Effects of Transverse Friction Massage on the Electromechanical Delay Components and Fractal Dimension of Surface Electromyography in Quadriceps Muscles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Setup
2.3. Experimental Procedure
2.4. Transverse Friction Massage Therapy
2.5. Signal Processing
2.5.1. Time Delays
2.5.2. Root Mean Square
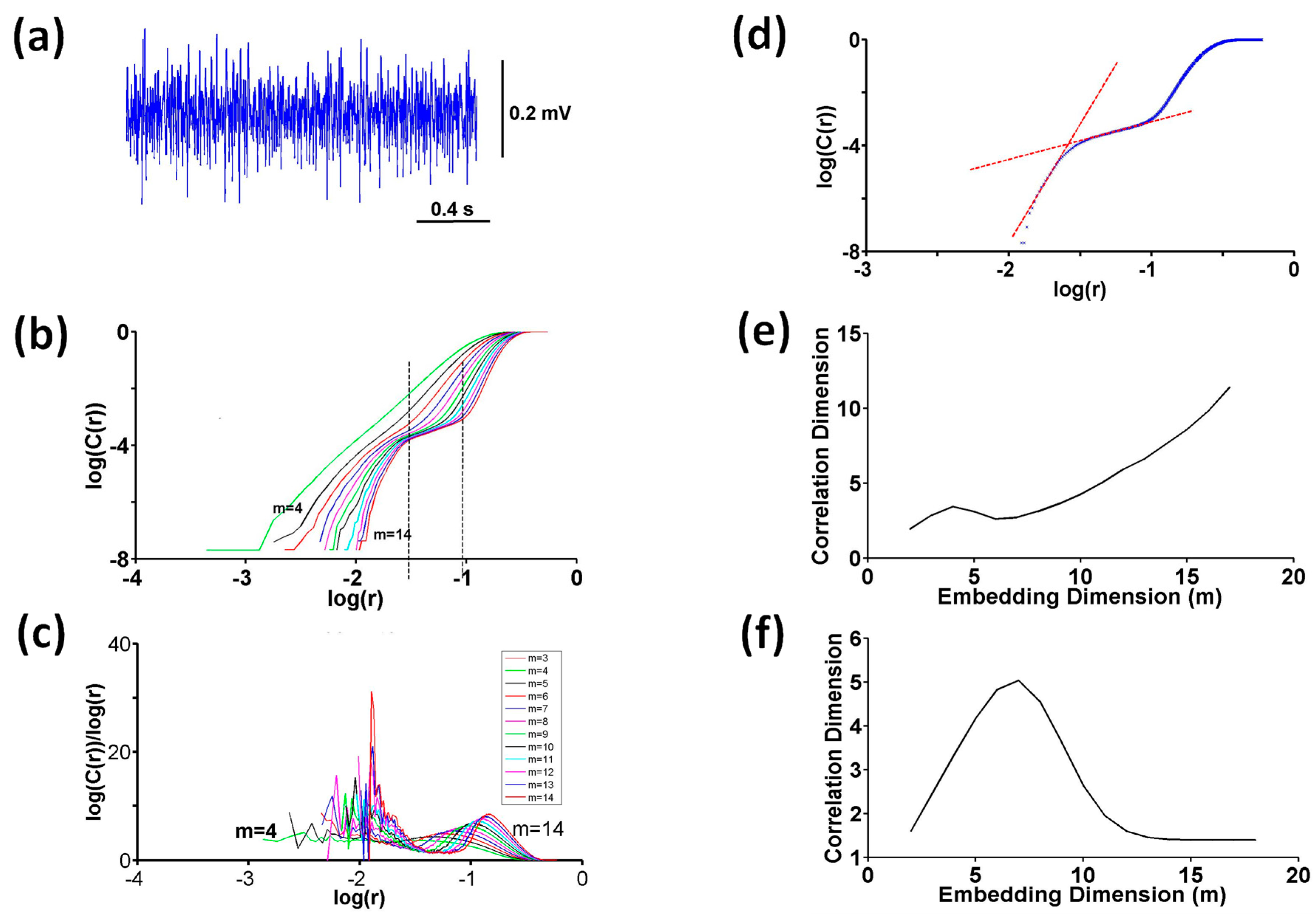
2.5.3. Correlation Dimension
Computation of Slope
Testing Nonlinearity
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AP | Action potential |
| EMD | Electromechanical delay |
| EMG | Electromyography |
| FD | Fractal dimension |
| m | Embedding dimension |
| MMG | Mechanomyography |
| MTJ | Musculotendinous junction |
| MUAPs | Motor unit action potentials |
| MUs | Motor units |
| MVIC | Maximum voluntary isometric contraction |
| RF | Rectus femoris muscle |
| RMS | Root mean square |
| ROM | Joint range of motion |
| TFM | Transverse friction massage |
| VM | Vastus medialis muscle |
| Δt(EMG–MMG) | Time delay between EMG and MMG |
| Δt(MMG–Force) | Time delay between MMG and force |
| Δt(EMG–Force) | Time delay between EMG and force |
References
- Sobeck, C.; Lenk, L.; Knipper, S.; Rhoda, A.; Stickler, L.; Stephenson, P. The effectiveness of functional massage on pain and range of motion measurements in patients with orthopedic impairments of the extremities. Int. Musculoskelet. Med. 2016, 38, 21–25. [Google Scholar] [CrossRef]
- Hernandez-reif, M.; Field, T.; Krasnegor, J.; Theakston, H. Lower back pain is reduced and range of motion increased after massage therapy. Int. J. Neurosci. 2001, 106, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.R.; da Silva Novaes, J.; Cavanaugh, M.T.; Hoogenboom, B.J.; Steele, J.; Vingren, J.L.; Škarabot, J. Quadriceps foam rolling and rolling massage increases hip flexion and extension passive range-of-motion. J. Bodyw. Mov. Ther. 2019, 23, 575–580. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, G.J.; Young, W.B.; Behm, D.G. Acute effects of two massage techniques on ankle joint flexibility and power of the plantar flexors. J. Sports Sci. Med. 2007, 6, 498–504. [Google Scholar] [PubMed]
- Boguszewski, D.; Szkoda, S.; Adamczyk, J.G.; Białoszewski, D. Sports massage therapy on the reduction of delayed onset muscle soreness of the quadriceps femoris. Hum. Mov. 2014, 15, 234–237. [Google Scholar] [CrossRef]
- Madoni, S.N.; Costa, P.B.; Coburn, J.W.; Galpin, A.J. Effects of foam rolling on range of motion, peak torque, muscle activation, and the hamstrings-to-quadriceps strength ratios. J. Strength Cond. Res. 2018, 32, 1821–1830. [Google Scholar] [CrossRef]
- Lee, H.M.; Wu, S.K.; You, J.Y. Quantitative application of transverse friction massage and its neurological effects on flexor carpi radialis. Man. Ther. 2009, 14, 501–507. [Google Scholar] [CrossRef]
- Begovic, H.; Zhou, G.Q.; Schuster, S.; Zheng, Y.P. The neuromotor effects of transverse friction massage. Man. Ther. 2016, 26, 70–76. [Google Scholar] [CrossRef]
