Simulated Cropping Season Effects on N Mineralization from Accumulated No-Till Crop Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Statistical Analysis
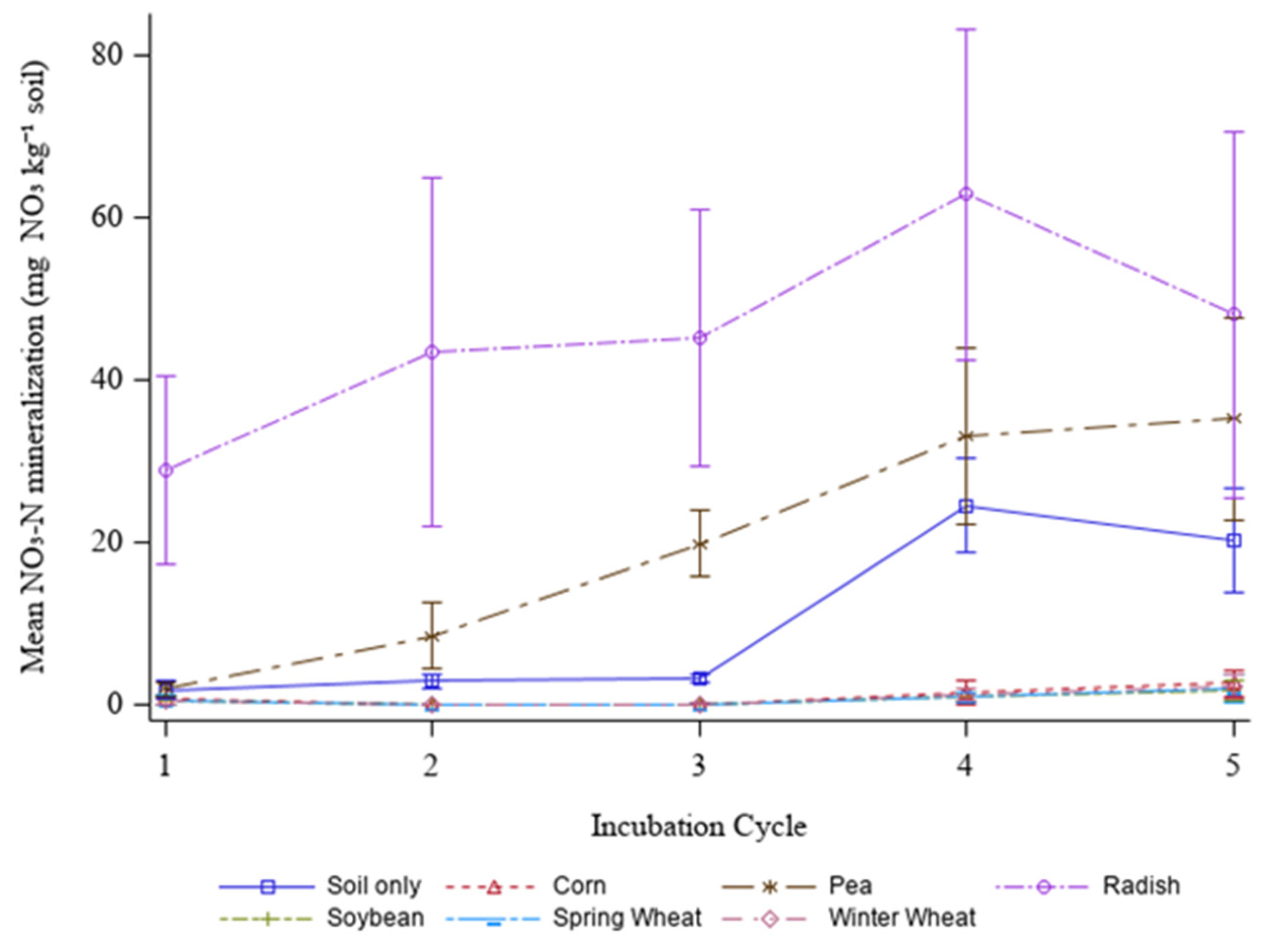
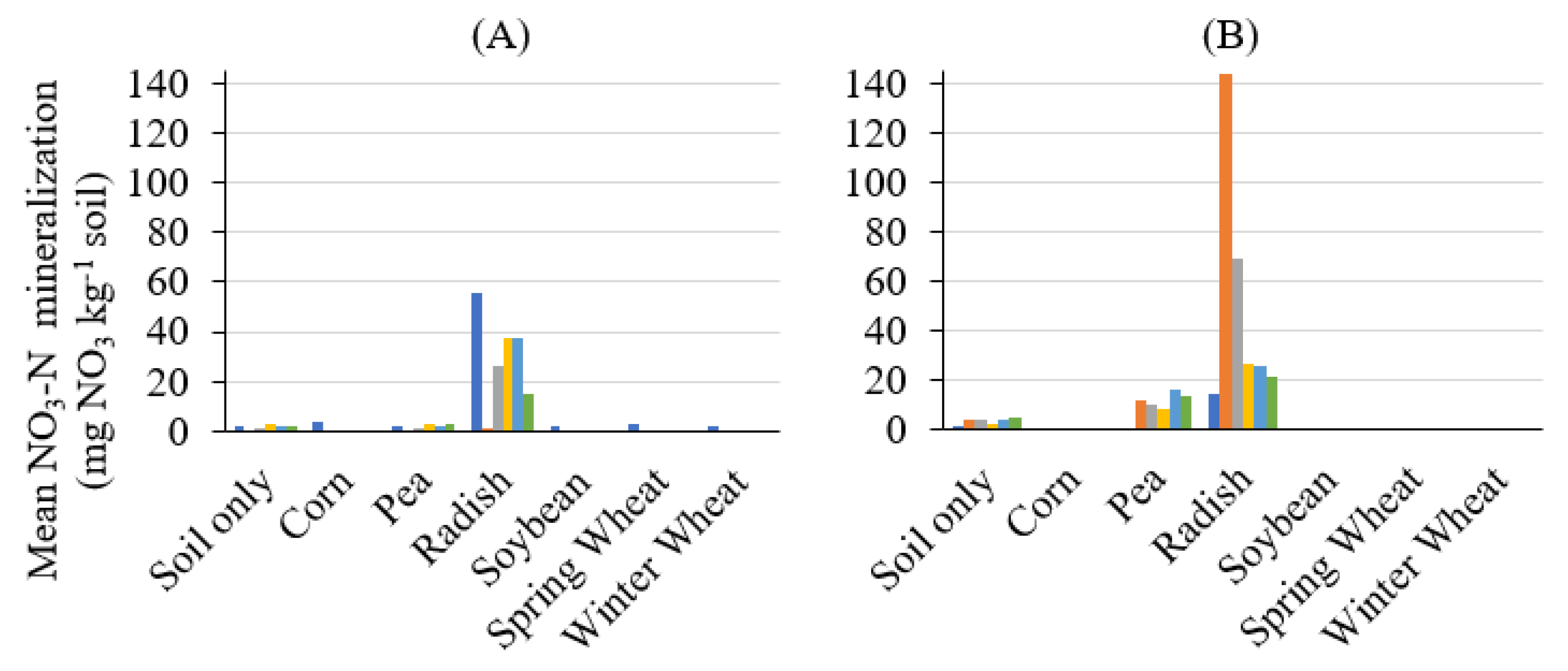
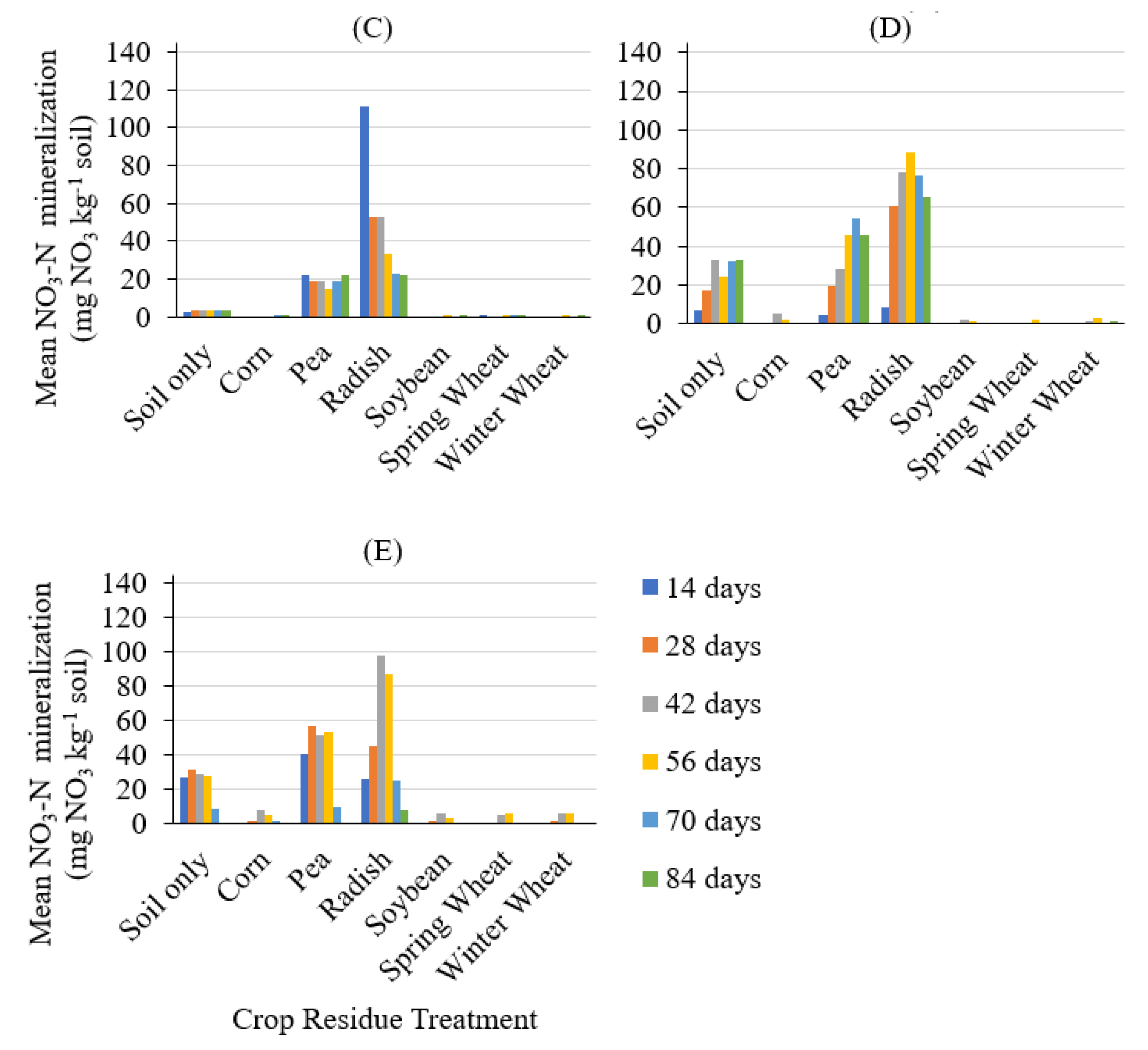
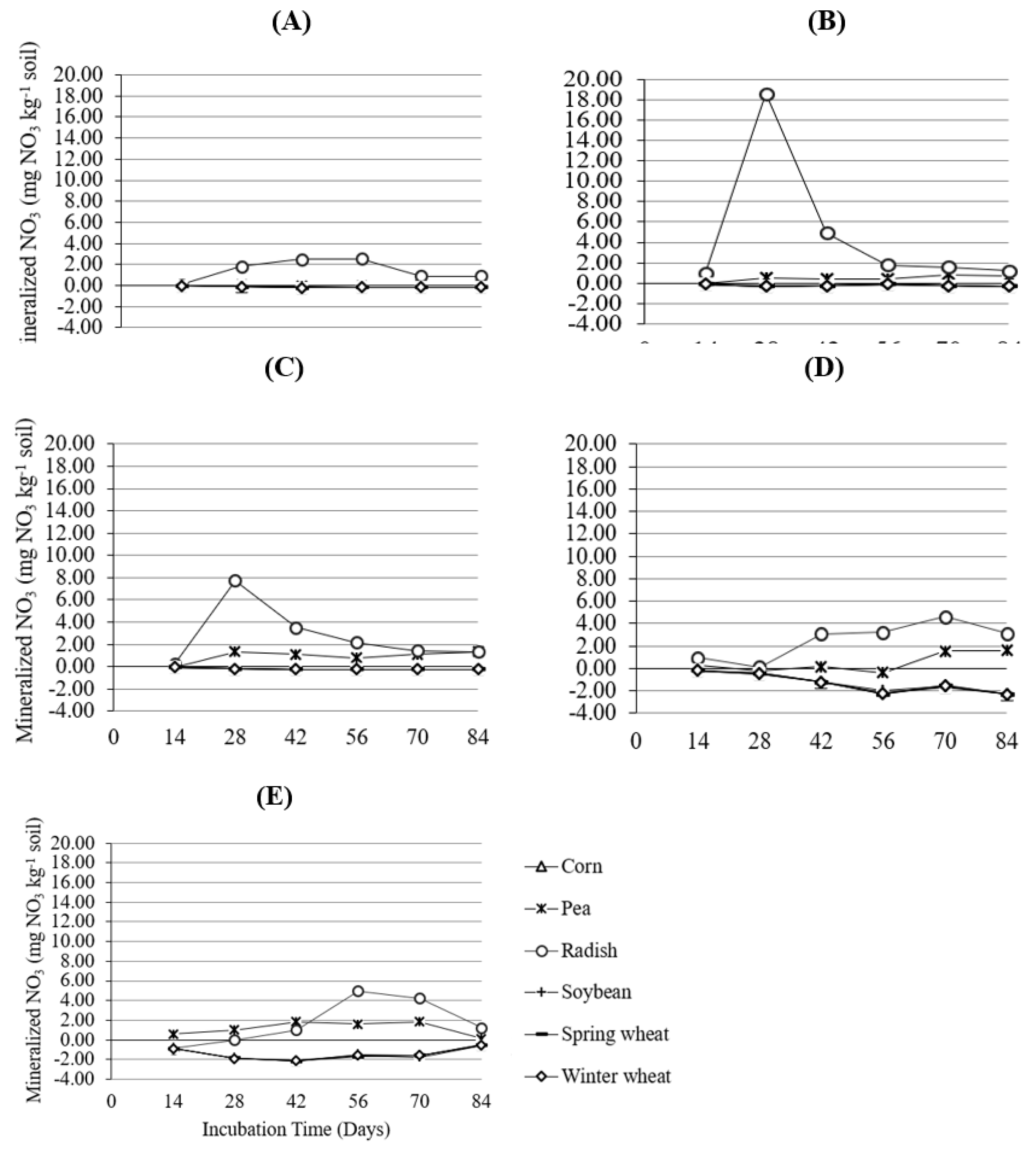
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larney, F.J.; Lindwall, C.W.; Izaurralde, R.C.; Moulin, A.P. Tillage systems for soil and water conservation on the Canadian prairies. In Conservation Tillage in Temperate Agroecosystems; Carter, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 305–328. ISBN 978-0873715713. [Google Scholar]
- Kolberg, R.L.; Kitchen, N.R.; Westfall, D.G.; Peterson, G.A. Cropping Intensity and Nitrogen Management Impact of Dryland No-Till Rotations in the Semi-Arid Western Great Plains. J. Prod. Agric. 1996, 9, 517–521. [Google Scholar] [CrossRef]
- Pedersen, P.; Lauer, J.G. Corn and Soybean Response to Rotation Sequence, Row Spacing, and Tillage System. Agron. J. 2003, 95, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Licht, M.A.; Al-Kaisi, M. Strip-tillage effect on seedbed soil temperature and other soil physical properties. Soil Tillage Res. 2005, 80, 233–249. [Google Scholar] [CrossRef]
