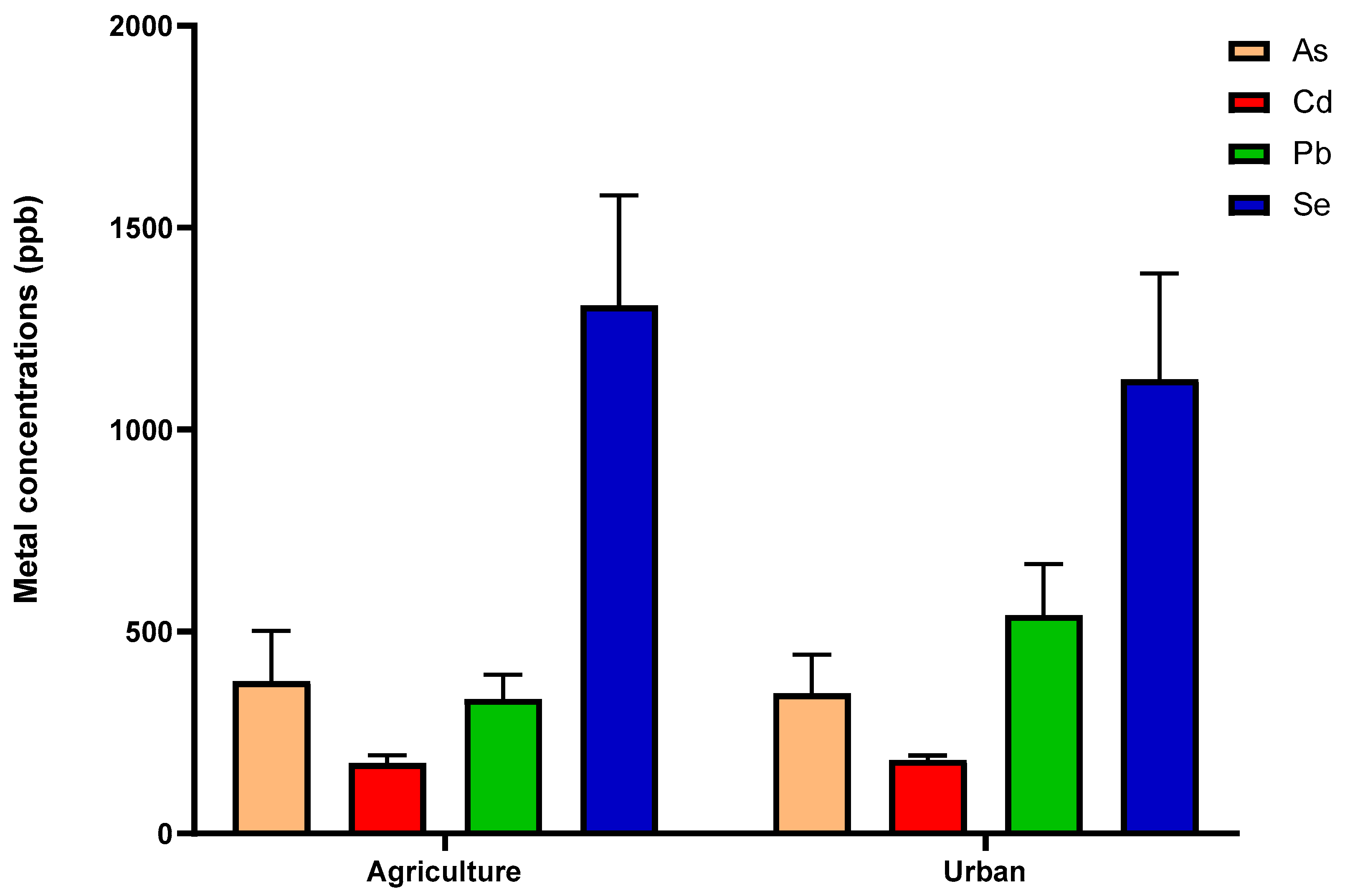
3.1. Selenium and Heavy Metals
| Heavy Metal | Agriculture | Urban | Multiple t-Test | |||
|---|---|---|---|---|---|---|
| Mean (ppb) | Mean (ppb) | ± SEM * | p-Value | t | df | |
| As | 377 | 347 | 159.5 | 0.99 | 0.009 | 20 |
| Cd | 174 | 182 | 21.91 | 0.99 | 0.012 | 20 |
| Pb | 333 | 540 | 144.8 | 0.16 | 1.432 | 21 |
| ** Se | 1307 | 1124 | 378.4 | 0.63 | 0.483 | 21 |
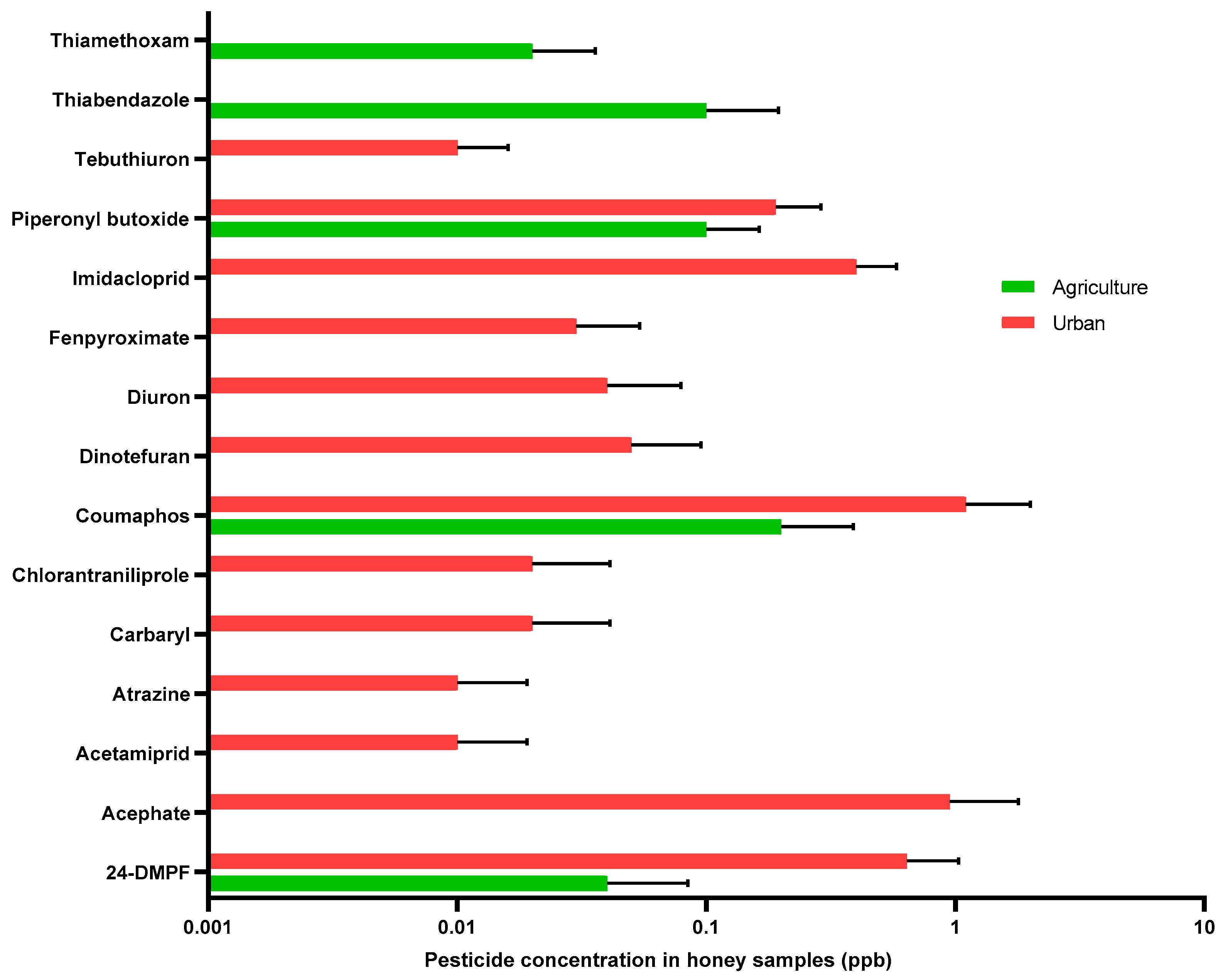
3.2. Pesticides
3.3. Risk Assessment
3.3.1. Heavy Metals
3.3.2. Pesticides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pesticide | Type | LOD-LOQ (ppb) Honey | LOD-LOQ (ppb) Pollen |
|---|---|---|---|
| 2,4-DMPF | Insecticide | 0.29–0.86 | 0.40–1.20 |
| 4-Hydroxy-chlorothalonil | Fungicide | 1.43–4.29 | 2.00–6.00 |
| Acephate | Insecticide | 0.71–2.14 | 1.00–3.00 |
| Acetamiprid | Insecticide | 0.07–0.21 | 0.1–0.30 |
| Ametryn | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Atrazine | Herbicide | 0.07–0.21 | 0.1–0.30 |
| Avermectin B1a | Acaricide | 0.43–1.29 | 0.60–1.80 |
| Azoxystrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Bendiocarb | Insecticide | 0.09–0.26 | 0.12–0.36 |
| Boscalid | Fungicide | 1.43–4.29 | 2.00–6.00 |
| Bromuconazole | Fungicide | 0.43–1.29 | 0.60–1.80 |
| Carbaryl | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Carbofuran | Insecticide | 0.03–0.09 | 0.04–0.12 |
| Chlorantraniliprole | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Chlorpyrifos | Insecticide | 4.29–12.86 | 6.00–18.00 |
| Clomazone | Herbicide | 0.11–0.34 | 0.16–0.48 |
| Clothianidin | Insecticide | 0.29–0.86 | 0.40–1.20 |
