Review on Detection Methods of Nitrogen Species in Air, Soil and Water
Abstract
:1. Introduction
2. Species of Nitrogen in Nature
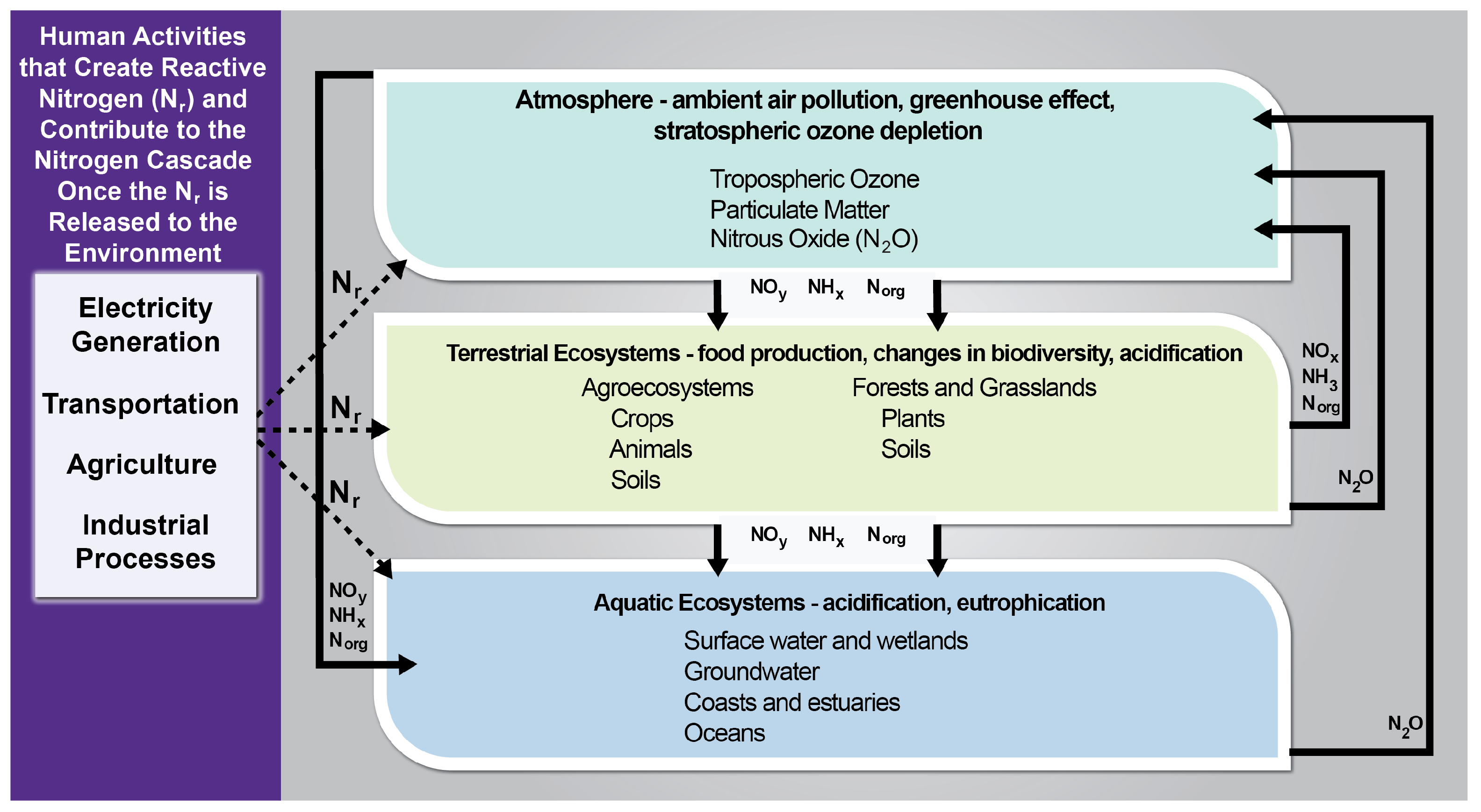
3. Nitrogen Species Detection in Air
3.1. Electrochemical Sensors for Nitrogen Species Detection in Air
Metal Oxide Semiconductors (MOS)
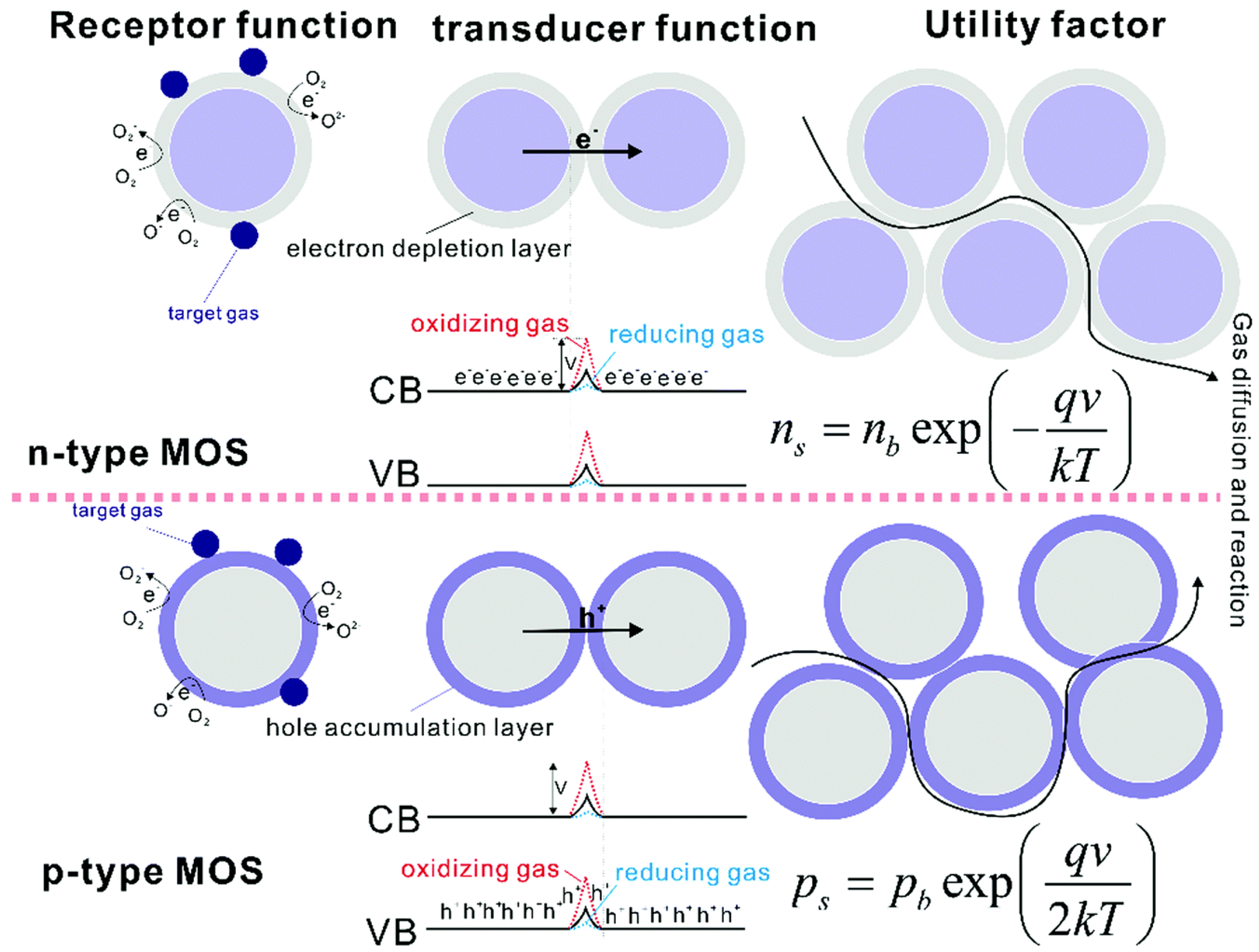
3.2. Optical Sensors for Nitrogen Species Detection in Air
3.2.1. Non-Dispersive InfraRed (NDIR)
3.2.2. Satellite Remote Sensing
4. Nitrogen Species Detection in Soil
4.1. Electrochemical Sensors for Nitrogen Species Detection in Soil
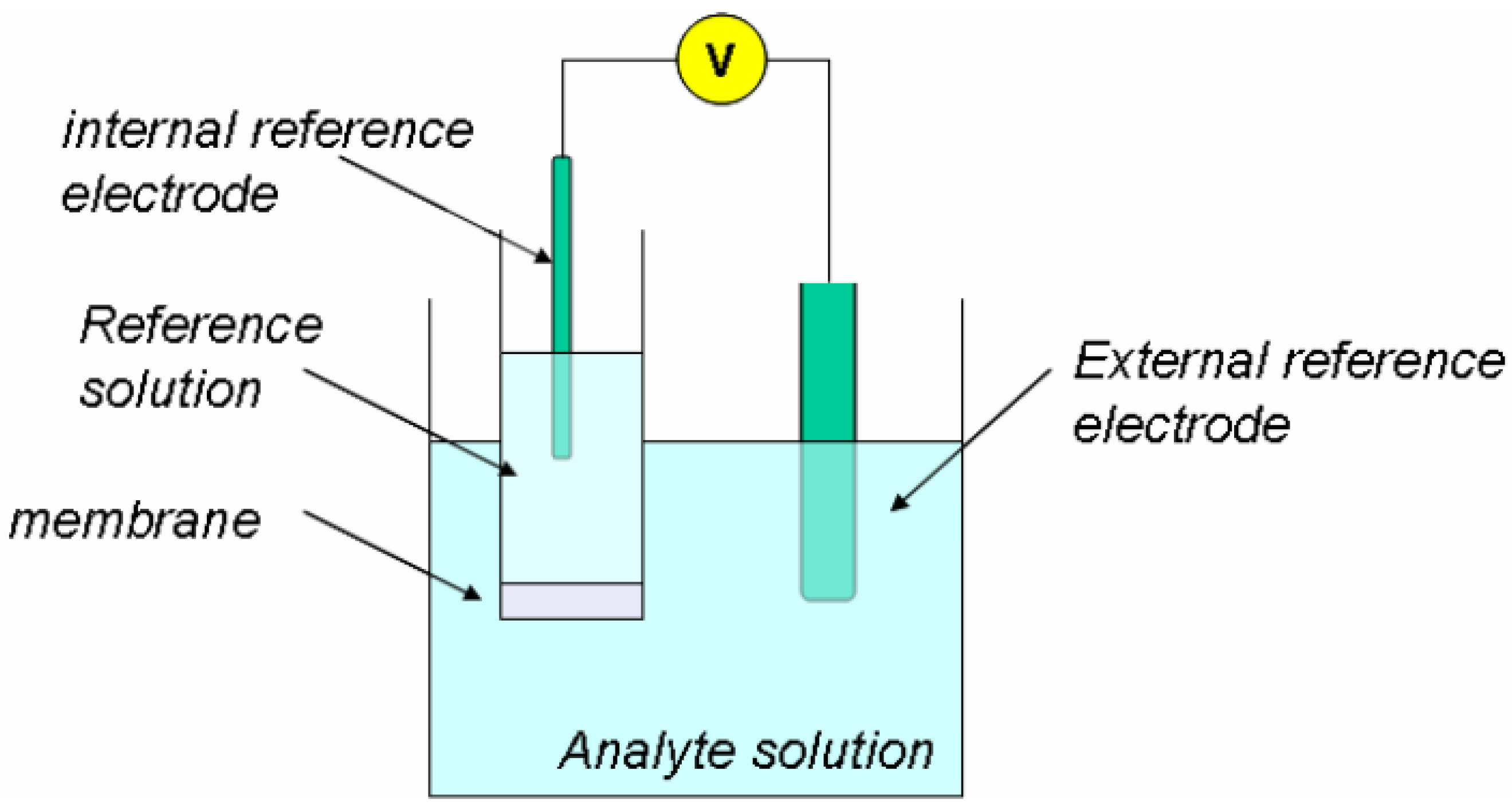
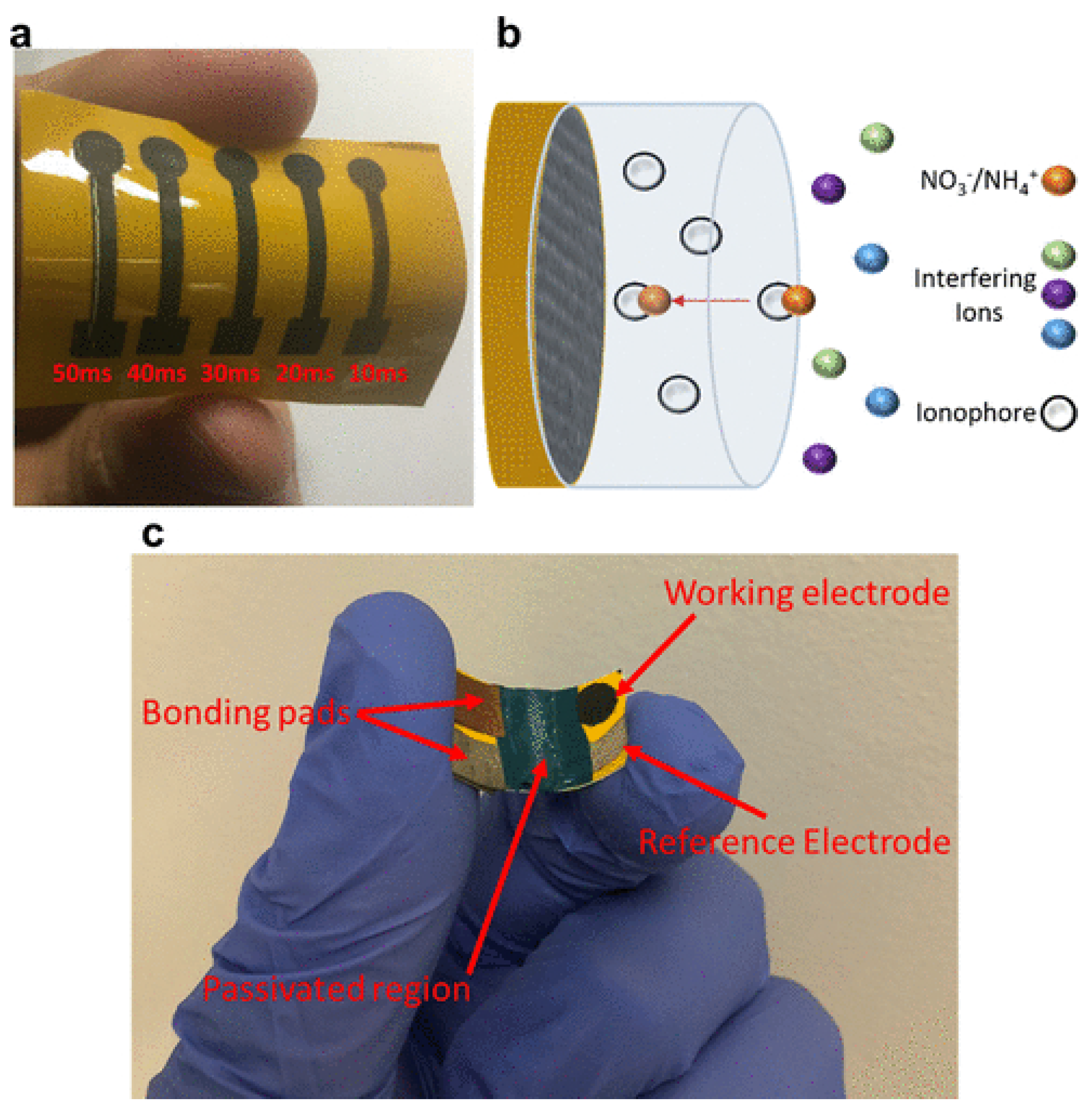
4.1.1. Ion-Selective Electrodes
4.1.2. Laser-Induced Graphene Sensor
4.2. Optical Sensors for Nitrogen Species Detection in Soil
4.2.1. Infrared Spectrum Analysis (IRS)
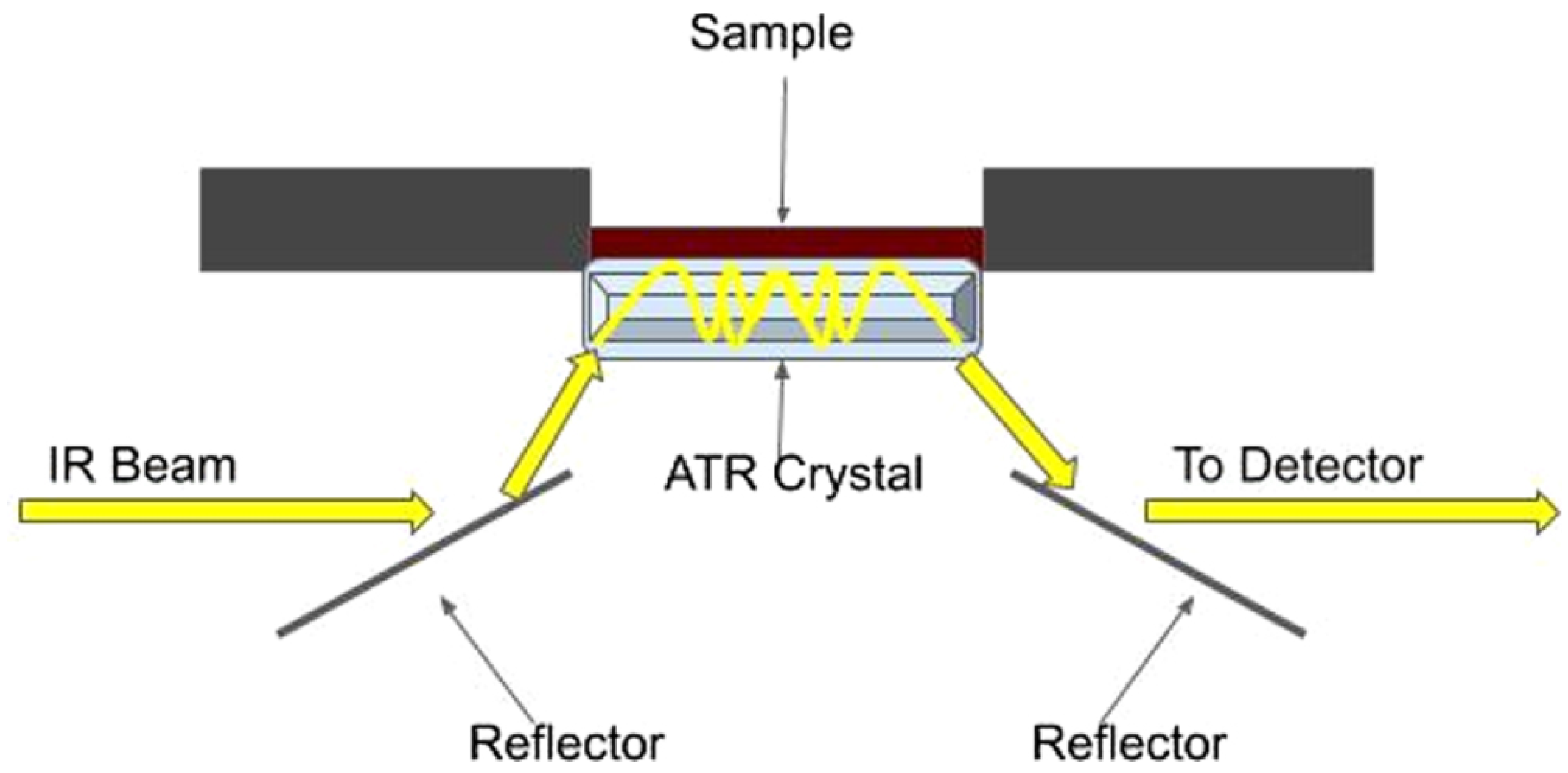
4.2.2. Attenuated Total Reflectance Spectroscopy
5. Nitrogen Species Detection in Water
5.1. Electrochemical Sensors for Nitrogen Species Detection in Water
5.1.1. Ion-Selective Electrodes
5.1.2. Gas Sensing Electrodes
5.2. Optical Sensors for Nitrogen Species Detection in Water
5.2.1. Spectrophotometry
5.2.2. Fluometry
5.2.3. Gas Diffusion Colorimetry
6. Discussion and Future Prospects
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zerkle, A.L.; Mikhail, S. The geobiological nitrogen cycle: From microbes to the mantle. Geobiology 2017, 15, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Pesticide 2018, 8, 1–27. [Google Scholar]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [Green Version]
- Greaver, T.L.; Sullivan, T.J.; Herrick, J.D.; Barber, M.C.; Baron, J.S.; Cosby, B.J.; Deerhake, M.E.; Dennis, R.L.; Dubois, J.J.B.; Goodale, C.L.; et al. Ecological effects of nitrogen and sulfur air pollution in the US: What do we know? Front. Ecol. Environ. 2012, 10, 365–372. [Google Scholar] [CrossRef]
- Cornell, S.E. Atmospheric nitrogen deposition: Revisiting the question of the importance of the organic component. Environ. Pollut. 2011, 159, 2214–2222. [Google Scholar] [CrossRef]
- Li, P.; Deng, Y.; Shu, H.; Lin, K.; Chen, N.; Jiang, Y.; Chen, J.; Yuan, D.; Ma, J. High-frequency underway analysis of ammonium in coastal waters using an integrated syringe-pump-based environmental-water analyzer (iSEA). Talanta 2019, 195, 638–646. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- de Hoyos-Vazquez, F.F.; de León, M.C.C.; Serrano-Nuñez, E.O.; Flores-Alamo, N.; Ríos, M.J.S. Development of a novel non-dispersive infrared multi sensor for measurement of gases in sediments. Sens. Actuators B Chem. 2019, 288, 486–492. [Google Scholar] [CrossRef]
