Spotted Fever Rickettsiosis in a Wildlife Researcher in Sabah, Malaysia: A Case Study
Abstract
:1. Introduction
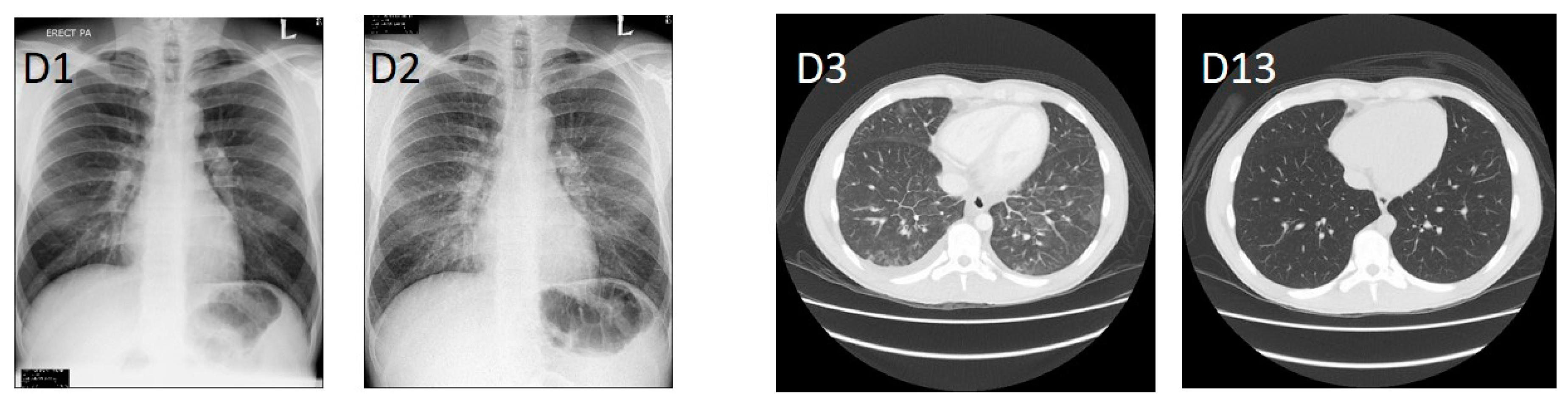
2. Clinical History
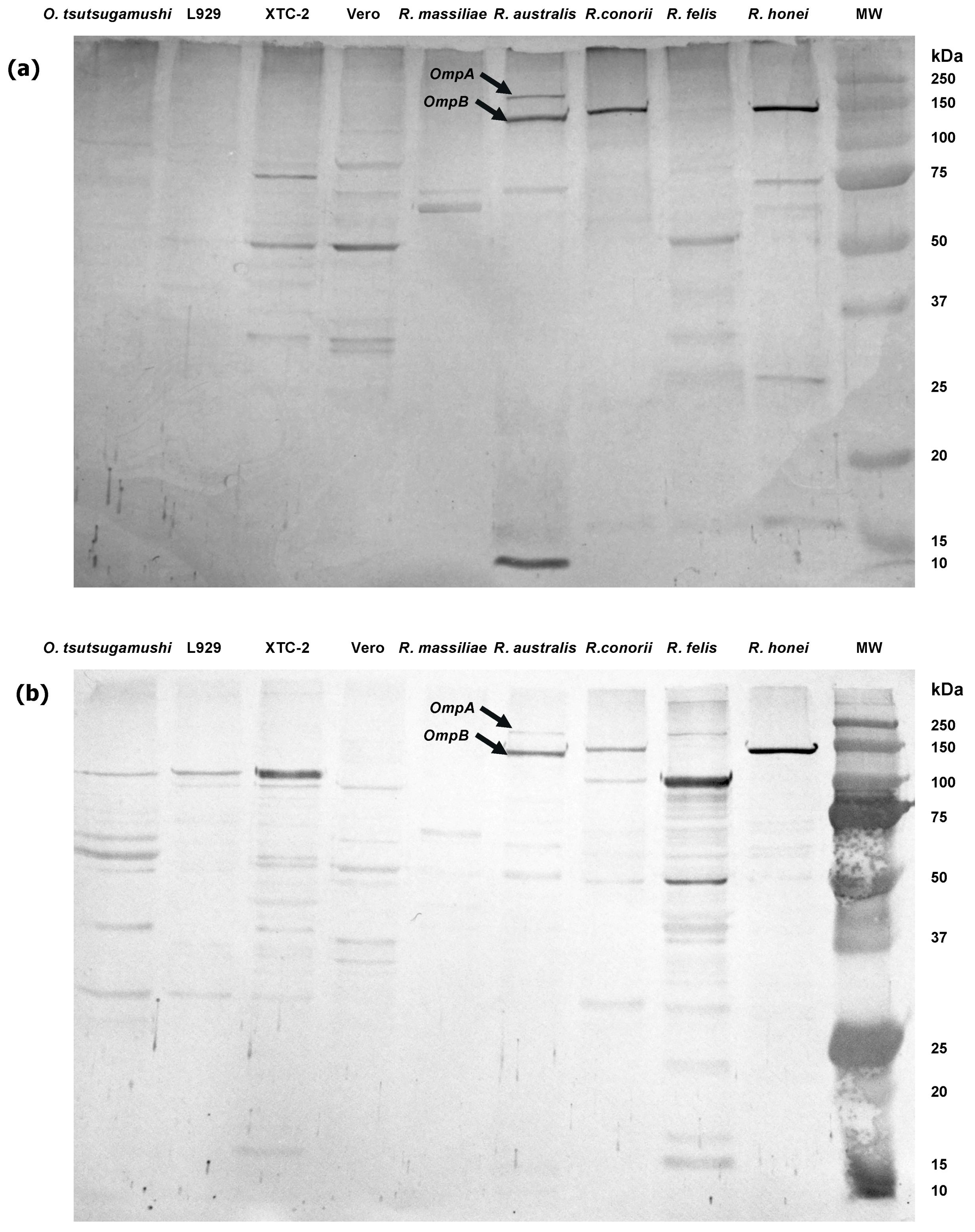
3. Laboratory Diagnosis
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Raoult, D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H. Rickettsiae and rickettsial infections: The current state of knowledge. Clin. Infect. Dis. 2007, 45, S39–S44. [Google Scholar] [CrossRef] [PubMed]
- Aung, A.K.; Spelman, D.W.; Murray, R.J.; Graves, S. Review article. Rickettsial infections in Southeast Asia: Implications for local populace and febrile returned travelers. Am. J. Trop. Med. Hyg. 2014, 91, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Jensenius, M.; Han, P.V.; Schlagenhauf, P.; Schwartz, E.; Parola, P.; Castelli, F.; Von Sonnenburg, F.; Loutan, L.; Leder, K.; Freedman, D.O. Acute and potentially life-threatening tropical diseases in western travelers—A GeoSentinel multicenter study, 1996–2011. Am. J. Trop. Med. Hyg. 2013, 88, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilde, H.; Suankratay, C. There is need for antigen-based rapid diagnostic tests to identify common acute tropical illnesses. J. Travel Med. 2007, 14, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Fuerst, P.; Richards, A. The historical case for and the future study of antibiotic-resistant scrub typhus. Trop. Med. Infect. Dis. 2017, 2, 63. [Google Scholar] [CrossRef]
- Botelho-Nevers, E.; Socolovschi, C.; Raoult, D.; Parola, P. Treatment of Rickettsia spp. infections: A review. Expert Rev. Anti-Infect. Ther. 2012, 10, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Marchette, N.J. Rickettsioses (tick typhus, Q-fever, urban typhus) in Malaya. J. Med. Entomol. 1966, 2, 339–371. [Google Scholar] [CrossRef] [PubMed]
- Tee, T.S.; Kamalanathan, M.; Suan, K.A.; Chun, S.S.; Ming, H.T.; Yasin, R.M.; Devi, S. Seroepidemiologic survey of Orientia tsutsugamushi, Rickettsia typhi, and TT118 spotted fever group rickettsiae in rubber estate workers in Malaysia. Am. J. Trop. Med. Hyg. 1999, 61, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.T.; Kamalanathan, M.; Rohani, M.Y. Antibody prevalence of Orientia tsutsugamushi, Rickettsia typhi and TT118 spotted fever group rickettsiae among Malaysian blood donors and febrile patients in the urban areas. Southeast Asian J. Trop. Med. Public Health 2003, 34, 165–170. [Google Scholar] [PubMed]
- Tay, S.T.; Rohani, M.Y. The use of the indirect immunoperoxidase test for the serodiagnosis of rickettsial diseases in Malaysia. Southeast Asian J. Trop. Med. Public Health 2002, 33, 314–320. [Google Scholar] [PubMed]
