Epidemiology and Spatiotemporal Patterns of Leprosy Detection in the State of Bahia, Brazilian Northeast Region, 2001–2014
Abstract
:1. Introduction
2. Methods
2.1. Study Area, Population and Design
2.2. Data Sources and Variables
2.3. Statistical Analyses
2.3.1. Epidemiological Analysis
2.3.2. Spatial Analyses in the Temporal Sections
3. Results
3.1. Epidemiological Analysis
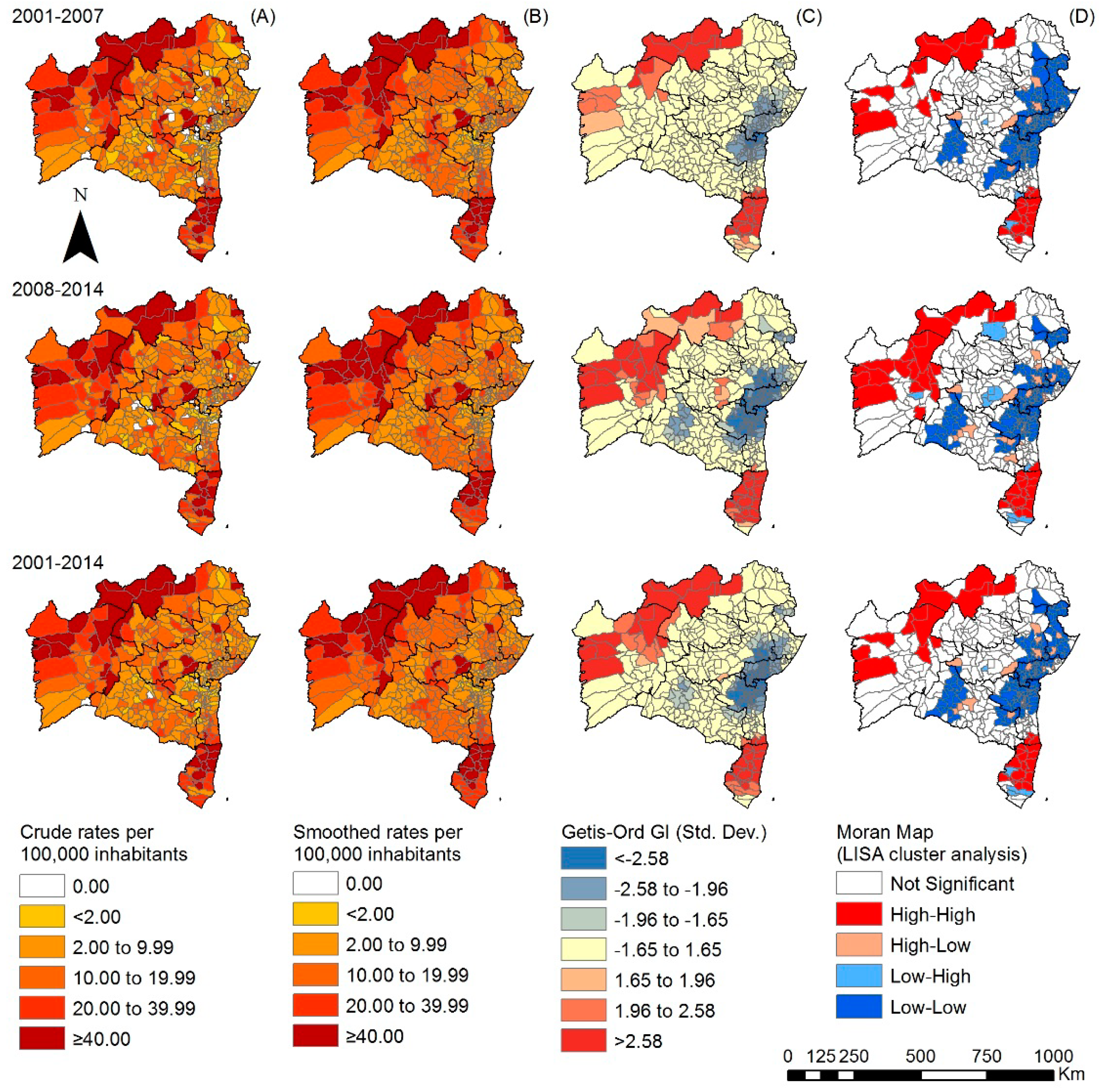
3.2. Spatiotemporal Analyses
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization, Regional Office for South-East Asia; Department of Control of Neglected Tropical Diseases, WHO. Global Leprosy Strategy 2016–2020: Accelerating towards a Leprosy-Free World; WHO SEARO: New Delhi, India, 2016; Available online: http://apps.who.int/iris/bitstream/handle/10665/208824/9789290225096_en.pdf?sequence=14&isAllowed=y (accessed on 8 July 2018).
