Microbiogeochemical Traits to Identify Nitrogen Hotspots in Permafrost Regions
Abstract
:1. Introduction
- Permafrost-affected soils are not generally N-limited, but there are N hotspots of N availability with more open N cycling and increased potential for N losses in the form of inorganic N leaching and N2O emissions.
- N hotspots can be identified by certain soil characteristics and microbial traits: different N forms, microbial net N turnover rates, especially N mineralization, nitrification and denitrification, and abundance of key functional N cycling genes.
2. Regulation of N Availability by Microbial N Processes in Permafrost-Affected Soils
2.1. Levels and Traits of N Availability
2.2. Key Microbial N Processes Determining N Availability in Permafrost-Affected Soils
2.2.1. Nitrification
2.2.2. Denitrification
3. Hotspots of N Availability and Properties
3.1. Bare Organic Lowland Soils in Permafrost Peatland
3.1.1. Habitat, Soil Characteristics and Inorganic N
3.1.2. C and N Mineralization
3.1.3. Gaseous N Loss
| Permafrost Peatland | Mineral Upland Soils in Thermokarst Landscapes | ||||
|---|---|---|---|---|---|
| Soil and Microbial Properties | Bare Surfaces * | Vegetated Surfaces * | Disturbed, Revegetated RTS ** | Undisturbed Fully Vegetated Site Next to RTS ** | |
| Slump Floor (SF) | Thaw Mound (TM) | ||||
| pH | 3.7 ± 0.5 [86,247] | 3.6 ± 0.3 [86,247] | 7.2 ± 1.5 [87,256] | 7.9 ± 0.0 [256] | 5.7 ± 0.3 [87] |
| C/N | 22 ± 4 [86,247] | 51 ± 16 [86,247] | 15 ± 1 [87,256] | 13 ± 0 [256] | 38 ± 4 [87] |
| WFPS (%) | 53 ± 25 [86,247] | 22 ± 7 [86,247] | 60 ± 11 [87,256] | 60 ± 3 [256] | 13 ± 6 [87] |
| SOM (%) | 95 ± 1 [86,247] | 98 ± 0 [86,247] | 11 ± 4 [87,256] | 8 ± 0 [256] | 27 ± 3 [87] |
| TN (%) | 2.4 ± 0.3 | 1.0 ± 0.3 | 0.30 ± 0.10 | 0.34 ± 0.01 | 0.29 ± 0.03 |
| [86,247] | [86,247] | [87,256] | [256] | [87] | |
| δ15N in bulk soil (‰) | n.d. | n.d. | 1.4 ± 0.5 [87,256] | 2.0 ± 0.1 [256] | 1.2 ± 0.3 [87] |
| Ammonium (µg N g dw−1) | 60.1 ± 14.4 | 19.6 ± 5.7 | 1.4 ± 1.8 | 0.0 ± 0.0 | 9.0 ± 11.7 |
| [86,202,205,216,247,255] | [86,202,205,216,247,255] | [87,256] | [256] | [87] | |
| Nitrat (µg N g dw−1) | 116.8 ± 2.8 | 4.4 ± 7.7 | 0.7 ± 0.1 | 81.6 ± 24.3 | 0.0 ± 0.0 |
| [86,202,205,216,247,255] | [86,202,205,216,247,255] | [87,256] | [256] | [87] | |
| DIN/TN (%) | 1.1 ± 1.3 [86,247] | 0.3 ± 0.1 [86,247] | 0.1 ± 0.1 [87,256] | 2.4 [256] | 0.3 [87] |
| Gross N mineralization | 16.8 ± 9.7 [86,202] | 9.0 ± 10.8 [86,202] | 15.1 ± 10.1 [87] | n.d. | b.d. |
| (µg N g dw−1 d−1) | |||||
| Net N mineralization | n.d. | n.d. | 4.0 ± 4.9 [87,256] | 1.4 ± 0.5 [256] | −20.9 ± 16.6 [87] |
| (µg N g dw−1 d−1), p.a.s. 0.8 [59] | |||||
| Gross nitrification | 8.4 ± 7.3 [120,202] | 0.2 ± 0.1 [202] | b.d. | n.d. | b.d. |
| (µg N g dw−1 d−1), p.a.s. 6.6 [59] | |||||
| Net nitrification | n.d. | n.d. | 1.6 ± 1.5 [87,256] | 1.4 ± 0.5 [256] | −6.0 ± 2.9 [87] |
| (µg N g dw−1 d−1), p.a.s. −0.4 [59] | |||||
| Net denitrification | 0.56 | <0.004 | 2.8 ± 1.8 [87] | n.d. | 0.01 ± 0.00 |
| with (without acetylene) (µg N g dw−1 d−1) | (0.37) [205] | (b.d.) [205] | (0.3 ± 0.4) [87,256] | (0.20 ± 0.02) | (0.01 ± 0.00) |
| [256] | [87] | ||||
| Functional nitrification gene | |||||
| AOA amoA (copies gdw−1) | 6.4 × 108 [120] | 8.0 × 106 [120] | 2.0 × 107 [87] | n.d. | 5.4 × 107 [87] |
| AOB amoA (copies gdw−1) | b.d. | b.d. | 3.3 × 107 [87] | n.d. | 4.2 × 106 [87] |
| Functional nitrification gene amoA (% of 16S rRNA) | |||||
| n.d. | n.d. | 7 [87] | n.d. | 1 [87] | |
