Melioidosis in the Western Indian Ocean and the Importance of Improving Diagnosis, Surveillance, and Molecular Typing
Abstract
:1. Introduction and History of Melioidosis in the Western Indian Ocean
2. Review of the Human Cases
2.1. Madagascar
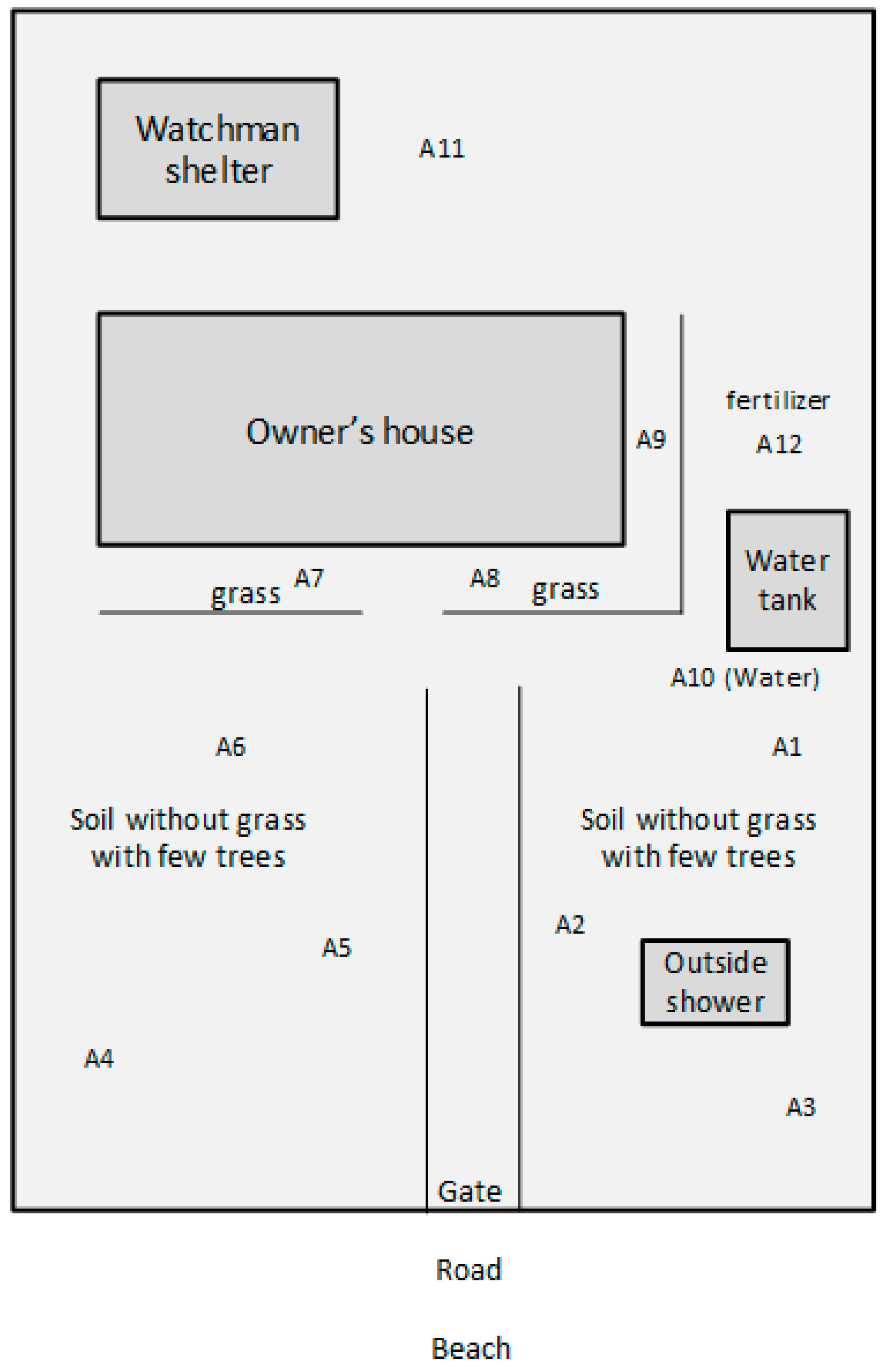
2.2. Soil Investigation in Mahajanga, Madagascar, in the Yard of Case #10
2.3. Mauritius
2.4. Réunion Island
2.5. Seychelles
2.6. Genetic and Genomic Relatedness between Strains Isolated in the Western Indian Ocean
3. Current Recommendations and Availability of Measures against Melioidosis
4. Awareness of Melioidosis
5. Current and Future Challenges
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B. Materials and Methods
Appendix B.1. Soil Sampling and Culture of B. pseudomallei from Soil and Water
Appendix B.2. Molecular Detection of B. pseudomallei from Soil
Appendix B.3. Identification of B. pseudomallei Isolates by PCR
Appendix B.4. MLSTF
References
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- Allou, N.; Martinet, O.; Allyn, J.; Bouchet, B.; Jaffar-Bandjee, M.-C.; Galas, T.; Traversier, N.; Belmonte, O. Emergence of melioidosis in the Indian Ocean region: Two new cases and a literature review. PLoS Negl. Trop. Dis. 2017, 11, e0006018. [Google Scholar] [CrossRef] [PubMed]
- The Map of Western Indian Ocean. Available online: https://www.lib.utexas.edu/maps/islands_oceans_poles/indian_ocean_w_96.jpg (accessed on 1 March 2018).
- Leelarasamee, A. Recent development in melioidosis. Curr Opin Infect Dis. 2004, 17, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Girard, G. Can pigs be a healthy carrier of Whitmore’s bacillus? Bull. Soc. Pathol. Exot. 1936, 29, 712–716. [Google Scholar]