- Martins-Melo, F.R.; Carneiro, M.; Ramos, A.N., Jr.; Heukelbach, J.; Ribeiro, A.L.P.; Werneck, G.L. The burden of Neglected Tropical Diseases in Brazil, 1990–2016: A subnational analysis from the Global Burden of Disease Study 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006559. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global leprosy update, 2016: Accelerating reduction of disease burden. Wkly. Epidemiol. Rec. 2017, 92, 501–520. [Google Scholar]
- Ministério da Saúde do Brasil, Secretaria de Vigilância em Saúde; Departamento de Vigilância das Doenças Transmissíveis. Diretrizes Para Vigilância, Atenção e Eliminação da Hanseníase Como Problema de Saúde Pública: Manual Técnico-Operacional; Ministério da Saúde: Brasília, Brazil, 2016. Available online: http://portalarquivos2.saude.gov.br/images/pdf/2016/fevereiro/04/diretrizes-eliminacao-hanseniase-4fev16-web.pdf (accessed on 8 July 2018).
- Souza, E.A.; Boigny, R.N.; Ferreira, A.F.; Alencar, C.H.; Oliveira, M.L.W.; Ramos, A.N., Jr. Programmatic vulnerability in leprosy control: Gender-related patterns in Bahia State, Brazil. Cad. Saúde Pública 2018, 34, e00196216. [Google Scholar] [PubMed]
- Romanholo, H.S.B.; Souza, E.A.; Ramos, A.N., Jr.; Kaiser, A.C.G.C.B.; Silva, I.O.D.; Brito, A.L.; Vasconcellos, C. Surveillance of intradomiciliary contacts of leprosy cases: Perspective of the client in a hyperendemic municipality. Rev. Bras. Enferm. 2018, 71, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde do Brasil, Secretaria de Vigilância em Saúde. Boletim Epidemiológico—Hanseníase; Ministério da Saúde: Brasília, Brazil, 2018; Volume 49. Available online: http://portalarquivos2.saude.gov.br/images/pdf/2018/janeiro/31/2018-004-Hanseniase-publicacao.pdf (accessed on 8 July 2018).
- Silva, L.J.D. The concept of space in infectious disease epidemiology. Cad. Saúde Pública 1997, 13, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Pescarini, J.M.; Strina, A.; Nery, J.S.; Skalinski, L.M.; Andrade, K.V.F.; Penna, M.L.F.; Brickley, E.B.; Rodrigues, L.C.; Barreto, M.L.; Penna, G.O. Socioeconomic risk markers of leprosy in high-burden countries: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2018, 12, e0006622. [Google Scholar] [CrossRef] [PubMed]
- Lana, F.C.F.; Pinheiro Amaral, E.; Moura Lanza, F.; Lamounier Lima, P.; Nascimento de Carvalho, A.C.; Gonçalves Diniz, L. [Hansen’s Disease in children under fifteen years-old in Jequitinhonha Valley, Minas Gerais, Brazil]. Rev. Bras. Enferm. 2007, 60, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.C.; Bonfim, C.V.D.; Brito, C.M.G.; Ferreira, A.T.; Gregório, V.R.D.N.; Oliveira, A.L.S.; Portugal, J.L.; Medeiros, Z.M. Spatial analysis of reported new cases and local risk of leprosy in hyper-endemic situation in Northeastern Brazil. Trop. Med. Int. Health. 2018, 23, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística, IBGE. Dados Populacionais; IBGE: Brasília, Brazil, 2014. Available online: https://www.ibge.gov.br/ (accessed on 8 July 2018).
- Alves, R.F.; Silva, R.P.; Ernesto, M.V.; Lima, A.G.B.; Souza, F.M. Gender and health: Men’s care in debate. Psicol. Teor. Prát. 2011, 13, 152–166. [Google Scholar]
- Instituto de Pesquisa Econômica Aplicada. IPEA. Programa das Nações Unidas para o Desenvolvimento, PNUD; Fundação João Pinheiro, FJP. IPEA Data; IPEA: Brasília, Brazil, 2013. Available online: http://www.ipeadata.gov.br/Default.aspx (accessed on 8 July 2018).