- Behm, D.G.; Peach, A.; Maddigan, M.; Aboodarda, S.J.; DiSanto, M.C.; Button, D.C.; Maffiuletti, N.A. Massage and stretching reduce spinal reflex excitability without affecting twitch contractile properties. J. Electromyogr. Kinesiol. 2013, 23, 1215–1221. [Google Scholar] [CrossRef]
- Huang, S.Y.; Di Santo, M.; Wadden, K.P.; Cappa, D.F.; Alkanani, T.; Behm, D.G. Short duration massage at the hamstrings musculotendinous junction induces greater range of motion. J. Strength Cond. Res. 2010, 24, 1917–1924. [Google Scholar] [CrossRef]
- Akazawa, N.; Okawa, N.; Kishi, M.; Nakatani, K.; Nishikawa, K.; Tokumura, D.; Matsui, Y.; Moriyama, H. Effects of long-term self-massage at the musculotendinous junction on hamstring extensibility, stiffness, stretch tolerance, and structural indices: A randomized controlled trial. Phys. Ther. Sport 2016, 21, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Thomson, D.; Gupta, A.; Arundell, J.; Crosbie, J. Deep soft-tissue massage applied to healthy calf muscle has no effect on passive mechanical properties: A randomized, single-blind, cross-over study. BMC. Sports Sci. Med. Rehabil. 2015, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, P.W.; Chua, Y.H.; Kawabata, M.; Burns, S.F.; Cai, C. Effect of post-exercise massage on passive muscle stiffness measured using myotonometry—A Double-Blind Study. J. Sports Sci. Med. 2018, 17, 599–606. [Google Scholar] [PubMed]
- Weerapong, P.; Hume, P.A.; Kolt, G.S. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2015, 35, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Sefton, J.E.M.; Yarar, C.; Carpenter, D.M.; Berry, J.W. Physiological and clinical changes after therapeutic massage of the neck and shoulders. Man. Ther. 2011, 16, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Eriksson Crommert, M.; Lacourpaille, L.; Heales, L.J.; Tucker, K.; Hug, F. Massage induces an immediate, albeit short-term, reduction in muscle stiffness. Scand. J. Med. Sci. Sports 2015, 25, e490–e496. [Google Scholar] [CrossRef] [PubMed]
- Arai, R.; Kuruma, H. Ineffectiveness of muscle reaction time and torque of quadriceps in treating hamstrings using deep transverse friction massage. Ann. Phys. Rehabil. Med. 2018, 61, e472. [Google Scholar] [CrossRef]
- Cè, E.; Longo, S.; McCoy, E.; Bisconti, A.V.; Tironi, D.; Limonta, E.; Rampichini, S.; Rabuffetti, M.; Esposito, F. Acute effects of direct inhibitory pressure over the biceps brachii myotendinous junction on skeletal muscle activation and force output. J. Electromyogr. Kinesiol. 2017, 37, 25–34. [Google Scholar] [CrossRef]
- Lei, M.; Wang, Z.; Feng, Z. Detecting nonlinearity of action surface EMG signal. Phys. Lett. A 2001, 290, 297–303. [Google Scholar] [CrossRef]
- Arjunan, S.P.; Kumar, D.K. Computation of fractal features based on the fractal analysis of surface Electromyogram to estimate force of contraction of different muscles. Comp. Method. Biomec. 2014, 17, 210–216. [Google Scholar] [CrossRef]