- Gallimand, M.; Dodin, A. A review of melioidosis worldwide. Bull. Soc. Pathol. Exot. 1982, 75, 375–383. [Google Scholar]
- Godoy, D.G.; Randle, A.J.; Simpson, D.M.; Aanensen, T.L.; Pitt, R.; Kinoshita; Spratt, B.G. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J. Clin. Microbiol. 2003, 41, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Mollaret, H.H. L’affaire du Jardin des Plantes ou comment la mélioïdose fît son apparition en France. Med. Mal. Infect. 1988, 11, 643–654. (In French) [Google Scholar] [CrossRef]
- Martinet, O.; Soo, A.M.P.; Knezynski, M.; Schlossmacher, P.; Jaffar-Bandjee, C.; Gaüzière, B.A. Melioidosis: About an acquired case in Madagascar and two nosocomial cases. Bull. Soc. Pathol. Exot. 2004, 97, 366–370. [Google Scholar]
- Borgherini, G.; Poubeau, P.; Paganin, F.; Picot, S.; Michault, A.; Thibault, F.; Arvin Berod, C. Melioidosis: An imported case from Madagascar. Internat. Soc. Trav. Med. 2006, 13, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Garin, G.; Djaomazala, I.; Dubois-Cauwelaert, N.; Mahafaly; Raharimanga, V.; Ralison, F.; Herindrainy, P.; Andriamalala, N.C.; Sarovich, D.S.; Mayo, M.; et al. Autochthonous melioidosis in humans, Madagascar, 2012 and 2013. Emerg. Infect. Dis. 2014, 20, 1739–1741. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Vallet, M.; Pierard, M.; Wattiau, P. Melioidosis—Belgium ex Madagascar. Available online: https://www.promedmail.org/post/1687746 (accessed on 8 January 2018).
- Köppen Climate Classification. Available online: https://www.britannica.com/science/Koppen-climate-classification (accessed on 1 March 2018).