- Ministério da Saúde. Histórico de Cobertura da Saúde da Família. Available online: http://dab.saude.gov.br/portaldab/historico_cobertura_sf.php (accessed on 2 September 2016).
- Altman, D.G. Confidence intervals for the number needed to treat. BMJ 1998, 317, 1309–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Environmental Systems Research Institute, ESRI. ArcGIS Desktop: Release 10; ESRI: Redlands, CA, USA, 2011. [Google Scholar]
- Machin, R.; Couto, M.T.; Sibele Nogueira da Silva, G.; Schraiber, L.B.; Gomes, R.; Santos Figueiredo, W.D.; Valença, O.A.; Pinheiro, T.F. Concepts of gender, masculinity and healthcare: A study of primary healthcare professionals. Ciênc. Saúde Coletiva 2011, 16, 4503–4512. [Google Scholar] [CrossRef]
- Ministério da Saúde, Secretaria de Vigilância em Saúde. Alerta Para o Exame Sistemático de Hanseníase na População Masculina e em Idosos; Ministério da Saúde: Brasília, Brasil, 2016. Available online: http://portalarquivos.saude.gov.br/images/pdf/2016/setembro/06/Nota-Informativa-Conjunta-n---01--SAS-e-SVS--para-publica----o.pdf (accessed on 15 April 2018).
- Nobre, M.L.; Illarramendi, X.; Dupnik, K.M.; Hacker, M.D.A.; Nery, J.A.D.C.; Jerônimo, S.M.B.; Sarno, E.N. Multibacillary leprosy by population groups in Brazil: Lessons from an observational study. PLoS Negl. Trop. Dis. 2017, 11, e0005364. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Silveira, F.; Abad-Franch, F. Sex bias in infectious disease epidemiology: Patterns and processes. PLoS ONE 2013, 8, e62390. [Google Scholar] [CrossRef] [PubMed]
- Van Brakel, W.H.; Sihombing, B.; Djarir, H.; Beise, K.; Kusumawardhani, L.; Yulihane, R.; Kurniasari, I.; Kasim, M.; Kesumaningsih, K.I.; Wilder-Smith, A. Disability in people affected by leprosy: The role of impairment, activity, social participation, stigma and discrimination. Glob. Health Action 2012, 5, e18394. [Google Scholar] [CrossRef] [PubMed]
- Lima-Costa, M.F.; Barreto, S.; Giatti, L.; Uchôa, E. Socioeconomic circumstances and health among the Brazilian elderly: A study using data from a National Household Survey. Cad. Saúde Pública 2003, 19, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Assis, I.S.; Arcoverde, M.A.M.; Ramos, A.C.V.; Alves, L.S.; Berra, T.Z.; Arroyo, L.H.; Queiroz, A.A.R.; Santos, D.T.D.; Belchior, A.S.; Alves, J.D.; et al. Social determinants, their relationship with leprosy risk and temporal trends in a tri-border region in Latin America. PLoS Negl. Trop. Dis. 2018, 12, e0006407. [Google Scholar]
- Agondi, R.C.; Rizzo, L.V.; Kalil, J.; Barros, M.T. Immunosenescence. Rev. Bras. Alerg. Imunopatol. 2012, 35, 167–168. [Google Scholar]
- Nogueira, P.S.F.; Marques, M.B.; Coutinho, J.F.V.; Maia, J.C.; Silva, M.J.D.; Moura, E.R.F. Factors associated with the functional capacity of older adults with leprosy. Rev. Bras. Enferm. 2017, 70, 711–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelarigo, J.G.T.; Prado, R.B.R.; Nardi, S.M.T.; Quaggio, C.M.D.P.; Camargo, L.H.S.; Marciano, L.H.S.C. Cognitive impairment, functional independence and depressive symptoms in elderly people with prior history of leprosy. Hansen. Int. 2014, 39, 30–39. [Google Scholar]
- Motta, L.B.D.; Aguiar, A.C.D.; Caldas, C.P. The Family Health Strategy and healthcare for the elderly: Experiences in three Brazilian cities. Cad. Saúde Pública 2011, 27, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Buss, P.M.; Pellegrini Filho, A. Health and its social determinants. Phys. Rev. Saúde Coletiva 2007, 17, 77–93. [Google Scholar] [CrossRef]
- Chor, D.; Lima, C.R.D.A. Epidemiologic aspects of racial inequalities in health in Brazil. Cad. Saúde Pública 2005, 21, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística, IBGE. Unidade da Federação: Bahia; IBGE: Brasília, Brasil, 2014. Available online: https://cidades.ibge.gov.br/brasil/ba (accessed on 8 July 2018).