- Xiao, S.; He, Y.; Dong, T.; Nie, P. Spectral analysis and sensitive waveband determination based on nitrogen detection of different soil types using near infrared sensors. Sensors 2018, 18, 523. [Google Scholar] [CrossRef] [Green Version]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Huang, N.M.; Zainal, Z.; Ahmad, S.A.A. Significance of nanomaterials in electrochemical sensors for nitrate detection: A review. Trends Environ. Anal. Chem. 2021, 31, 135. [Google Scholar] [CrossRef]
- Burton, L.; Jayachandran, K.; Bhansali, S. Review—The “Real-Time” Revolution for In situ Soil Nutrient Sensing. J. Electrochem. Soc. 2020, 167, 037569. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Gan, F.; Wu, K.; Ma, F.; Du, C. In Situ Determination of Nitrate in Water Using Fourier Transform Mid-Infrared Attenuated Total Reflectance Spectroscopy Coupled with Deconvolution Algorithm. Molecules 2020, 25, 5838. [Google Scholar] [CrossRef]
- Morellos, A.; Pantazi, X.E.; Moshou, D.; Alexandridis, T.; Whetton, R.; Tziotzios, G.; Wiebensohn, J.; Bill, R.; Mouazen, A.M. Machine learning based prediction of soil total nitrogen, organic carbon and moisture content by using VIS-NIR spectroscopy. Biosyst. Eng. 2016, 152, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, M.Z.; Zheng, L.H.; Zhao, Y.; Pei, X. Soil nitrogen content forecasting based on real-time NIR spectroscopy. Comput. Electron. Agric. 2016, 124, 29–36. [Google Scholar] [CrossRef]
- Bernhard, A. The Nitrogen Cycle: Processes, Players, and Human Impact. Nat. Educ. Knowl. 2010, 3, 25. [Google Scholar]
- Robertson, G.; Groffman, P. Nitrogen Transformations. In Soil Microbiology, Ecology and Biochemistry; Academic Press: Cambridge, MA, USA, 2007; pp. 341–364. [Google Scholar] [CrossRef]
- Grace, P.R.; Robertson, G.P.; Millar, N.; Colunga-Garcia, M.; Basso, B.; Gage, S.H.; Hoben, J. The contribution of maize cropping in the Midwest USA to global warming: A regional estimate. Agric. Syst. 2011, 104, 292–296. [Google Scholar] [CrossRef]
- Basso, B.; Dumont, B.; Cammarano, D.; Pezzuolo, A.; Marinello, F.; Sartori, L. Environmental and economic benefits of variable rate nitrogen fertilization in a nitrate vulnerable zone. Sci. Total Environ. 2016, 545–546, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.M.R.; Leach, A.M.; de Vries, W. Consequences of human modification of the global nitrogen cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [Green Version]
- Galloway, J.N.; Schlesinger, W.H.; Clark, C.M.; Grimm, N.B.; Jackson, R.B.; Law, B.E.; Thornton, P.E.; Townsend, A.R.; Martin, R. Ch. 15: Biogeochemical Cycles. In Climate Change Impacts in the United States: The Third National Climate Assessment; Technical Report, U.S. Global Change Research Program; National Climate Assessment: Washington, DC, USA, 2014. [Google Scholar] [CrossRef]
- Lu, Z.; Pang, Y.; Li, S.; Wang, Y.; Yang, Z.; Ma, D.; Wu, R. Phosphorene: A promising metal free cathode material for proton exchange membrane fuel cell. Appl. Surf. Sci. 2019, 479, 590–594. [Google Scholar] [CrossRef]