| Coumaphos | Insecticide | 1.43–4.29 | 2.00–6.00 |
| Cyanazine | Herbicide | 0.14–0.43 | 0.20–0.60 |
| Cyantraniliprole | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Cyflufenamid | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Cyprodinil | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Cyromazine | Insecticide | 0.71–2.14 | 1.00–3.00 |
| Difenoconazole | Fungicide | 0.07–0.21 | 0.1–0.30 |
| Diflubenzuron | Acaricide | 2.86–8.57 | 4.00–12.00 |
| Dimoxystrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Dinotefuran | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Diuron | Herbicide | 0.29–0.86 | 0.40–1.20 |
| Fenamidone | Fungicide | 0.07–0.21 | 0.1–0.30 |
| Fenbuconazole | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Fenhexamid | Fungicide | 2.86–8.57 | 4.00–12.00 |
| Fenpyroximate | Acaricide | 0.07–0.21 | 0.10–0.30 |
| Fipronil | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Fluazifop | Herbicide | 0.43–1.29 | 0.60–1.80 |
| Fluazinam | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Fludioxonil | Fungicide | 0.43–1.29 | 0.60–1.80 |
| Flufenacet | Herbicide | 0.29–0.86 | 0.40–1.20 |
| Flumioxazin | Herbicide | 7.14–21.43 | 10.00–30.00 |
| Fluometuron | Herbicide | 0.29–0.86 | 0.40–1.20 |
| Fluopicolide | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Fluopyram | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Fluoxastrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Flupyradifurone | Insecticide | 0.29–0.86 | 0.40–1.20 |
| Fluxapyroxad | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Fumagillin | Fungicide | 1.43–4.29 | 2.00–6.00 |
| Hexaflumuron | Insecticide | 2.86–8.57 | 4.00–12.00 |