- Tay, S.T.; Ho, T.M.; Rohani, M.Y.; Devi, S. Antibodies to Orientia tsutsugamushi, Rickettsia typhi and spotted fever group rickettsiae among febrile patients in rural areas of Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 280–284. [Google Scholar] [CrossRef]
- Kho, K.L.; Koh, F.X.; Singh, H.K.L.; Zan, H.A.M.; Ponnampalavanar, S.; Kukreja, A.; Tay, S.T. spotted fever group rickettsioses and murine typhus in a Malaysian teaching hospital. Am. J. Trop. Med. Hyg. 2016, 95, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Sagin, D.D.; Ismail, G.; Nasian, L.M.; Jok, J.J.; Pang, E.K.H. Rickettsial infection in five remote Orang Ulu villages in upper Rejang River, Sarawak, Malaysia. Southeast Asian J. Trop. Med. Public Health 2000, 31, 733–735. [Google Scholar] [PubMed]
- Taylor, A.C.; Hii, J.; Kelly, D.J.; Davis, D.R.; Lewis, G.E. A serological survey of scrub, tick, and endemic typhus in Sabah, East Malaysia. Southeast Asian J. Trop. Med. Public Health 1986, 17, 613–619. [Google Scholar] [PubMed]
- Reller, M.E.; Grigg, M.; William, T.; Yeo, T.; Clemens, E.G.; Dumler, J.S. Rickettsial infections as a major etiology of acute febrile illness: A prospective study in northern Sabah, Borneo, East Malaysia [abstract]. In: American Society of Tropical Medicine and Hygiene Sixty-Sixth Annual Meeting, 2017 November 5–9; Baltimore, Maryland, USA; abstract number 1982. Am. J. Trop. Med. Hyg. 2017, 97, 1–674. [Google Scholar] [CrossRef]
- Watanabe, S.; Masangkay, J.S.; Nagata, N.; Morikawa, S.; Mizutani, T.; Fukushi, S.; Alviola, P.; Omatsu, T.; Ueda, N.; Iha, K.; et al. Bat coronaviruses and experimental infection of bats, the Philippines. Emerg. Infect. Dis. 2010, 16, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.L.; Firth, C.; Street, C. Identification of a severe acute respiratory syndrome coronavirus-like virus in a leaf-nosed bat in Nigeria. mBio 2010, 1, e00208-10. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Palacios, G.; Towner, J.S.; Jabado, O.; Kapoor, V.; Venter, M.; Grolla, A.; Briese, T.; Paweska, J.; Swanepoel, R.; et al. Rapid molecular strategy for filovirus detection and characterization. J. Clin. Microbiol. 2007, 45, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.J.; Leger, J.A.S.; Pugliares, K.; Ip, H.S.; Chan, J.M.; Carpenter, Z.W.; Navarrete-Macias, I.; Sanchez-Leon, M.; Saliki, J.T.; Pedersen, J.; et al. Emergaence of fatal avian influenza in New England harbour seals. mBio 2012, 3, e00166-12. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Chern, S.W.W.; Li, Y.; Pallansch, M.A.; Anderson, L.J. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J. Clin. Microbiol. 2008, 46, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Allan Nix, W.; Oberste, M.S.; Pallansch, M.A. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 2006, 44, 2698–2704. [Google Scholar] [CrossRef]