- Binions, R.; Naik, A.J. Metal oxide semiconductor gas sensors in environmental monitoring. Semicond. Gas Sens. 2013, 433–466. [Google Scholar] [CrossRef] [Green Version]
- Procek, M.; Pustelny, T. Analysis of the responses of metal-Oxide semiconductor nanostructures to nitrogen dioxide. Acta Phys. Pol. A 2013, 124, 529–533. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Malkov, I.; Garshev, A.; Mordvinova, N.; Lebedev, O.I.; Dolenko, S.; Efitorov, A.; Grigoriev, T.; Rumyantseva, M.; Gaskov, A. Chemically modified nanocrystalline SnO2-based materials for nitrogen-containing gases detection using gas sensor array. J. Alloys Compd. 2017, 691, 514–523. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Y.; Xu, J.; Debliquy, M. Room temperature conductive type metal oxide semiconductor gas sensors for NO2 detection. Sens. Actuators A Phys. 2019, 289, 118–133. [Google Scholar] [CrossRef]
- Peterson, P.J.; Aujla, A.; Grant, K.H.; Brundle, A.G.; Thompson, M.R.; Hey, J.V.; Leigh, R.J. Practical use of metal oxide semiconductor gas sensors for measuring nitrogen dioxide and ozone in urban environments. Sensors 2017, 17, 1653. [Google Scholar] [CrossRef]
- Venkatanarayanan, A.; Spain, E. Review of Recent Developments in Sensing Materials. Compr. Mater. Process. 2014, 13, 47–101. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.V.; Choi, I.Y.; Son, Y.S.; Kim, J.C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Thompson, J.E. Crowd-sourced air quality studies: A review of the literature & portable sensors. Trends Environ. Anal. Chem. 2016, 11, 23–34. [Google Scholar] [CrossRef]
- Martin, R.V.; Parrish, D.D.; Ryerson, T.B.; Nicks, D.K., Jr.; Chance, K.; Kurosu, T.P.; Jacob, D.J.; Sturges, E.D.; Fried, A.; Wert, B.P. Evaluation of GOME satellite measurements of tropospheric NO2 and HCHO using regional data from aircraft campaigns in the southeastern United States. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Ordóñez, C.; Richter, A.; Steinbacher, M.; Zellweger, C.; Nüß, H.; Burrows, J.P.; Prévôt, A.S.H. Comparison of 7 years of satellite-borne and ground-based tropospheric NO2 measurements around Milan, Italy. J. Geophys. Res. Atmos. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Streets, D.G.; He, K.; Wang, Y.; Richter, A.; Burrows, J.P.; Uno, I.; Jang, C.J.; Chen, D.; Yao, Z.; et al. NOx emission trends for China, 1995–2004: The view from the ground and the view from space. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef] [Green Version]
- Bucsela, E.J.; Perring, A.E.; Cohen, R.C.; Boersma, K.F.; Celarier, E.A.; Gleason, J.F.; Wenig, M.O.; Bertram, T.H.; Wooldridge, P.J.; Dirksen, R.; et al. Comparison of tropospheric NO2 from in situ aircraft measurements with near-real-time and standard product data from OMI. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Lamsal, L.N.; Martin, R.V.; van Donkelaar, A.; Steinbacher, M.; Celarier, E.A.; Bucsela, E.; Dunlea, E.J.; Pinto, J.P. Ground-level nitrogen dioxide concentrations inferred from the satellite-borne Ozone Monitoring Instrument. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef] [Green Version]