- Phinyomark, A.; Quaine, F.; Charbonnier, S.; Serviere, C.; Tarpin-Bernard, F.; Laurillau, Y. EMG feature evaluation for improving myoelectric pattern recognition robustness. Expert Syst. Appl. 2013, 40, 4832–4840. [Google Scholar] [CrossRef]
- Chen, W.; Zhuang, J.; Yu, W.; Wang, Z. Measuring complexity using FuzzyEn, ApEn, and SampEn. Med. Eng. Phys. 2009, 31, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Y.; Wang, J. The analysis of surface EMG signals with the wavelet-based correlation dimension method. Comput. Math. Methods Med. 2014, 2014, 284308. [Google Scholar] [CrossRef] [Green Version]
- Ancillao, A.; Galli, M.; Rigoldi, C.; Albertini, G. Linear correlation between fractal dimension of surface EMG signal from Rectus Femoris and height of vertical jump. Chaos Soliton Fractals 2014, 66, 120–126. [Google Scholar] [CrossRef]
- Rissanen, S.M.; Kankaanpää, M.; Meigal, A.; Tarvainen, M.P.; Nuutinen, J.; Tarkka, I.M.; Airaksinen, O.; Karjalainen, P.A. Surface EMG and acceleration signals in Parkinson’s disease: Feature extraction and cluster analysis. Med. Biol. Eng. Comput. 2008, 46, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.R.; Camic, C.L. Fatigue-mediated loss of complexity is contraction-type dependent in vastus lateralis electromyographic signals. Sports 2019, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Meigal, A.I.; Rissanen, S.; Tarvainen, M.P.; Karjalainen, P.A.; Iudina-Vassel, I.A.; Airaksinen, O.; Kankaanpää, M. Novel parameters of surface EMG in patients with Parkinson’s disease and healthy young and old controls. J. Electromyogr. Kinesiol. 2009, 19, e206–e213. [Google Scholar] [CrossRef]
- Boccia, G.; Dardanello, D.; Beretta-Piccoli, M.; Cescon, C.; Coratella, G.; Rinaldo, N.; Barbero, M.; Lanza, M.; Schena, F.; Rainoldi, A. Muscle fiber conduction velocity and fractal dimension of EMG during fatiguing contraction of young and elderly active men. Physiol. Meas. 2016, 37, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beretta-Piccoli, M.; D’Antona, G.; Barbero, M.; Fisher, B.; Dieli-Conwright, C.M.; Clijsen, R.; Cescon, C. Evaluation of central and peripheral fatigue in the quadriceps using fractal dimension and conduction velocity in young females. PLoS ONE 2015, 4, e0123921. [Google Scholar] [CrossRef]
- Beretta-Piccoli, M.; Calanni, L.; Negro, M.; Ricci, G.; Bettio, C.; Barbero, M.; Berardinelli, A.; Siciliano, G.; Tupler, R.; Soldini, E.; et al. Increased resistance towards fatigability in patients with facioscapulohumeral muscular dystrophy. Eur. J. Appl. Physiol. 2021, 121, 1617–1629. [Google Scholar] [CrossRef]