- Alghamdi, R.S.; Daigh, A.L.M.; DeJong-Hughes, J.; Wick, A.F. Soil temperature and water contents among vertical tillage, strip tillage, and chisel plowing in the Upper Great Plains. Can. J. Soil Sci. 2021, 101, 596–616. [Google Scholar] [CrossRef]
- Daigh, A.L.M.; DeJong-Hughes, J.; Gatchell, D.H.; Derby, N.E.; Alghamdi, R.; Leitner, Z.R.; Wick, A.; Acharya, U. Crop and Soil Responses to On-Farm Conservation Tillage Practices in the Upper Midwest. Agric. Environ. Lett. 2019, 4, 190012. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, R.S.; Cihacek, L.; Daigh, A.L.M.; Rahman, S. Post-harvest crop residue contribution to soil N availability or unavailability in North Dakota. Agrosyst. Geosci. Environ. 2022, 5, e20233. [Google Scholar] [CrossRef]
- Bakermans, W.A.P.; De Wit, C.T.G. Crop husbandry on naturally compacted soils. Neth. J. Agric. Sci. 1970, 18, 225–246. [Google Scholar] [CrossRef]
- Bandel, V.A.; Dzienia, S.; Stanford, G.; Legg, G.J.O. N Behavior Under No-Till vs. Conventional Corn Culture. I. First-Year Results Using Unlabeled N Fertilizer 1. Agron. J. 1975, 67, 782–786. [Google Scholar] [CrossRef]