| Imidacloprid | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Indoxacarb | Insecticide | 0.43–1.29 | 0.60–1.80 |
| Malaoxon | Insecticide | 0.03–0.09 | 0.04–0.12 |
| Mandipropamid | Fungicide | 0.06–0.17 | 0.08–0.24 |
| Metalaxyl | Fungicide | 0.07–0.21 | 0.10–0.30 |
| Metazachlor | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Metconazole | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Methiocarb | Insecticide | 0.29–0.86 | 0.40–1.20 |
| Methoprotryne | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Methoxyfenozide | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Metobromuron | Herbicide | 0.43–1.29 | 0.60–1.80 |
| Metolachlor | Herbicide | 0.14–0.43 | 0.20–0.60 |
| Mevinphos | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Myclobutanil | Fungicide | 0.07–0.21 | 0.10–0.30 |
| Napropamide | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Penthiopyrad | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Phenmedipham | Herbicide | 0.14–0.43 | 0.20–0.60 |
| Phosmet | Insecticide | 1.43–4.29 | 2.00–6.00 |
| Picoxystrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Piperonyl butoxide | pesticide synergist | 0.03–0.09 | 0.04–0.12 |
| Profenophos | Insecticide | 0.57–1.71 | 0.80–2.40 |
| Prometon | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Prometryn | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Propazine | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Propiconazole | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Pyraclostrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Pyrimethanil | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Spinetoram | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Spinosad | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Spirotetramat | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Sulfentrazone | Herbicide | 2.86–8.57 | 4.00–12.00 |
| Sulfoxaflor | Insecticide | 1.43–4.29 | 2.00–6.00 |
| Tebuconazole | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Tebufenozide | Insecticide | 0.03–0.09 | 0.04–0.12 |
| Tebuthiuron | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Terbutryn | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Tetraconazole | Fungicide | 0.29–0.86 | 0.40- 1.20 |
| Tetramethrin | Insecticide | 0.43–1.29 | 0.60–1.80 |
| Thiabendazole | Fungicide | 0.07–0.21 | 0.10–0.30 |
| Thiacloprid | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Thiamethoxam | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Thiobencarb | Herbicide | 0.43–1.29 | 0.60–1.80 |
| Thiophanate-methyl | Fungicide | 0.07–0.21 | 0.10–0.30 |
| Triadimefon | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Trifloxystrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Triflumizole | Fungicide | 0.07–0.21 | 0.10–0.30 |
References
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Miller-Struttmann, N. The Complex Causes of Worldwide Bee Declines. Available online: https://phys.org/news/2016-01-complex-worldwide-bee-declines.html (accessed on 20 March 2022).
- The State of Food and Agriculture. 2009. Available online: https://www.fao.org/3/i0680e/i0680e.pdf (accessed on 2 March 2023).
- ARS Honey Bee Health. Available online: https://www.ars.usda.gov/oc/br/ccd/index/ (accessed on 10 February 2022).