- Arantes, C.K.; Garcia, M.L.R.; Filipe, M.S.; Nardi, S.M.T.; Paschoal, V.D.A. Health services assessment of early leprosy diagnosis. Epidemiol. Serv.Saúde 2010, 19, 155–164. [Google Scholar]
- Souza, C.; Rodrigues, M. Magnitude, trend and spatialization of leprosy on minors of fifteen years in the state of Bahia, with focus on risk areas: An ecological study. Hygeia 2015, 11, 201–212. [Google Scholar]
- Souza, E.A.; Ferreira, A.F.; Boigny, R.N.; Alencar, C.H.; Heukelbach, J.; Martins-Melo, F.R.; Barbosa, J.C.; Ramos, A.N., Jr. Leprosy and gender in Brazil: Trends in an endemic area of the Northeast region, 2001–2014. Rev. Saúde Pública 2018, 52, 20. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Miranda, W.; Chiaravalloti Neto, F.; Barrozo, L.V. Socio-economic and environmental effects influencing the development of leprosy in Bahia, north-eastern Brazil. Trop. Med. Int. Health 2014, 19, 1504–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, L.R.; Duarte, E.C.; Garcia, L.P. Trends of main indicators of leprosy in Brazilian municipalities with high risk of leprosy transmission, 2001–2012. BMC Infect. Dis. 2016, 16, 472. [Google Scholar] [CrossRef] [PubMed]
- Mauro, V.; Biggeri, M.; Deepak, S.; Trani, J.-F. The effectiveness of community-based rehabilitation programmes: An impact evaluation of a quasi-randomised trial. J. Epidemiol. Community Health 2014, 68, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Alencar, C.H.; Ramos, A.N., Jr.; dos Santos, E.S.; Richter, J.; Heukelbach, J. Clusters of leprosy transmission and of late diagnosis in a highly endemic area in Brazil: Focus on different spatial analysis approaches. Trop. Med. Int. Health 2012, 17, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ayres, J.; França Júnior, I.; Calazans, G.J.; Saletti Filho, H.C.; Czeresnia, D.; Freitas, C. The vulnerability concept and the practices of health: New perspectives and challenges. In Promoção da Saúde: Conceitos, Reflexões, Tendências, 2nd ed.; Czeresnia, D., Freitas, C.M., Eds.; Editora Fiocruz: Rio de Janeiro, Brazil, 2003; pp. 117–139. ISBN 9788575411834. [Google Scholar]
- Assunção, R.M.; Schmertmann, C.P.; Potter, J.E.; Cavenaghi, S.M. Empirical Bayes’ estimation of demographic schedules for small areas. Demography 2005, 42, 537–558. [Google Scholar] [CrossRef] [PubMed]



| Variables | Cases (Total) | Average Cases Annually (2001–2014) n (%) | Detection Rate a | 95% CI | RR | 95% CI | p Value |
|---|---|---|---|---|---|---|---|
| Gender b | |||||||
| Male | 20,132 | 1438 (50.3) | 20.75 | 19.70–21.85 | 1.03 | 0.96–1.11 | 0.3900 |
| Female | 19,922 | 1423 (49.7) | 20.08 | 19.07–21.15 | Ref | - | - |
| Age group (years) b | |||||||
| <15 | 3219 | 230 (8.0) | 5.44 | 4.78–6.19 | Ref | - | - |