- Lamsal, L.N.; Martin, R.V.; van Donkelaar, A.; Celarier, E.A.; Bucsela, E.J.; Boersma, K.F.; Dirksen, R.; Luo, C.; Wang, Y. Indirect validation of tropospheric nitrogen dioxide retrieved from the OMI satellite instrument: Insight into the seasonal variation of nitrogen oxides at northern midlatitudes. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef]
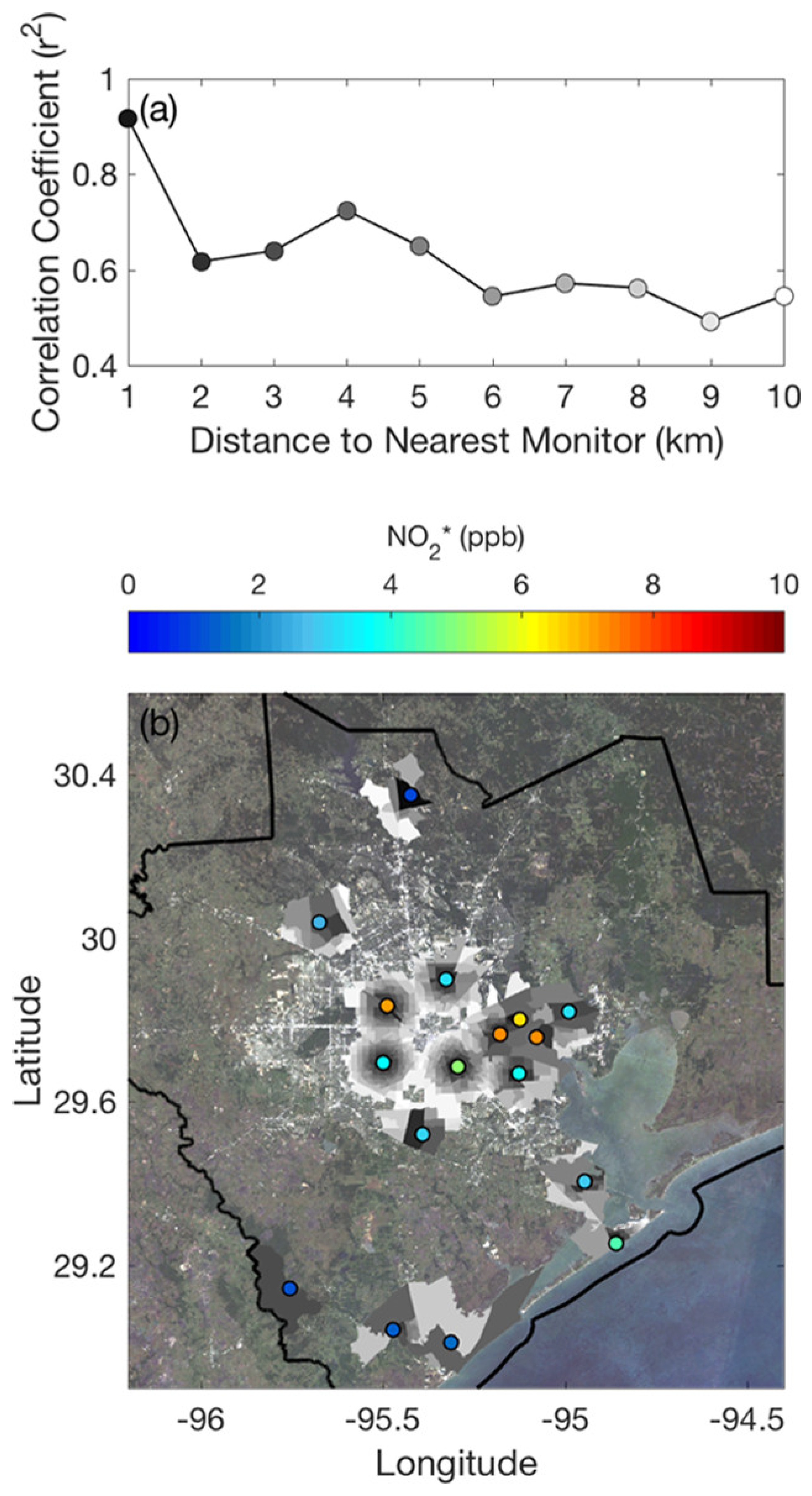
- Demetillo, M.A.G.; Navarro, A.; Knowles, K.K.; Fields, K.P.; Geddes, J.A.; Nowlan, C.R.; Janz, S.J.; Judd, L.M.; Al-Saadi, J.; Sun, K.; et al. Observing Nitrogen Dioxide Air Pollution Inequality Using High-Spatial-Resolution Remote Sensing Measurements in Houston, Texas. Environ. Sci. Technol. 2020, 54, 9882–9895. [Google Scholar] [CrossRef]
- Omrani, H.; Omrani, B.; Parmentier, B.; Helbich, M. Spatio-temporal data on the air pollutant nitrogen dioxide derived from Sentinel satellite for France. Data Brief 2020, 28, 105089. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Krupnick, A. Using Satellite Data to Fill the Gaps in the US Air Pollution Monitoring Network. Resources for the Future Working Paper; 2018. Available online: https://media.rff.org/documents/RFF20WP-18-21_0.pdf (accessed on 3 February 2022).
- Ryu, H.; Thompson, D.; Huang, Y.; Li, B.; Lei, Y. Electrochemical sensors for nitrogen species: A review. Sens. Actuators Rep. 2020, 2, 100022. [Google Scholar] [CrossRef]
- Ion-Selective Electrodes. 9 June 2020. Available online: https://chem.libretexts.org/@go/page/78061 (accessed on 3 February 2022).
- Garland, N.T.; Mclamore, E.S.; Cavallaro, N.D.; Mendivelso-Perez, D.; Smith, E.A.; Jing, D.; Claussen, J.C. Flexible Laser-Induced Graphene for Nitrogen Sensing in Soil. ACS Appl. Mater. Interfaces 2018, 10, 39124–39133. [Google Scholar] [CrossRef] [Green Version]
- Yunus, Y.; Satriyo, P.; Munawar, A.A. Rapid Prediction of Soil Quality Indices Using Near Infrared Spectroscopy Rapid Prediction of Soil Quality Indices Using Near Infrared Spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2021, 365, 012043. [Google Scholar] [CrossRef]
- Sanderman, J.; Savage, K.; Dangal, S.R. Mid-infrared spectroscopy for prediction of soil health indicators in the United States. Soil Sci. Soc. Am. J. 2020, 84, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Seybold, C.A.; Ferguson, R.; Wysocki, D.; Bailey, S.; Anderson, J.; Nester, B.; Schoeneberger, P.; Wills, S.; Libohova, Z.; Hoover, D.; et al. Application of Mid-Infrared Spectroscopy in Soil Survey. Soil Sci. Soc. Am. J. 2019, 83, 1746–1759. [Google Scholar] [CrossRef]