- Bandel, V.A. Nitrogen fertilization of no-tillage corn. In Proceedings of the Abstr. Am. Soc. Agron. N.E. Branch Meeting. Rutgers Univ., New Brunswick, NJ, USA, 24–27 June 1979; pp. 15–20. [Google Scholar]
- Alghamdi, R.S.; Cihacek, L.J. Do post-harvest crop residues in no-till systems provide for nitrogen needs of following crops? Agron. J. 2021, 114, 835–852. [Google Scholar] [CrossRef]
- Miller, K.S.; Geisseler, D. Temperature sensitivity of nitrogen mineralization in agricultural soils. Biol. Fertil. Soils 2018, 54, 853–860. [Google Scholar] [CrossRef]
- Schoenau, J.J.; Campbell, C.A. Impact of crop residues on nutrient availability in conservation tillage systems. Can. J. Plant Sci. 1996, 76, 621–626. [Google Scholar] [CrossRef]
- Blevins, R.L.; Smith, M.S.; Thomas, G.W. Changes in soil properties under no-tillage. In No-Tillage Agriculture Principles and Practices; Phillips, R.E., Phillips, S.H., Eds.; Van Nostrand Reinhold Company Inc.: New York, NY, USA, 1984; pp. 190–230. ISBN 978-0442277314. [Google Scholar]
- Green, C.J.; Blackmer, A.M. Residue Decomposition Effects on Nitrogen Availability to Corn following Corn or Soybean. Soil Sci. Soc. Am. J. 1995, 59, 1065–1070. [Google Scholar] [CrossRef]