| 15–29 | 9330 | 666 (23.3) | 15.87 | 14.70–17.12 | 2.91 | 2.51–3.39 | <0.0001 |
| 30–39 | 7092 | 507 (17.7) | 26.15 | 23.99–28.55 | 4.81 | 4.11–5.62 | <0.0001 |
| 40–49 | 6706 | 479 (16.7) | 32.85 | 30.04–35.93 | 6.03 | 5.16–7.06 | <0.0001 |
| 50–59 | 6052 | 432 (15.1) | 43.86 | 39.89–48.16 | 8.05 | 6.86–9.45 | <0.0001 |
| 60–69 | 4015 | 287 (10.0) | 44.30 | 39.49–49.76 | 8.14 | 6.85–9.68 | <0.0001 |
| ≥70 | 3640 | 260 (9.1) | 46.03 | 40.76–51.97 | 8.45 | 7.08–10.09 | <0.0001 |
| Ethnicity b | |||||||
| Caucasian | 6914 | 494 (20.1) | 16.10 | 14.74–17.58 | Ref | - | - |
| Afro-Brazilian | 6188 | 442 (18.0) | 22.22 | 20.24–24.39 | 1.38 | 1.33–1.43 | <0.0001 |
| Asian | 345 | 25 (1.0) | 26.97 | 18.50–40.40 | 1.68 | 1.50–1.87 | <0.0001 |
| Mixed/Pardo-Brazilian | 20,799 | 1486 (60.5) | 19.15 | 18.20–20.20 | 1.19 | 1.16–1.22 | <0.0001 |
| Amerindian | 156 | 11 (0.5) | 18.48 | 10.20–32.70 | 1.15 | 1.00–1.35 | 0.0881 |
| City size (by inhabitants) | |||||||
| Small (<100,000) | 21,348 | 1525 (53.3) | 18.10 | 17.21–19.03 | 1.34 | 1.21–1.49 | <0.0001 |
| Medium (100,000–500,000) | 12,279 | 877 (30.7) | 37.66 | 35.25–40.23 | 2.80 | 2.50–3.13 | <0.0001 |
| Large (>500,000) | 6433 | 460 (16.1) | 13.45 | 12.29–14.75 | Ref | - | - |
| Residing in the state capital | |||||||
| Yes | 4962 | 354 (12.4) | 12.96 | 11.66–14.36 | Ref | - | - |
| No | 35,098 | 2507 (87.6) | 22.22 | 21.37–23.11 | 1.72 | 1.54–1.92 | <0.0001 |
| Region b | |||||||
| North | 7916 | 565 (19.8) | 55.65 | 51.21–60.38 | 6.18 | 4.85–7.88 | <0.0001 |
| Northeast | 1030 | 74 (2.6) | 8.93 | 7.16–11.28 | Ref | - | - |
| South | 2791 | 199 (7.0) | 11.87 | 10.31–13.62 | 1.32 | 1.01–1.72 | 0.0422 |
| South-west | 2635 | 188 (6.6) | 10.76 | 9.32–12.40 | 1.20 | 0.91–1.57 | 0.1912 |
| East | 7677 | 548 (19.2) | 12.27 | 11.28–13.33 | 1.36 | 1.07–1.74 | 0.0121 |
| Central East | 4457 | 318 (11.1) | 15.37 | 13.76–17.13 | 1.71 | 1.33–2.20 | <0.0001 |
| West | 5576 | 398 (13.9) | 47.67 | 43.18–52.55 | 5.30 | 4.13–6.79 | <0.0001 |
| Far south | 6252 | 447 (15.6) | 58.91 | 53.75–64.69 | 6.56 | 5.13–8.39 | <0.0001 |
| Central North | 1725 | 123 (4.3) | 15.98 | 13.40–19.00 | 1.78 | 1.33–2.37 | 0.0001 |
| Total | 40,060 | 2861 (100.0) | 20.41 | 19.68–21.17 | - | - | - |
| Variables | Cases | Average Annual (2001–2014) n (%) | Detection Rate in Children a | 95% CI | RR | 95% CI | p Value |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 1585 | 113 (49.2) | 5.51 | 4.59–6.63 | Ref | - | - |
| Female | 1634 | 117 (50.8) | 5.90 | 4.92–7.07 | 1.35 | 1.22–1.50 | <0.0001 |
| Ethnicity b | |||||||
| Caucasian | 448 | 32 (16.4) | 3.51 | 2.49–4.95 | Ref | - | - |
| Afro-Brazilian | 475 | 34 (17.4) | 7.25 | 5.19–10.12 | 2.06 | 1.81–2.35 | <0.0001 |