- Ozturk, N.; Begovic, H.; Demir, P.; Yagcioglu, S.; Can, F. Effects of stretching on the fractal dimension of rectus femoris and vastus medialis muscles. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1–3 June 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Rampichini, S.; Vieira, T.M.; Castiglioni, P.; Merati, G. Complexity analysis of surface electromyography for assessing the myoelectric manifestation of muscle fatigue: A review. Entropy 2020, 22, 529. [Google Scholar] [CrossRef]
- Arjunan, S.P.; Kumar, D.K. Fractals and electromyograms. In The Fractal Geometry of the Brain; Di Ieva, A., Ed.; Springer Series in Computational Neuroscience; Springer: New York, NY, USA, 2016; pp. 445–455. [Google Scholar] [CrossRef]
- Begovic, H.; Can, F.; Yagcioglu, S.; Ozturk, N. Passive stretching-induced changes detected during voluntary muscle contractions. Physiother. Theory Pract. 2020, 36, 731–740. [Google Scholar] [CrossRef]
- Ozturk, N.; Begovic, H.; Demir, P.; Yagcioglu, S.; Can, F. Rectus Femoris and Vastus Medialis Muscles Exhibit Different Dynamics in Processing of Isometric Voluntary Contractions: A Fractal Analysis Study. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), İstanbul, Turkey, 26–28 June 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Grassberger, P.; Procaccia, I. Characterization of strange attractors. Phys. Rev. Lett. 1983, 50, 346–349. [Google Scholar] [CrossRef]
- Grassberger, P.; Procaccia, I. Measuring the strangeness of strange attractors. Phys. D 1983, 9, 189–208. [Google Scholar] [CrossRef]
- Grassberger, P. Grassberger-Procaccia algorithm. Scholarpedia 2007, 2, 3043. [Google Scholar] [CrossRef]
- Takens, F. Detecting strange attractors in turbulence. In Dynamical Systems and Turbulence, Warwick 1980: Proceedings of a Symposium Held at the University of Warwick 1979/80; Lecture Notes in Mathematics; Rand, D.A., Young, L.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1980; Volume 898, pp. 366–381. [Google Scholar]
- Henry, B.; Lovell, N.; Camacho, F. Nonlinear dynamics time series analysis. Nonlinear Biomed. Signal Process. Dyn. Anal. Model. 2000, 2, 1–39. [Google Scholar]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 14–37. [Google Scholar]
- Lei, M.; Meng, G. Nonlinear analysis of surface EMG signals. In Computational Intelligence in Electromyography Analysis—A Perspective on Current Applications and Future Challenges; Naik, G.R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 120–171. [Google Scholar] [CrossRef] [Green Version]
- Theiler, J.; Eubank, S.; Longtin, A.; Galdrikian, B.; Farmer, J. Testing for nonlinearity in time series: The method of surrogate data. Phys. D 1992, 58, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Zunino, L.; Kulp, C.W. Detecting nonlinearity in short and noisy time series using the permutation entropy. Phys. Lett. A 2017, 381, 3627–3635. [Google Scholar] [CrossRef]
- Esposito, F.; Limonta, E.; Ce, E. Passive stretching effects on electromechanical delay and time course of recovery in human skeletal muscle: New insights from an electromyographic and mechanomyographic combined approach. Eur. J. Appl. Physiol. 2011, 111, 485–495. [Google Scholar] [CrossRef]