- Reda, R.; Saffaj, T.; Ilham, B.; Saidi, O.; Issam, K.; Brahim, L.; Hadrami, E.M.E. A comparative study between a new method and other machine learning algorithms for soil organic carbon and total nitrogen prediction using near infrared spectroscopy. Chemom. Intell. Lab. Syst. 2019, 195, 103873. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; van Wesemael, B.; Aitkenhead, M.; Bachmann, M.; Barthès, B.; Dor, E.B.; Brown, D.J.; Clairotte, M.; Csorba, A.; et al. Soil Spectroscopy: An Alternative to Wet Chemistry for Soil Monitoring. Adv. Agron. 2015, 132, 139–159. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on Water Quality Detection by UV-Vis Spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Ramírez, P.B.; Calderón, F.J.; Haddix, M.; Lugato, E.; Cotrufo, M.F. Using Diffuse Reflectance Spectroscopy as a High Throughput Method for Quantifying Soil C and N and Their Distribution in Particulate and Mineral-Associated Organic Matter Fractions. Front. Environ. Sci. 2021, 9, 634472. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Janik, L.J.; Rossel, R.A.V.; MacDonald, L.M.; McLaughlin, M.J. The performance of visible, near-, and mid-infrared reflectance spectroscopy for prediction of soil physical, chemical, and biological properties. Appl. Spectrosc. Rev. 2014, 49, 139–186. [Google Scholar] [CrossRef]
- Nie, P.; Dong, T.; He, Y.; Qu, F. Detection of Soil Nitrogen Using Near Infrared Sensors Based on Soil Pretreatment and Algorithms. Sensors 2017, 17, 1102. [Google Scholar] [CrossRef]
- Shao, Y.; Du, C.; Zhou, J.; Ma, F.; Zhu, Y.; Yang, K.; Tian, C. Quantitative analysis of different nitrogen isotope labelled nitrates in paddy soil using mid-infrared attenuated total reflectance spectroscopy. Anal. Methods 2017, 9, 5388–5394. [Google Scholar] [CrossRef]
- Borenstein, A.; Linker, R.; Shmulevich, I.; Shaviv, A. Determination of Soil Nitrate and Water Content Using Attenuated Total Reflectance Spectroscopy. Am. Soc. Agric. Biol. Eng. 2006, 60, 1267–1272. [Google Scholar] [CrossRef]
- Linker, R.; Shaviv, A. Nitrate Determination Using Anion Exchange Membrane and Mid-Infrared Spectroscopy. Appl. Spectrosc. 2006, 60, 1008–1012. [Google Scholar] [CrossRef]
- Linker, R.; Shmulevich, I.; Kenny, A.; Shaviv, A. Soil identification and chemometrics for direct determination of nitrate in soils using FTIR-ATR mid-infrared spectroscopy. Chemosphere 2005, 61, 652–658. [Google Scholar] [CrossRef]
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A guide for selecting the most appropriate method for ammonium determination in water analysis. TrAC Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- Wen, Y.; Mao, Y.; Kang, Z.; Luo, Q. Application of an ammonium ion-selective electrode for the real-time measurement of ammonia nitrogen based on pH and temperature compensation. Measurement 2019, 137, 98–101. [Google Scholar] [CrossRef]