| Asian | 25 | 2 (0.9) | 8.05 | 2.21–29.34 | 2.05 | 1.37–3.06 | 0.0005 |
| Mixed/Pardo-Brazilian | 1767 | 126 (64.6) | 5.15 | 4.33–6.14 | 1.47 | 1.33–1.63 | <0.0001 |
| Amerindian | 21 | 2 (0.8) | 12.38 | 3.40–45.13 | 2.65 | 1.71–4.10 | <0.0001 |
| City size (inhabitants) | |||||||
| Small (<100,000) | 1735 | 124 (53.9) | 4.89 | 4.10–5.83 | 1.76 | 1.36–2.28 | <0.0001 |
| Medium (100,000–500,000) | 970 | 69 (30.1) | 10.42 | 8.24–13.19 | 4.70 | 3.65–6.06 | <0.0001 |
| Large (>500,000) | 514 | 37 (16.0) | 4.43 | 3.22–6.11 | 9.85 | 7.71–12.59 | <0.0001 |
| Residing in the state capital | |||||||
| Yes | 422 | 30 (13.1) | 4.39 | 3.08–6.27 | Ref | - | - |
| No | 2797 | 200 (86.9) | 5.97 | 5.20-6.86 | 1.35 | 1.22–1.50 | <0.0001 |
| Region | |||||||
| North | 731 | 52 (22.7) | 16.70 | 12.74–21.89 | 8.25 | 6.47–10.53 | <0.0001 |
| Northeast | 71 | 5 (2.2) | 2.00 | 0.86–4.69 | Ref | - | - |
| South | 197 | 14 (6.1) | 2.84 | 1.69–4.77 | 1.40 | 1.07–1.84 | 0.0141 |
| South-west | 120 | 9 (3.7) | 1.85 | 0.97–3.52 | 0.87 | 0.65–1.16 | 0.3442 |
| East | 683 | 49 (21.2) | 4.30 | 3.25–5.68 | 2.11 | 1.65–2.69 | <0.0001 |
| Central East | 308 | 22 (9.6) | 3.58 | 2.36–5.41 | 1.76 | 1.36–2.28 | <0.0001 |
| West | 362 | 26 (11.2) | 9.60 | 6.56–14.07 | 4.70 | 3.65–6.06 | <0.0001 |
| Far south | 659 | 47 (20.5) | 19.99 | 15.03–26.58 | 9.85 | 7.72–12.59 | <0.0001 |
| Central North | 88 | 6 (2.7) | 2.59 | 1.19–5.65 | 1.33 | 0.98–1.82 | 0.0706 |
| Total | 3219 | 230 (100.0) | 5.71 | 5.02–6.49 | - | - | - |
| Variables | Cases | Average Annual (2001–2014) n (%) | Detection Rate a | 95% CI | RR | 95% CI | p Value |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 1351 | 97 (70.3) | 14.00 | 11.48–17.08 | 2.43 | 2.20–2.67 | <0.0001 |
| Female | 570 | 41 (29.7) | 5.80 | 4.28–7.87 | Ref | - | - |
| Age group (years) | |||||||
| <15 | 56 | 4 (2.9) | 0.98 | 0.38–2.52 | Ref | - | - |
| 15–29 | 300 | 21 (15.6) | 5.00 | 3.27–7.64 | 5.39 | 4.05–7.17 | <0.0001 |
| 30–39 | 310 | 22 (16.1) | 11.40 | 7.53–17.26 | 12.07 | 9.08–16.05 | <0.0001 |
| 40–49 | 316 | 23 (16.4) | 15.80 | 10.53–23.71 | 16.35 | 12.31–21.73 | <0.0001 |
| 50–59 | 323 | 23 (16.8) | 23.30 | 15.53–34.96 | 24.73 | 18.62–32.84 | <0.0001 |
| 60–69 | 286 | 20 (14.9) | 30.90 | 20.00–47.73 | 33.33 | 25.03–44.39 | <0.0001 |
| ≥70 | 330 | 24 (17.2) | 42.50 | 28.56–63.24 | 44.08 | 33.21–58.51 | <0.0001 |
| Ethnicity b | |||||||
| Caucasian | 342 | 24 (20.0) | 7.80 | 5.24–11.61 | Ref | - | - |
| Afro-Brazilian | 353 | 25 (20.7) | 12.60 | 8.54–18.60 | 1.59 | 1.37–1.85 | <0.0001 |
| Asian | 15 | 1 (0.9) | 10.90 | 1.92–61.74 | 1.47 | 0.88–2.47 | 0.1420 |
| Mixed/Pardo-Brazilian | 986 | 70 (57.7) | 9.00 | 7.12–11.37 | 1.14 | 1.00–1.29 | 0.0364 |