- Li, L.-J.; Han, X.-Z.; You, M.-Y.; Yuan, Y.-R.; Ding, X.-L.; Qiao, Y.-F. Carbon and nitrogen mineralization patterns of two contrasting crop residues in a Mollisol: Effects of residue type and placement in soils. Eur. J. Soil Biol. 2013, 54, 1–6. [Google Scholar] [CrossRef]
- Satchell, J.E. Litter—Interface of animate/inanimate matter. In Biology of Plant Litter Decomposition; Dickinson, C.H., Pugh, G.J.F., Eds.; Academic Press: London, UK; Academic Press: New York, NY, USA, 1974; Volume 1, pp. xiv–xl. ISBN 9780323151061. [Google Scholar]
- Stemmer, M.; Von Lützow, M.; Kandeler, E.; Pichlmayer, F.; Gerzabek, M.H. The effect of maize straw placement on mineralization of C and N in soil particle size fractions. Eur. J. Soil Sci. 1999, 50, 73–85. [Google Scholar] [CrossRef]
- Vanhie, M.; Deen, W.; Lauzon, J.D.; Hooker, D.C. Effect of increasing levels of maize (Zea mays L.) residue on no-till soybean (Glycine max Merr.) in Northern production regions: A review. Soil Tillage Res. 2015, 150, 201–210. [Google Scholar] [CrossRef]
- Coppens, F.; Garnier, P.; Findeling, A.; Merckx, R.; Recous, S. Decomposition of mulched versus incorporated crop residues: Modelling with PASTIS clarifies interactions between residue quality and location. Soil Biol. Biochem. 2007, 39, 2339–2350. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Kwaw-Mensah, D.; Ci, E. Effect of Nitrogen Fertilizer Application on Corn Residue Decomposition in Iowa. Agron. J. 2017, 109, 2415–2427. [Google Scholar] [CrossRef] [Green Version]