- Grozinger, C.M.; Flenniken, M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019, 64, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Feinsinger, P. Habitat Fragmentation, Native Insect Pollinators, and Feral Honey Bees in Argentine. Ecol. Appl. 1994, 4, 378–392. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A Worldwide Survey of Neonicotinoids in Honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef] [Green Version]
- Tison, L.; Hahn, M.-L.; Holtz, S.; Rößner, A.; Greggers, U.; Bischoff, G.; Menzel, R. Honey Bees’ Behavior Is Impaired by Chronic Exposure to the Neonicotinoid Thiacloprid in the Field. Environ. Sci. Technol. 2016, 50, 7218–7227. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2014, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.-F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Wernecke, A.; Frommberger, M.; Forster, R.; Pistorius, J. Lethal Effects of Various Tank Mixtures Including Insecticides, Fungicides and Fertilizers on Honey Bees under Laboratory, Semi-Field and Field Conditions. J. Consum. Prot. Food Saf. 2019, 14, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Polykretis, P.; Delfino, G.; Petrocelli, I.; Cervo, R.; Tanteri, G.; Montori, G.; Perito, B.; Branca, J.; Morucci, G.; Gulisano, M. Evidence of Immunocompetence Reduction Induced by Cadmium Exposure in Honey Bees (Apis Mellifera). Environ. Pollut. 2016, 218, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Monchanin, C.; Drujont, E.; Devaud, J.-M.; Lihoreau, M.; Barron, A.B. Metal Pollutants Have Additive Negative Effects on Honey Bee Cognition. J. Exp. Biol. 2021, 24, jeb241869. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet Effects on Honeybee Immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairbrother, A.; Purdy, J.; Anderson, T.; Fell, R. Risks of Neonicotinoid Insecticides to Honeybees. Environ. Toxicol. Chem. 2014, 33, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The Neonicotinoids Thiacloprid, Imidacloprid, and Clothianidin Affect the Immunocompetence of Honey Bees (Apis Mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Moroń, D.; Grześ, I.M.; Skórka, P.; Szentgyörgyi, H.; Laskowski, R.; Potts, S.G.; Woyciechowski, M. Abundance and Diversity of Wild Bees Along Gradients of Heavy Metal Pollution. J. Appl. Ecol. 2011, 49, 118–125. [Google Scholar] [CrossRef]
- Hladun, K.R.; Di, N.; Liu, T.; Trumble, J.T. Metal Contaminant Accumulation in the Hive: Consequences for whole-colony Health and Brood Production in the Honey Bee (Apis mellifera L.). Environ. Toxicol. Chem. 2016, 35, 322–329. [Google Scholar] [CrossRef]
- Sivakoff, F.S.; Gardiner, M.M. Soil Lead Contamination Decreases Bee Visit Duration at Sunflowers. Urban Ecosyst. 2017, 20, 1221–1228. [Google Scholar] [CrossRef]
- Zhou, X.; Taylor, M.P.; Davies, P.J.; Prasad, S. Identifying Sources of Environmental Contamination in European Honey Bees (Apis mellifera) Using Trace Elements and Lead Isotopic Compositions. Environ. Sci. Technol. 2018, 52, 991–1001. [Google Scholar] [CrossRef]
- Aghamirlou, H.M.; Khadem, M.; Rahmani, A.; Sadeghian, M.; Mahvi, A.H.; Akbarzadeh, A.; Nazmara, S. Heavy Metals Determination in Honey Samples Using Inductively Coupled Plasma-Optical Emission Spectrometry. J. Environ. Health Sci. Eng. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Shalaby, T.A.; Prokisch, J.; Fári, M. Selenium in agriculture: Water, air, soil, plants, food, animals and nanoselenium. In CO2 Sequestration, Biofuels and Depollution; Springer: Cham, Switzerland, 2015; pp. 153–232. [Google Scholar]