| Amerindian | 13 | 1 (0.8) | 16.60 | 2.93–94.02 | 1.93 | 1.11–3.37 | 0.0196 |
| City size (inhabitants) | |||||||
| Small (<100,000) | 974 | 70 (50.7) | 8.30 | 6.57–10.49 | 1.16 | 1.02–1.31 | 0.0222 |
| Medium (100,000–500,000) | 605 | 43 (31.5) | 18.50 | 13.74–24.92 | 2.60 | 2.27–2.96 | <0.0001 |
| Large (>500,000) | 342 | 24 (17.8) | 7.00 | 4.70–10.42 | Ref | - | - |
| Residing in the state capital | |||||||
| Yes | 254 | 18 (13.2) | 6.60 | 4.18–10.43 | Ref | - | - |
| No | 1667 | 119 (86.8) | 10.50 | 8.78–12.56 | 1.59 | 1.39–1.82 | <0.0001 |
| Region | |||||||
| North | 322 | 23 (16.8) | 22.60 | 15.06–32.91 | 5.02 | 3.74–6.73 | <0.0001 |
| Northeast | 52 | 4 (2.7) | 4.90 | 1.91–12.60 | Ref | - | - |
| South | 174 | 12 (9.1) | 7.10 | 4.06–12.41 | 1.64 | 1.20–2.24 | 0.0017 |
| South-west | 187 | 13 (9.7) | 7.40 | 4.33–12.66 | 1.69 | 1.25–2.30 | 0.0008 |
| East | 387 | 28 (20.1) | 6.30 | 4.36–9.11 | 1.37 | 1.03–1.83 | 0.0324 |
| Central East | 220 | 16 (11.5) | 7.70 | 4.74–12.51 | 1.68 | 1.24–2.28 | 0.0007 |
| West | 121 | 9 (6.3) | 10.80 | 5.68–20.53 | 2.29 | 1.66–3.17 | <0.0001 |
| Far south | 357 | 26 (18.6) | 34.30 | 23.41–50.26 | 7.46 | 5.58–9.98 | <0.0001 |
| Central North | 101 | 7 (5.3) | 9.10 | 4.41–18.78 | 2.07 | 1.49–2.90 | <0.0001 |
| Total | 1921 | 137 (100.0) | 9.80 | 8.29–11.58 | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim de Souza, E.; Fuentes Ferreira, A.; Heukelbach, J.; Nzundu Boigny, R.; Alencar, C.H.; Novaes Ramos, A., Jr. Epidemiology and Spatiotemporal Patterns of Leprosy Detection in the State of Bahia, Brazilian Northeast Region, 2001–2014. Trop. Med. Infect. Dis. 2018, 3, 79. https://doi.org/10.3390/tropicalmed3030079
Amorim de Souza E, Fuentes Ferreira A, Heukelbach J, Nzundu Boigny R, Alencar CH, Novaes Ramos A Jr. Epidemiology and Spatiotemporal Patterns of Leprosy Detection in the State of Bahia, Brazilian Northeast Region, 2001–2014. Tropical Medicine and Infectious Disease. 2018; 3(3):79. https://doi.org/10.3390/tropicalmed3030079
Chicago/Turabian StyleAmorim de Souza, Eliana, Anderson Fuentes Ferreira, Jorg Heukelbach, Reagan Nzundu Boigny, Carlos Henrique Alencar, and Alberto Novaes Ramos, Jr. 2018. "Epidemiology and Spatiotemporal Patterns of Leprosy Detection in the State of Bahia, Brazilian Northeast Region, 2001–2014" Tropical Medicine and Infectious Disease 3, no. 3: 79. https://doi.org/10.3390/tropicalmed3030079
APA StyleAmorim de Souza, E., Fuentes Ferreira, A., Heukelbach, J., Nzundu Boigny, R., Alencar, C. H., & Novaes Ramos, A., Jr. (2018). Epidemiology and Spatiotemporal Patterns of Leprosy Detection in the State of Bahia, Brazilian Northeast Region, 2001–2014. Tropical Medicine and Infectious Disease, 3(3), 79. https://doi.org/10.3390/tropicalmed3030079