- Al-Kaisi, M.M.; Guzman, J.G. Effects of tillage and nitrogen rate on decomposition of transgenic Bt and near-isogenic non-Bt maize residue. Soil Tillage Res. 2013, 129, 32–39. [Google Scholar] [CrossRef]
- Reis, E.M.; Baruffi, D.; Remor, L.; Zanatta, M. Decomposition of corn and soybean residues under field conditions and their role as inoculum source. Summa Phytopathol. 2011, 37, 65–67. [Google Scholar] [CrossRef] [Green Version]
- Kirschbaum, M.U.F. The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Vigil, M.F.; Kissel, D.E. Rate of Nitrogen Mineralized from Incorporated Crop Residues as Influenced by Temperature. Soil Sci. Soc. Am. J. 1995, 59, 1636–1644. [Google Scholar] [CrossRef]
- Aher, G.; Cihacek, L.J.; Cooper, K. An Evaluation of C and N of Fresh and Aged Crop Residue from Mixed Long-Term No-Till Cropping Systems. J. Plant Nutr. 2016, 40, 177–186. [Google Scholar] [CrossRef]
- NOAA (National Oceanic and Atmospheric Administration). Climate of North Dakota. 2020. Available online: https://www.ncdc.noaa.gov/climatenormals/clim60/states/Clim_ND_01.pdf (accessed on 29 January 2020).