- Petitjean, M.; Maton, B.; Fourment, A. Summation of elementary phonomyograms during isometric twitches in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Housh, T.J.; Hill, E.C.; Keller, J.L.; Johnson, G.O.; Schmidt, R.J. Effects of intensity on muscle-specific voluntary electromechanical delay and relaxation electromechanical delay. J. Sports Sci. 2018, 36, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Sözen, H.; Cè, E.; Bisconti, A.V.; Rampichini, S.; Longo, S.; Coratella, G.; Shokohyar, S.; Doria, C.; Borrelli, M.; Limonta, E.; et al. Differences in electromechanical delay components induced by sex, age and physical activity level: New insights from a combined electromyographic, mechanomyographic and force approach. Sport Sci. Health 2019, 15, 623–633. [Google Scholar] [CrossRef]
- Chakraborty, M.; Parbat, D. Fractals, chaos and entropy analysis to obtain parametric features of surface electromyography signals during dynamic contraction of biceps muscles under varying load. In Proceedings of the 2017 2nd International Conference for Convergence in Technology (I2CT), Mumbai, India, 21 December 2017; pp. 222–229. [Google Scholar] [CrossRef]
- Beretta-Piccoli, M.; Boccia, G.; Ponti, T.; Clijsen, R.; Barbero, M.; Cescon, C. Relationship between isometric muscle force and fractal dimension of surface electromyogram. Biomed. Res. Int. 2018, 2018, 5373846. [Google Scholar] [CrossRef] [Green Version]
- Marri, K.; Swaminathan, R. Analysis of Biceps Brachii Muscles in Dynamic Contraction Using sEMG Signals and Multifractal DMA Algorithm. Int. J. Signal Process. Syst. 2016, 4, 79–85. [Google Scholar] [CrossRef]
- Phinyomark, A.; Phukpattaranont, P.; Limsakul, C. Feature reduction and selection for EMG signal classification. Expert Syst. Appl. 2012, 39, 7420–7431. [Google Scholar] [CrossRef]
- Gitter, J.A.; Czerniecki, M.J. Fractal analysis of the electromyographic interference pattern. J. Neurosci. Meth. 1995, 58, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Dardanello, D.; Rainoldi, A.; Boccia, G. Motor unit firing rates and synchronisation affect the fractal dimension of simulated surface electromyogram during isometric/isotonic contraction of vastus lateralis muscle. Med. Eng. Phys. 2016, 38, 1530–1533. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Xiao, S. Fractal dimension of surface emg and its determinants. In Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, ‘Magnificent Milestones and Emerging Opportunities in Medical Engineering’ (Cat. No.97CH36136). Chicago, IL, USA, 30 October–2 November 1997; Volume 4, pp. 1570–1573. [Google Scholar] [CrossRef]
- De Luca, C.J. Myoelectrical manifestations of localized muscular fatigue in humans. Crit. Rev. Biomed. Eng. 1984, 11, 251–279. [Google Scholar]