- Moureau, G.; Temmam, S.; Gonzalez, J.P.; Charrel, R.N.; Grard, G.; de Lamballerie, X. A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector-Borne Zoonotic Dis. 2007, 7, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Aitichou, M.; Saleh, S.S.; McElroy, A.K.; Schmaljohn, C.; Ibrahim, M.S. Identification of Dobrava, Hantaan, Seoul, and Puumala viruses by one-step real-time RT-PCR. J. Virol. Methods 2005, 124, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Raboni, S.M.; Rubio, G.; De Borba, L.; Zeferino, A.; Skraba, I.; Goldenberg, S.; Dos Santos, C.N. Clinical survey of hantavirus in southern Brazil and the development of specific molecular diagnosis tools. Am. J. Trop. Med. Hyg. 2005, 72, 800–804. [Google Scholar] [PubMed]
- Stenos, J.; Ross, B.; Feng, H.M.; Crocquet-Valdes, P.; Walker, D. Protein characterization of Australian spotted fever group rickettsiae and monoclonal antibody typing of Rickettsia honei. J. Clin. Microbiol. 1997, 35, 261–263. [Google Scholar] [PubMed]
- Reynolds, G.; Payne, J.; Sinun, W.; Mosigil, G.; Walsh, R.P.D. Changes in forest land use and management in Sabah, Malaysian Borneo, 1990–2010, with a focus on the Danum Valley region. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Adrus, M.; Ahamad, M.; Abdullah, M.T. Detection of rickettsiae in engorged ticks from small mammals in Malaysia. Borneo J. Resour. Sci. Technol. 2014, 4, 34–41. [Google Scholar]
- Kernif, T.; Socolovschi, C.; Wells, K.; Lakim, M.B.; Inthalad, S.; Slesak, G.; Boudebouch, N.; Beaucournu, J.C.; Newton, P.N.; Raoult, D.; et al. Bartonella and Rickettsia in arthropods from the Lao PDR and from Borneo, Malaysia. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Health Informatics Centre. Health Indicators 2016: Indicators for Monitoring and Evaluation of Strategy Health for All; MOH/S/RAN/18.16(AR); Health Informatics Centre, Planning Division, Ministry of Health Malaysia: Putrajaya, Malaysia, 2016. [Google Scholar]
- Blacksell, S.D.; Bryant, N.J.; Paris, D.H.; Doust, J.A.; Sakoda, Y.; Day, N.P.J. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: A lack of consensus leads to a lot of confusion. Clin. Infect. Dis. 2007, 44, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Fong, T. Prevalence of erythrocyte G6PD deficiency in Sabah. Mod. Med. Asia 1977, 13, 14–16. [Google Scholar] [PubMed]