- Čuvardić, M.S. Selenium in soil. Zb. Matice Srp. Za Prir. Nauk. 2003, 23–37. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burden, C.M.; Elmore, C.; Hladun, K.R.; Trumble, J.T.; Smith, B.H. Acute exposure to selenium disrupts associative conditioning and long-term memory recall in honey bees (Apis mellifera). Ecotoxicol. Environ. Saf. 2016, 127, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hladun, K.R.; Smith, B.H.; Mustard, J.A.; Morton, R.R.; Trumble, J.T. Selenium Toxicity to Honey Bee (Apis mellifera L.) Pollinators: Effects on Behaviors and Survival. PLoS ONE 2012, 7, e34137. [Google Scholar] [CrossRef]
- Sabatino, L.; Scordino, M.; Pantò, V.; Chiappara, E.; Traulo, P.; Gagliano, G. Survey of Neonicotinoids and Fipronil in Corn Seeds for Agriculture. Food Addit. Contam. Part B 2013, 6, 11–16. [Google Scholar] [CrossRef]
- Pohorecka, K.; Skubida, P.; Semkiw, P.; Miszczak, A.; Teper, D.; Sikorski, P.; Zagibajło, K.; Skubida, M.; Zdańska, D.; Bober, A. Effects of Exposure of Honey Bee Colonies to Neonicotinoid seed–treated Maize Crops. J. Apic. Sci. 2013, 57, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Castilhos, D.; Dombroski, J.L.D.; Bergamo, G.C.; Gramacho, K.P.; Gonçalves, L.S. Neonicotinoids and Fipronil Concentrations in Honeybees Associated With Pesticide Use in Brazilian Agricultural Areas. Apidologie 2019, 50, 657–668. [Google Scholar] [CrossRef]
- Zawislak, J.; Adamczyk, J.; Johnson, D.R.; Lorenz, G.; Black, J.; Hornsby, Q.; Stewart, S.D.; Joshi, N. Comprehensive Survey of Area-Wide Agricultural Pesticide Use in Southern United States Row Crops and Potential Impact on Honey Bee Colonies. Insects 2019, 10, 280. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, M.; Pinion, C., Jr.; Klyza, J.; Zimeri, A.M. Pesticide Contamination in Central Kentucky Urban Honey: A Pilot Study. J. Environ. Health 2019, 82, 8–13. [Google Scholar]
- Sadowska, M.; Gogolewska, H.; Pawelec, N.; Sentkowska, A.; Krasnodębska-Ostręga, B. Comparison of the Contents of Selected Elements and Pesticides in Honey Bees with Regard to Their Habitat. Environ. Sci. Pollut. Res. 2018, 26, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naccari, C.; Macaluso, A.; Giangrosso, G.; Naccari, F.; Ferrantelli, V. Risk Assessment of Heavy Metals and Pesticides in Honey From Sicily (Italy). J. Food Res. 2014, 3, 107. [Google Scholar] [CrossRef] [Green Version]
- The Urban and Rural Classifications. Available online: https://www2.census.gov/geo/pdfs/reference/GARM/Ch12GARM.pdf (accessed on 13 May 2022).
- Colorado Revised Statutes 2016 TITLE 25.5. Available online: https://leg.colorado.gov/sites/default/files/images/olls/crs2016-title-25.5.pdf (accessed on 14 September 2022).
- Utaipanon, P.; Holmes, M.J.; Chapman, N.C.; Oldroyd, B.P. Estimating the Density of Honey Bee (Apis mellifera) Colonies Using Trapped Drones: Area Sampled and Drone Mating Flight Distance. Apidologie 2019, 50, 578–592. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Vandame, R.; Castro-Chan, R.; Penilla-Navarro, R.; Gómez, J.; Sánchez, D. Organochlorine Pesticides in Honey and Pollen Samples from Managed Colonies of the Honey Bee Apis mellifera Linnaeus and the Stingless Bee Scaptotrigona Mexicana Guérin from Southern, Mexico. Insects 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kılıç Altun, S.; Dinç, H.; Paksoy, N.; Temamoğulları, F.K.; Savrunlu, M. Analyses of Mineral Content and Heavy Metal of Honey Samples from South and East Region of Turkey by Using ICP-MS. Int. J. Anal. Chem. 2017, 2017, 6391454. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Jones, J.B.; Workman, S.M. Optical Emission Spectrometry. In Agronomy Monographs; ACSESS: Hoboken, NJ USA, 2015; pp. 29–65. [Google Scholar] [CrossRef]
- EN 15662—European Standards. Available online: https://www.en-standard.eu/csn-en-15662-foods-of-plant-origin-multimethod-for-the-determination-of-pesticide-residues-using-gc-and-lc-based-analysis-following-acetonitrile-extraction-partitioning-and-clean-up-by-dispersive-spe-modular-quechers-method/ (accessed on 2 March 2023).