- Pincivero, D.M.; Green, R.C.; Mark, J.D.; Campy, R.M. Gender and muscle differences in EMG amplitude and median frequency, and variability during maximal voluntary contractions of the quadriceps femoris. J. Electromyogr. Kinesiol. 2000, 10, 189–196. [Google Scholar] [CrossRef]
- Lindstrom, L.H.; Magnusson, R.I. Interpretation of myoelectric power spectra: A model and its applications. Proc. IEEE 1977, 65, 653–662. [Google Scholar] [CrossRef]
- Stulen, F.B.; De Luca, C.J. Frequency parameters of the myoelectric signal as a measure of muscle conduction velocity. IEEE Trans. Biomed. Eng. 1981, 7, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjunan, S.P.; Kumar, D.K.; Wheeler, K.; Shimada, H. Spectral properties of surface electromyogram signal and change in muscle conduction velocity during isometric muscle contraction. SIViP 2015, 9, 261–266. [Google Scholar] [CrossRef]
- Bigland-Ritchie, B.; Donovan, E.F.; Roussos, C.S. Conduction velocity and EMG power spectrum changes in fatigue of sustained maximal efforts. J. Appl. Physiol. 1981, 51, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.M.; Vaughan, C.L.; Nolan, P.J.; O’Malley, M.J. Spectral compression of the electromyographic signal due to decreasing muscle fiber conduction velocity. IEEE Trans. Rehabil. Eng. 2000, 8, 353–361. [Google Scholar] [CrossRef]
- Filligoi, G. Chaos theory and sEMG. In Cyber Journals: Multidisciplinary Journals in Science and Technology, Journal of Selected Areas in Bioengineering (JSAB); January Edition; 2011; pp. 143–154. Available online: http://www.cyberjournals.com/Papers/Jan2011/13.pdf (accessed on 7 August 2023).
- Webber, C.L., Jr.; Zbilut, J.P. Recurrence quantification analysis of nonlinear dynamical systems. Tutor. Contemp. Nonlinear Methods Behav. Sci. 2005, 94, 26–94. [Google Scholar]
- Kantz, H.; Schreiber, T. Dimension estimates and physiological data. Chaos 1995, 5, 143–154. [Google Scholar] [CrossRef]
- Schreiber, T.; Kantz, H. Noise in chaotic data: Diagnosis and treatment. Chaos 1995, 5, 133–142. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturk, N.; Begovic, H.; Demir, P.; Can, F.; Yagcioglu, S. Effects of Transverse Friction Massage on the Electromechanical Delay Components and Fractal Dimension of Surface Electromyography in Quadriceps Muscles. Fractal Fract. 2023, 7, 620. https://doi.org/10.3390/fractalfract7080620
Ozturk N, Begovic H, Demir P, Can F, Yagcioglu S. Effects of Transverse Friction Massage on the Electromechanical Delay Components and Fractal Dimension of Surface Electromyography in Quadriceps Muscles. Fractal and Fractional. 2023; 7(8):620. https://doi.org/10.3390/fractalfract7080620
Chicago/Turabian StyleOzturk, Necla, Haris Begovic, Pinar Demir, Filiz Can, and Suha Yagcioglu. 2023. "Effects of Transverse Friction Massage on the Electromechanical Delay Components and Fractal Dimension of Surface Electromyography in Quadriceps Muscles" Fractal and Fractional 7, no. 8: 620. https://doi.org/10.3390/fractalfract7080620