- Khoo, K.K. The treatment of malaria in glucose-6-phosphate dehydrogenase deficient patients in Sabah. Ann. Trop. Med. Parasitol. 1981, 75, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Rathi, N.; Rathi, A. Rickettsial infections: Indian perspective. Indian Pediatr. 2010, 47, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
| Test | D1 * | D3 | D5 | D7 | D13 | D15 | Reference Range |
|---|---|---|---|---|---|---|---|
| Haemoglobin, g/L | 156 | 132 | 132 | 125 | 140 | 129 | 130–180 |
| White blood cells, cells/L | 4.6 | 3.8 | 6.6 | 10.1 | 7.6 | 5.8 | 4.0–11.0 × 109 |
| Lymphocytes, cells/L | 0.6 | 0.4 | 2.1 | 6.7 | 5.5 | 4.1 | 1.5–4.0 × 109 |
| Platelets, cells/L | 62 | 38 | 106 | 204 | 408 | 374 | 140–400 × 109 |
| Albumin, g/L | 39 | 30 | 24 | 26 | 38 | 38 | 35–52 |
| Bilirubin, µmol/L | 26 | 16 | 18 | 9 | 12 | 10 | <21 |
| Alkaline phosphatase, U/L | 84 | 74 | 125 | 136 | 114 | 100 | 30–120 |
| Gamma-glutamyl transferase, U/L | 147 | 119 | 164 | 159 | 136 | 114 | <50 |
| Aspartate transferase, U/L | 37 | 43 | 206 | 88 | 38 | 33 | <45 |
| Alanine transaminase, U/L | 35 | 36 | 154 | 112 | 68 | 54 | <55 |
| C-reactive protein, mg/L | N/T | N/T | 93.99 | 35.34 | 2.55 | N/T | >=5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado Lynn, M.; William, T.; Tanganuchitcharnchai, A.; Jintaworn, S.; Thaipadungpanit, J.; Lee, M.H.; Jalius, C.; Daszak, P.; Goossens, B.; Hughes, T.; et al. Spotted Fever Rickettsiosis in a Wildlife Researcher in Sabah, Malaysia: A Case Study. Trop. Med. Infect. Dis. 2018, 3, 29. https://doi.org/10.3390/tropicalmed3010029
Salgado Lynn M, William T, Tanganuchitcharnchai A, Jintaworn S, Thaipadungpanit J, Lee MH, Jalius C, Daszak P, Goossens B, Hughes T, et al. Spotted Fever Rickettsiosis in a Wildlife Researcher in Sabah, Malaysia: A Case Study. Tropical Medicine and Infectious Disease. 2018; 3(1):29. https://doi.org/10.3390/tropicalmed3010029
Chicago/Turabian StyleSalgado Lynn, Milena, Timothy William, Ampai Tanganuchitcharnchai, Suthatip Jintaworn, Janjira Thaipadungpanit, Mei Ho Lee, Cyrlen Jalius, Peter Daszak, Benoît Goossens, Tom Hughes, and et al. 2018. "Spotted Fever Rickettsiosis in a Wildlife Researcher in Sabah, Malaysia: A Case Study" Tropical Medicine and Infectious Disease 3, no. 1: 29. https://doi.org/10.3390/tropicalmed3010029
APA StyleSalgado Lynn, M., William, T., Tanganuchitcharnchai, A., Jintaworn, S., Thaipadungpanit, J., Lee, M. H., Jalius, C., Daszak, P., Goossens, B., Hughes, T., & Blacksell, S. D. (2018). Spotted Fever Rickettsiosis in a Wildlife Researcher in Sabah, Malaysia: A Case Study. Tropical Medicine and Infectious Disease, 3(1), 29. https://doi.org/10.3390/tropicalmed3010029