- Novak, R.T.; Glass, M.B.; Gee, J.E.; Gal, D.; Mayo, M.J.; Currie, B.J.; Wilkins, P.P. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J. Clin. Microbiol. 2006, 44, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Issack, M.I.; Bundhun, C.D.; Gokhool, H. Melioidosis in Mauritius. Emerg. Infect. Dis. 2005, 11, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Borgherini, G.; Camuset, G.; Foucher, A.; Maiza, J.C.; Thibault, F.M.; Picot, S.; Poubeau, P. The first autocthonous case of human melioidosis in Réunion Island. Med. Mal. Inf. 2015, 45, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Bibi, J.; Biscornet, L.; Bermingham, A.; von Gottberg, A. First identification of Burkholderia pseudomallei in Seychelles. In Proceedings of the 1st International Forum Public Health Surveillance and Response in Island Territories, Saint Denis, La Réunion, 11–13 June 2013; p. 124. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing-EUCAST. Available online: http://www.eucast.org/ (accessed on 1 March 2018).
- Sarovich, D.S.; Garin, B.; De Smet, B.; Kaestli, M.; Mayo, M.; Vandamme, P.; Jacobs, J.; Lompo, P.; Tahita, M.C.; Tinto, H.; et al. Phylogenomic analysis reveals an Asian origin for African Burkholderia pseudomallei and further supports melioidosis endemicity in Africa. mSphere 2016, 1, e00089-15. [Google Scholar] [CrossRef] [PubMed]
- Chewapreecha, C.; Holden, M.T.; Vehkala, M.; Välimäki, N.; Yang, Z.; Harris, S.R.; Mather, A.E.; Tuanyok, A.; De Smet, B.; Le Hello, S.; et al. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat. Microbiol. 2017, 2, 16263. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.E.; Gulvik, C.A.; Elrod, M.G.; Batra, D.; Rowe, L.A.; Sheth, M.; Hoffmaster, A.R. Phylogeography of Burkholderia pseudomallei isolates, Western Hemisphere. Emerg. Infect. Dis. 2017, 23, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Currie, B.J. Melioidosis: Epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005, 18, 383–416. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Melioidosis. Lancet 2003, 361, 1715–1722. [Google Scholar] [CrossRef]
- Kaestli, M.; Harrington, G.; Mayo, M.; Chatfield, M.D.; Harrington, I.; Hill, A.; Munksgaard, N.; Gibb, K.; Currie, B.J. What drives the occurrence of the melioidosis bacterium Burkholderia pseudomallei in domestic gardens? PLoS Negl. Trop. Dis. 2015, 9, e0003635. [Google Scholar] [CrossRef] [PubMed]
- Keluangkhot, V.; Pethsouvanh, R.; Strobel, M. Melioidosis. Med. Mal. Infect. 2005, 35, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Vlieghe, E.; Kruy, L.; De Smet, B.; Kham, C.; Veng, C.H.; Phe, T.; Koole, O.; Thai, S.; Lynen, L.; Jacobs, J. Melioidosis, Phnom Penh, Cambodia. Emerg. Infect. Dis. 2011, 17, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Houghton, R.L.; Reed, D.E.; Hubbard, M.A.; Dillon, M.J.; Chen, H.; Currie, B.J.; Mayo, M.; Sarovich, D.S.; Theobald, V.; et al. Development of a prototype lateral flow immunoassay (LFI) for the rapid diagnosis of melioidosis. PLoS Negl. Trop. Dis. 2014, 8, e2727. [Google Scholar] [CrossRef] [PubMed]
- Kaestli, M.; Mayo, M.; Harrington, G.; Ward, L.; Hill, J.; Watt, F.; Cheng, A.C.; Currie, B.J. Landscape changes influence the occurrence of the melioidosis bacterium Burkholderia pseudomallei in soil in northern Australia. PLoS Negl. Trop. Dis. 2009, 3, e364. [Google Scholar] [CrossRef] [PubMed]