- Carter, M.R. Conservation Tillage in Temperate Agrosystems; CRC Press: Boca Raton, FL, USA, 1994; ISBN 0-87371-571-3. [Google Scholar]
- Shields, J.A.; Paul, E.A.; Lowe, W.E. Factors influencing the stability of labelled microbial materials in soils. Soil Biol. Biochem. 1974, 6, 31–37. [Google Scholar] [CrossRef]
- Rovira, A.; Greacen, E. The effect of aggregate disruption on the activity of microorganisms in the soil. Aust. J. Agric. Res. 1957, 8, 659–673. [Google Scholar] [CrossRef]
- Witkamp, M. Environmental effects on microbial turnover of some mineral elements: Part I—Abiotic factors. Soil Biol. Biochem. 1969, 1, 167–176. [Google Scholar] [CrossRef]
- Gasser, J.K.R. Use of Deep-Freezing in the Preservation and Preparation of Fresh Soil Samples. Nature 1958, 181, 1334–1335. [Google Scholar] [CrossRef]
- Soulides, D.A.; Allison, F.E. Effect of drying and freezing soils on carbon dioxide production, available mineral nutrients, aggregation, and bacterial population. Soil Sci. 1961, 91, 291–298. [Google Scholar] [CrossRef]
- Ivarson, K.C.; Sowden, F.J. Effect of frost action and storage of soil at freezing temperatures on the free amino acids, free sugars and respiratory activity of soil. Can. J. Soil Sci. 1970, 50, 191–198. [Google Scholar] [CrossRef]
- Ross, D.J. Effects of freezing and thawing of some grassland topsoils on oxygen uptakes and dehydrogenase activities. Soil Biol. Biochem. 1972, 4, 115–117. [Google Scholar] [CrossRef]
- Schimel, J.P.; Clein, J.S. Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biol. Biochem. 1996, 28, 1061–1066. [Google Scholar] [CrossRef]
- Franzen, D.W.; Wick, A.F.; Bu, H.; Ressler, L.; Bell, J.; Berti, M.T.; Gasch, C. Nitrogen non-cycling from cover crops grown before corn and spring wheat—unexpected early project results. In Proceedings of the 48th North Central Extension-Industry Soil Fertility Conference, Des Moines, IA, USA, 14–15 November 2018; pp. 21–26. [Google Scholar]
- Soil Survey Staff. Forman Soil Series. Natural Resources Conservation Service, United States Department of Agriculture. Official Soil Series Descriptions; 1998. Available online: https://soilseries.sc.egov.usda.gov/osdname.aspx (accessed on 30 March 2022).
- Stanford, G.; Smith, S.J. Nitrogen Mineralization Potentials of Soils. Soil Sci. Soc. Am. J. 1972, 36, 465–472. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/ACCESS® 9.4 Interface to ADABAS: Reference; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Knops, J.M.H.; Bradley, K.L.; Wedin, D.A. Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol. Lett. 2002, 5, 454–466. [Google Scholar] [CrossRef] [Green Version]

| Residue Treatment | Scientific Name | Abbreviation | C/N Ratio 1 |
|---|---|---|---|
| Corn | Zea mays L. | CR | 73 |
| Soybean | Glycine max L. | S | 53 |
| Forage radish | Raphanus sativus L. | R | 8 |
| Winter pea | Pisum sativum L. | P | 18 |
| Spring wheat | Triticum aestivum L. | SW | 76 |
| Winter wheat | Triticum aestivum L. | WW | 101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, R.S.; Cihacek, L.; Wen, Q. Simulated Cropping Season Effects on N Mineralization from Accumulated No-Till Crop Residues. Nitrogen 2022, 3, 149-160. https://doi.org/10.3390/nitrogen3020011
Alghamdi RS, Cihacek L, Wen Q. Simulated Cropping Season Effects on N Mineralization from Accumulated No-Till Crop Residues. Nitrogen. 2022; 3(2):149-160. https://doi.org/10.3390/nitrogen3020011
Chicago/Turabian StyleAlghamdi, Rashad S., Larry Cihacek, and Qian Wen. 2022. "Simulated Cropping Season Effects on N Mineralization from Accumulated No-Till Crop Residues" Nitrogen 3, no. 2: 149-160. https://doi.org/10.3390/nitrogen3020011