- Ding, L.; Ding, J.; Ding, B.; Qin, W. Solid-contact Potentiometric Sensor for the Determination of Total Ammonia Nitrogen in Seawater. Int. J. Electrochem. Sci. 2017, 12, 3296–3308. [Google Scholar] [CrossRef]
- Athavale, R.; Dinkel, C.; Wehrli, B.; Bakker, E.; Crespo, G.A.; Brand, A. Robust Solid-Contact Ion Selective Electrodes for High-Resolution In Situ Measurements in Fresh Water Systems. Environ. Sci. Technol. Lett. 2017, 4, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Crespo, G.A. Recent Advances in Ion-selective membrane electrodes for in situ environmental water analysis. Electrochim. Acta 2017, 245, 1023–1034. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. TrAC Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Kysar, J.S. An Electrochemical Ammonia Sensor on Paper Substrate. J. Electrochem. Soc. 2017, 164, B113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, W.; Fang, Y.; Jia, D.; Wang, Y.; Song, Y.; Zhang, C. A supra-cubane-like Mo/S/Cu cluster: Cation-directed synthesis, crystal structure and nonlinear optical property. Polyhedron 2015, 101, 1–5. [Google Scholar] [CrossRef]
- Nxumalo, N.L.; Madikizela, L.M.; Kruger, H.G.; Onwubu, S.C.; Mdluli, P.S. Development of a paper-based microfluidic device for the quantification of ammonia in industrial wastewater. Water SA 2020, 46, 506–513. [Google Scholar] [CrossRef]
- Phansi, P.; Sumantakul, S.; Wongpakdee, T.; Fukana, N.; Ratanawimarnwong, N.; Sitanurak, J.; Nacapricha, D. Membraneless Gas-Separation Microfluidic Paper-Based Analytical Devices for Direct Quantitation of Volatile and Nonvolatile Compounds. Am. Chem. Soc. 2016, 88, 8749–8756. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Y.; Liu, S.; Song, W.G.; Gao, H.W. Using a PC camera to determine the concentration of nitrite, ammonia nitrogen, sulfide, phosphate, and copper in water. Anal. Methods 2018, 10, 2096–2101. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Yuan, D.; Yang, Z.; Shi, X.; Li, H.; Jin, H.; Ran, L. Development of analytical methods for ammonium determination in seawater over the last two decades. TrAC Trends Anal. Chem. 2019, 119, 115627. [Google Scholar] [CrossRef]
- Lin, K.; Zhu, Y.; Zhang, Y.; Lin, H. Determination of ammonia nitrogen in natural waters: Recent advances and applications. Trends Environ. Anal. Chem. 2019, 24, e00073. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; Lin, K.; Chen, Z.; Chen, N.; Liao, K.; Yuan, D. Optimization of a salinity-interference-free indophenol method for the determination of ammonium in natural waters using o-phenylphenol. Talanta 2017, 179, 608–614. [Google Scholar] [CrossRef]