- Kaestli, M.; Mayo, M.; Harrington, G.; Hill, J.; Watt, F.; Gal, D.; Currie, B.J. Sensitive and specific DNA extraction and real-time PCR for detection of Burkholderia pseudomallei in the soil in tropical northern Australia. Appl. Environ. Microbiol. 2007, 73, 6891–6897. [Google Scholar] [CrossRef] [PubMed]
| Year | 2004 | 2005 | 2012 | 2013 | 2013 | 2016 | |
|---|---|---|---|---|---|---|---|
| Case | #1 | #5 | #8 | #9 | #10 | #13 | |
| Reference | [9] | [10] | [11] | [11] | [12] | [2] | |
| Sex | Male | Male | Male | Male | Male | Male | |
| Age | 60 | 58 | 52 | 45 | 44 | 63 | |
| Strain ID (sequence type) * | 4419 (ST-1260) | 4420 (ST-1433) | 3240 (ST-1053) | 3241 (ST-1054) | 4416 (ST-1043) | 2017-012 (ST1430) | |
| Allele profile | 1, 12, 34, 2, 5, 2, 1 | 4, 1, 34, 2, 5, 2, 1 | 4, 12, 3, 2, 5, 2, 1 | 4, 12, 34, 1, 5, 2, 1 | 4, 1, 3, 2, 5, 2, 1 | 4, 2 ,3, 1, 5 ,2, 1 | |
| Occupation | Not known | Retired | Rural rice farmer | Rice, sugar cane, and tobacco farmer | Gardener | Not known | |
| Risk factors | Heavy drinker (1 L/day) and smoker (20 cigarettes/day); cachexia | Smoker (35 cigarette packs/year); unremarkable medical history | Diabetes | Diabetes. A history of furunculosis for several months | No diabetes mellitus | ||
| 1st admission | CHD Félix Guyon, Saint Denis, Réunion Island (24.05.2004) | Hospital in Antananarivo (March 2005) | Androva University Hospital in Mahajanga (July 2012) | Androva University Hospital in Mahajanga (May 2013) | CHU-AP, Mons, Belgium (16.03.2013) | CHD Félix Guyon, Saint Denis, Réunion Island | |
| 2nd admission | Groupe Hospitalier Sud Réunion, Saint Pierre, Réunion Island | CHU-AP, Mons, Belgium (April 2013) due to identification of the causative agent and symptoms | |||||
| Previous history | Lived in Madagascar (Mahajanga); lived for an unknown period of time in Vietnam 20 or 30 years before the onset of the disease | Spent most of his life in France but lived for the past 5 years in Madagascar before hospitalisation in Antananarivo. He had also travelled for short periods in Tunisia, Turkey and Mauritius. The first symptoms appeared during the last days of a stay (couple of weeks) in Mahajanga | Frequent travels to Mahajanga for entertainment (beach sports and fishing) lasting usually for 3 weeks every 3 or 4 months including during the rainy season | Had consulted the Hospital in Mahajanga 4 days before being admitted to CHD Félix Guyon, Saint Denis | |||
| Outcome | Discharged | Clinical improvement and discharged | Died 3 days later | Died 2 weeks after admission and two days after ceftazidime treatment | Discharged a few days after his clinical status remained stable, with decreasing inflammatory syndrome and fever. | Discharged a few days after his clinical status remained stable, with decreasing inflammatory syndrome and fever. | Died on day 1 after his admission |
| Case [Ref.] | Year | Place of Diagnosis | City (Country) Visited within 12 Months of Diagnosis | Allele Profile | MLST Type | Remark | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 [9] | 2004 | Réunion Island | Mahajanga (Madagascar) | 1 | 12 | 34 | 2 | 5 | 2 | 1 | 1260 | ||
| #2 [9] | 2004 | Réunion Island * | 0 | Not typed, nosocomial cases | |||||||||
| #3 [9] | 2004 | Réunion Island * | 0 | Not typed, nosocomial cases | |||||||||
| #4 [15] | 2004 | Mauritius | 0 | 4 | 12 | 34 | 2 | 5 | 2 | 1 | 1549 | ST1549 is a single locus variant of ST1053 (case #8), ST1054 (case #9), ST1260 (case #1), and 1433 (case #5) | |