- United States Environmental Protection Agency; Office of Pesticide Programs. Assigning Values to Non-Detected /Non-Quantified Pesticide Residues in Human Health Food Exposure Assessments; Office of Pesticide Programs, US Environmental Protection Agency: Washington, DC, USA, 2000.
- Knowlton, G.F.; Sturtevant, A.P.; Sorenson, C.J. Adult Honey Bee Losses in Utah as Related to Arsenic Poisoning; Bulletin No. 340-; Utah Agricultural Experiment Station: Logan, UT, USA, 1950. [Google Scholar]
- Di, N.; Hladun, K.R.; Zhang, K.; Liu, T.-X.; Trumble, J.T. Laboratory Bioassays on the Impact of Cadmium, Copper and Lead on the Development and Survival of Honeybee (Apis mellifera L.) Larvae and Foragers. Chemosphere 2016, 152, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Pettis, J.S.; Collins, A.M.; Wilbanks, R.; Feldlaufer, M.F. Effects of Coumaphos on Queen Rearing in the Honey Bee, Apis mellifera. Apidologie 2004, 35, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Nakar, R.; Koteswara Rao, S.; Sridevi, D.; Vidyasagar, B. Contact Toxicity of Certain Conventional Insecticides to European Honeybee, Apis mellifera Linnaeus. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3359–3365. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Zhu, Y.C.; Adamczyk, J.; Luttrell, R. Influences of Acephate and Mixtures With Other Commonly Used Pesticides on Honey Bee (Apis mellifera) Survival and Detoxification Enzyme Activities. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 209, 9–17. [Google Scholar] [CrossRef]
- Abbo, P.M.; Kawasaki, J.K.; Hamilton, M.; Cook, S.C.; DeGrandi-Hoffman, G.; Li, W.F.; Liu, J.; Chen, Y.P. Effects of Imidacloprid and Varroa Destructor on Survival and Health of European Honey Bees, Apis mellifera. Insect Sci. 2016, 24, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Hatjina, F.; Papaefthimiou, C.; Charistos, L.; Dogaroglu, T.; Bouga, M.; Emmanouil, C.; Arnold, G. Sublethal Doses of Imidacloprid Decreased Size of Hypopharyngeal Glands and Respiratory Rhythm of Honeybees in Vivo. Apidologie 2013, 44, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Sonnet, P.E.; Lye, T.L.; Sackett, R.R. Effects of Selected Herbicides on the Toxicity of Several Insecticides to Honey Bees. Environ. Entomol. 1978, 7, 254–256. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Li, Y.; Zhang, S.; Shi, P.; Li-Byarlay, H.; Luo, S. Pesticide Residues in Beebread and Honey in Apis Cerana Cerana and Their Hazards to Honey Bees and Human. Ecotoxicol. Environ. Saf. 2022, 238, 113574. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.; Maccagnani, B. Honey bees as bioindicators of environmental pollution. Bull. Insectology 2003, 56, 137–139. [Google Scholar]
- VanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef] [Green Version]
- Frazier, J.; Mullin, C.; Frazier, M.; Ashcraft, S. Pesticides and their involvement in colony collapse disorder. Am. Bee J. 2011, 151, 779–781. [Google Scholar]
- Zhu, Y.-C.; Wang, Y.; Portilla, M.; Parys, K.; Li, W. Risk and Toxicity Assessment of a Potential Natural Insecticide, Methyl Benzoate, in Honey Bees (Apis mellifera L.). Insects 2019, 10, 382. [Google Scholar] [CrossRef] [Green Version]