- Zhou, L.; Boyd, C.E. Comparison of Nessler, phenate, salicylate and ion selective electrode procedures for determination of total ammonia nitrogen in aquaculture. Aquaculture 2015, 450, 187–193. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, R.; Bian, X.; Zhou, C.; Zhao, Y.; Zhang, S.; Wu, F.; Waterhouse, G.I.; Wu, L.Z.; Tung, C.H.; et al. Ammonia Detection Methods in Photocatalytic and Electrocatalytic Experiments: How to Improve the Reliability of NH3 Production Rates? Adv. Sci. 2019, 6, 1802109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Liang, Y.; Li, S.; Guo, Q.; Wu, C. A Modified o-Phthalaldehyde Fluorometric Analytical Method for Ultratrace Ammonium in Natural Waters Using EDTA-NaOH as Buffer. J. Anal. Methods Chem. 2014, 2014, 728068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Pan, Y.; Guo, Q.; Hu, H.; Wu, C.; Zhang, Q. A novel analytical method for trace ammonium in freshwater and seawater using 4-methoxyphthalaldehyde as fluorescent reagent. J. Anal. Methods Chem. 2015, 2015, 387207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhang, T.; Liang, Y.; Pan, Y. Toward sensitive determination of ammonium in field: A novel fluorescent probe, 4,5-dimethoxyphthalaldehyde along with a hand-held portable laser diode fluorometer. Sens. Actuators B Chem. 2018, 276, 356–361. [Google Scholar] [CrossRef]
- Engel, L.; Benito-Altamirano, I.; Tarantik, K.R.; Pannek, C.; Dold, M.; Prades, J.D.; Wöllenstein, J. Printed sensor labels for colorimetric detection of ammonia, formaldehyde and hydrogen sulfide from the ambient air. Sens. Actuators B Chem. 2020, 330, 129281. [Google Scholar] [CrossRef]
- Segundo, R.A.; Mesquita, R.B.R.; Ferreira, M.T.S.O.B.; Teixeira, C.F.C.P.; Bordalo, A.A.; Rangel, A.O.S.S. Development of a sequential injection gas diffusion system for the determination of ammonium in transitional and coastal waters. Anal. Methods 2011, 3, 2049–2055. [Google Scholar] [CrossRef]
- Sukaram, T.; Sirisakwisut, P.; Sirirak, J.; Nacapricha, D.; Chaneam, S. Environmentally friendly method for determination of ammonia nitrogen in fertilisers and wastewaters based on flow injection-spectrophotometric detection using natural reagent from orchid flower. Int. J. Environ. Anal. Chem. 2018, 98, 907–920. [Google Scholar] [CrossRef]
- Peters, J.J.; Almeida, M.I.G.; Šraj, L.O.; McKelvie, I.D.; Kolev, S.D. Development of a micro-distillation microfluidic paper-based analytical device as a screening tool for total ammonia monitoring in freshwaters. Anal. Chim. Acta 2019, 1079, 120–128. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical Sensors for Detecting Toxic Gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Chen, J.; Wang, K.; Mei, J.; Tan, T.; Wang, G.; Liu, K.; Gao, X. Highly precise measurement of atmospheric N2O and CO using improved White cell and RF current perturbation. Sens. Actuators B Chem. 2022, 352, 130995. [Google Scholar] [CrossRef]
- Monteiro-Silva, F.; Jorge, P.A.S.; Martins, R.C. Optical Sensing of Nitrogen, Phosphorus and Potassium: A Spectrophotometrical Approach toward Smart Nutrient Deployment. Chemosensors 2019, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Sabri, N.; Aljunid, S.A.; Salim, M.S.; Ahmad, R.B.; Kamaruddin, R. Toward Optical Sensors: Review and Applications. J. Phys. Conf. Ser. 2013, 423, 012064. [Google Scholar] [CrossRef]