| #5 [10] | 2005 | Madagascar (1st admission)/Réunion Island (2nd admission) | Antananarivo and Mahajanga (Madagascar) | 4 | 1 | 34 | 2 | 5 | 2 | 1 | 1433 | ST1433 is a single locus variant of ST1549 (case #4) | |
| #6 [This study] | 2006 | Mauritius | Bangladeshi worker | Not typed | |||||||||
| #7 [16] | 2012 | Réunion Island | None | Not typed | |||||||||
| #8 [11] | 2012 | Madagascar | Mahajanga (Madagascar) | 4 | 12 | 3 | 2 | 5 | 2 | 1 | 1053 | ST1053 is a single locus variant of ST1043 (case #10), ST1432 (case #11), ST1549 (case #4) | |
| #9 [11] | 2013 | Madagascar | Mahajanga (Madagascar) | 4 | 12 | 34 | 1 | 5 | 2 | 1 | 1054 | ST1054 is a single locus variant of ST1433 (case #5), ST1549 (case #4) | |
| #10 [12] | 2013 | Belgium | Mahajanga (Madagascar) | 4 | 1 | 3 | 2 | 5 | 2 | 1 | 1043 | ST1043 is a single locus variant of ST1053 (case #8), ST1432 (case #11), ST1433 (case #5) | |
| #11 [17] | 2013 | Seychelles ** | Unknown | 4 | 2 | 3 | 2 | 5 | 2 | 1 | 1432 | ST1432 is a single locus variant of ST1043 (case #10), ST1430 (soil E1) | |
| #12 [17] | 2013 | Seychelles ** | Unknown | Not typed | |||||||||
| #13 [2] | 2016 | Réunion Island | Mahajanga (Madagascar) | 4 | 2 | 3 | 1 | 5 | 2 | 1 | 1430 | Same ST than E1. ST1430 is a single locus variant of ST1432 (case #11). | |
| #14 [2] | 2017 | Réunion Island | Southeast Asia | Not typed | |||||||||
| Environmental isolates | |||||||||||||
| E1 [This study] | 2014 | Soil from the garden of case 9 (A4) | N/A | 4 | 2 | 3 | 1 | 5 | 2 | 1 | 1430 | Same ST as case #13. ST1430 is a single locus variant of ST1432 (case #11). | |
| E2 [This study] | 2014 | Soil from the garden of case 9 (A8) | Typing in progress | ||||||||||
| E3 [This study] | 2014 | Soil from the garden of case 9 (A12) | N/A | 1 | 2 | 3 | 1 | 5 | 1 | 1 | 1431 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakotondrasoa, A.; Issack, M.I.; Garin, B.; Biot, F.; Valade, E.; Wattiau, P.; Allou, N.; Belmonte, O.; Bibi, J.; Price, E.P.; et al. Melioidosis in the Western Indian Ocean and the Importance of Improving Diagnosis, Surveillance, and Molecular Typing. Trop. Med. Infect. Dis. 2018, 3, 30. https://doi.org/10.3390/tropicalmed3010030
Rakotondrasoa A, Issack MI, Garin B, Biot F, Valade E, Wattiau P, Allou N, Belmonte O, Bibi J, Price EP, et al. Melioidosis in the Western Indian Ocean and the Importance of Improving Diagnosis, Surveillance, and Molecular Typing. Tropical Medicine and Infectious Disease. 2018; 3(1):30. https://doi.org/10.3390/tropicalmed3010030
Chicago/Turabian StyleRakotondrasoa, Andriniaina, Mohammad Iqbal Issack, Benoît Garin, Fabrice Biot, Eric Valade, Pierre Wattiau, Nicolas Allou, Olivier Belmonte, Jastin Bibi, Erin P. Price, and et al. 2018. "Melioidosis in the Western Indian Ocean and the Importance of Improving Diagnosis, Surveillance, and Molecular Typing" Tropical Medicine and Infectious Disease 3, no. 1: 30. https://doi.org/10.3390/tropicalmed3010030
APA StyleRakotondrasoa, A., Issack, M. I., Garin, B., Biot, F., Valade, E., Wattiau, P., Allou, N., Belmonte, O., Bibi, J., Price, E. P., & Collard, J.-M. (2018). Melioidosis in the Western Indian Ocean and the Importance of Improving Diagnosis, Surveillance, and Molecular Typing. Tropical Medicine and Infectious Disease, 3(1), 30. https://doi.org/10.3390/tropicalmed3010030