- Démares, F.; Gibert, L.; Creusot, P.; Lapeyre, B.; Proffit, M. Acute Ozone Exposure Impairs Detection of Floral Odor, Learning, and Memory of Honey Bees, through Olfactory Generalization. Sci. Total. Environ. 2022, 827, 154342. [Google Scholar] [CrossRef]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, Fungicide and Drug Interactions in Honey Bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Takahashi, O.; Oishi, S. Reproductive and Neurobehavioural Effects in Three-Generation Toxicity Study of Piperonyl Butoxide Administered to Mice. Food Chem. Toxicol. 1992, 30, 1015–1019. [Google Scholar] [CrossRef]
- Devine, G.; Denholm, I. An Unconventional Use of Piperonyl Butoxide for Managing the Cotton Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 1998, 88, 601–610. [Google Scholar] [CrossRef]
- Johnson, R.M.; Pollock, H.S.; Berenbaum, M.R. Synergistic Interactions Between In-Hive Miticides in Apis mellifera. J. Econ. Entomol. 2009, 102, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.R.; Johnson, R.M. Xenobiotic Detoxification Pathways in Honey Bees. Curr. Opin. Insect Sci. 2015, 10, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Heavy Metal | Agriculture | Urban | Multiple t-Test | |||
|---|---|---|---|---|---|---|
| Mean (ppb) | Mean (ppb) | ±SEM * | p-Value | t | df | |
| As | 271 | 188 | 148.9 | 0.46 | 0.7423 | 18 |
| Cd | 53 | 36 | 30.67 | 0.60 | 0.5296 | 19 |
| Pb | 539 | 409 | 174.0 | 0.64 | 0.4628 | 19 |
| Se | 1061 | 1006 | 500.4 | 0.91 | 0.1086 | 19 |
| Metal | Matrix Conc. Range (ppb) | Metal Conc./Range in Other Research (ppb) | Effect | Reference | |
|---|---|---|---|---|---|
| Pollen | Honey | ||||
| As | 1-1243 | 1-1280 | 10–50 | Slows down learning and reduces long-term memory | [15] |
| 3000 | Lethal | [45] | |||
| Pb | 5-624 | 1-1168 | 60 | Slows down learning and reduces long-term memory | [15] |
| 1120–larvae 345,000–foragers | Lethal | [46] | |||
| Cd | 79-258 | 1-298 | 275–larvae 78,000–foragers | Lethal | [46] |
| 100–1000 | Immuno-competence reduction | [14] | |||
| Se | 20-7460 | 1-3491 | 500–700 | Disrupts foraging behavior | [47] |
| Pesticide | Matrix Avg. of Conc. (ppb) | Pesticide Conc./Range in Other Research (ppb) | Effect | Reference | |
|---|---|---|---|---|---|
| Pollen | Honey | ||||
| Coumaphos | 0.2 | 1.1 | 1 × 106 | Failure of queen development | [48] |
| Acephate | ND | 0.93 | 15 × 105 | Lethal | [49] |
| 6970 | Inhibit detoxification enzyme | [50] | |||
| Chlorpyrifos | 17.30–26.01 | ND | 25 × 105 | Lethal | [49] |
| Imidacloprid | 0.14–1.34 | 0.37 | 50 25 | Lethal Negatively affect development and behavior | [51] |
| 2–3 | Negatively affect the development of the hypopharyngeal glands | [52] | |||
| Atrazine | 2.03–3.44 | 0.01 | 46,700–65,300 | Lethal | [53] |
| Tebuconazole | 0.38–3.77 | 0.11–11.83 | 51 | Lethal | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, M.M.; Boone, R.B. Assessment of Spatial Variations in Pesticide, Heavy Metal, and Selenium Residues in Honey Bee (Apis mellifera L.) Products. Sci 2023, 5, 24. https://doi.org/10.3390/sci5020024
Awad MM, Boone RB. Assessment of Spatial Variations in Pesticide, Heavy Metal, and Selenium Residues in Honey Bee (Apis mellifera L.) Products. Sci. 2023; 5(2):24. https://doi.org/10.3390/sci5020024
Chicago/Turabian StyleAwad, Mai M., and Randall B. Boone. 2023. "Assessment of Spatial Variations in Pesticide, Heavy Metal, and Selenium Residues in Honey Bee (Apis mellifera L.) Products" Sci 5, no. 2: 24. https://doi.org/10.3390/sci5020024