- Berger, K.; Verrelst, J.; Féret, J.B.; Wang, Z.; Wocher, M.; Strathmann, M.; Danner, M.; Mauser, W.; Hank, T. Crop nitrogen monitoring: Recent progress and principal developments in the context of imaging spectroscopy missions. Remote Sens. Environ. 2020, 242, 111758. [Google Scholar] [CrossRef]
- Fabbri, C.; Mancini, M.; dalla Marta, A.; Orlandini, S.; Napoli, M. Integrating satellite data with a Nitrogen Nutrition Curve for precision top-dress fertilization of durum wheat. Eur. J. Agron. 2020, 120, 126148. [Google Scholar] [CrossRef]
- Feng, W.; Guo, B.B.; Zhang, H.Y.; He, L.; Zhang, Y.S.; Wang, Y.H.; Zhu, Y.J.; Guo, T.C. Remote estimation of above ground nitrogen uptake during vegetative growth in winter wheat using hyperspectral red-edge ratio data. Field Crops Res. 2015, 180, 197–206. [Google Scholar] [CrossRef]
- Roodenko, K.; Hinojos, D.; Hodges, K.L.; Veyan, J.F.; Chabal, Y.J.; Clark, K.; Katzir, A.; Robbins, D. Non-dispersive infrared (NDIR) sensor for real-time nitrate monitoring in wastewater treatment. In Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications XIX; Gannot, I., Ed.; SPIE: Bellingham, WA USA, 2019; p. 15. [Google Scholar] [CrossRef]
- Drozdowska, K.; Welearegay, T.; Österlund, L.; Smulko, J. Combined chemoresistive and in situ FTIR spectroscopy study of nanoporous NiO films for light-activated nitrogen dioxide and acetone gas sensing. Sens. Actuators B Chem. 2021, 353, 131125. [Google Scholar] [CrossRef]
- Al-Jalal, A.; Al-Basheer, W.; Gasmi, K.; Romadhon, M.S. Measurement of low concentrations of NO2 gas by differential optical absorption spectroscopy method. Measurement 2019, 146, 613–617. [Google Scholar] [CrossRef]





| Environment | Sensing Method | Detected Components | References |
|---|---|---|---|
| Soil | IRS Spectroscopy | Soil Nitrogen | [47,48,49,50] |
| Soil | ATR Spectroscopy | Nitrate | [56] |
| Water | ATR Spectroscopy | Nitrate | [15,90] |
| Water | UV Spectroscopy | Nitrate | [52] |
| Air | FTIR Spectroscopy | NO2 | [91] |
| Air | NDIR | NO2 | [33] |
| Air | TDLAS | NO2 | [84] |
| Air | LED Absorption Spectroscopy | NO2 | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousuf, M.F.; Mahmud, M.S. Review on Detection Methods of Nitrogen Species in Air, Soil and Water. Nitrogen 2022, 3, 101-117. https://doi.org/10.3390/nitrogen3010008
Yousuf MF, Mahmud MS. Review on Detection Methods of Nitrogen Species in Air, Soil and Water. Nitrogen. 2022; 3(1):101-117. https://doi.org/10.3390/nitrogen3010008
Chicago/Turabian StyleYousuf, Md Faishal, and Md Shaad Mahmud. 2022. "Review on Detection Methods of Nitrogen Species in Air, Soil and Water" Nitrogen 3, no. 1: 101-117. https://doi.org/10.3390/nitrogen3010008