| amoA (% of N genes) | n.d. | n.d. | 3.4 ± 1.0 [87] | n.d. | 0.5 ± 1.0 [87] |
| Functional denitrification gene (% of 16S rRNA [205], N genes [87]) | |||||
| narG | 7.6 ± 2.8 [205] | 0.04 ± 0.01 [205] | 42 ± 0.9 [87] | n.d. | 45 ± 9.3 [87] |
| nirS + nirK | 0.34 ± 0.08 [205] | 0.88 ± 0.13 [205] | 29 ± 0.1 [87] | n.d. | 15 ± 0.6 [87] |
| (nirS + nirK)/nosZ (%/%) | 0.20 × 103 [205] | 8.88 × 103 [205] | 2.4 ± 0.2 [87] | n.d. | 0.5 ± 0.1 [87] |
| N2O fluxes | 1.98 ± 3.19 | 0.01 ± 0.03 | 1.64 ± 2.61 [87] | n.d. | −0.001 ± 0.018 [87] |
| (mg N m−2 d−1) | [86,120,202,247,255] | [86,120,202,247,255] | |||
3.1.4. Microbial Based N Processes
3.2. Disturbed Mineral Upland Soils in Hillslope Thermokarst Landscapes
3.2.1. Habitat and Soil Characteristics
3.2.2. C and N Mineralization and Inorganic N
3.2.3. Gaseous N2O Loss and Based Microbial N Processes
3.2.4. Lateral N Loss
3.3. Bare Soils in the Transition between Terrestrial and Aquatic Ecosystems

3.4. Animal-Influenced Soils
3.5. Wildfire-Affected Soils
4. Hotspots of N Availability and Climate Change
4.1. Contribution of N Hotspots to Climate Change
4.2. Impact of Climate Change on N Hotspots
5. Conclusions and Perspectives
- Soil properties: Low C/N below a threshold of 25 [29,86,87,120,202,234,247,255,256], high δ15N of bulk soil [234,304], WFPS around 60–70% [82,86,87,120,202,217,247,256] and high DIN [28,29,86,87,120,188,189,202,208,210,213,214,234,247,255,256,292,304], sometimes expressed as higher DIN/DON ratio [256]. Nitrogen loss often correlates with dominance of nitrate over ammonium [29,87,213,216,217,247,255,256], but at some sites a higher ammonium content was found in association with higher N2O emission [86,188,189,248,306]. Therefore, the total inorganic N content would be a better indicator for N loss.
- Microbial process rates measured in situ or in laboratory incubations correlated with N loss and were therefore appropriate traits of high N availability. They were gross [202,216] and net mineralization, the sum of ammonification and nitrification [87,304], gross [111,120,202] and net nitrification [29,69,87,119,188,304,307] and denitrification [189,205,234,254,256]. Since gross N turnover rates are also indicative of the total amount of circulating N and theses rates were higher than currently expected in this ecosystem [59], we propose to use net rates as an indicator of N limitation status instead. Net rates could provide a better indication of how much N might be lost from the tight N cycling system of permafrost-affected soils. However, net rates estimated using laboratory incubation techniques are highly dependent on conditions such as temperature and have inherent biases related to functional groups, so they do not characterize the contribution of, e.g., nitrifying organisms in the natural habitat [379]. Recently, however, in situ N2O flux rates from an N hotspot were shown to be highly correlated with anaerobic N2O production in laboratory incubation tests, as well as with net mineralization and net nitrification [87], so laboratory tests could be a good tool for predicting field fluxes.
- The abundance of functional genes or the detection of known 16S rRNA gene sequences of nitrifiers [119,120,188], denitrifiers [189,218], or both [86] have been correlated with high N2O losses in N hotspots. For nitrifiers, especially ammonia-oxidizing bacteria (AOB) and archaea (AOA), the gene amoA was mostly applied. However, gene AOA amoA abundance also dominated in permafrost-affected soils [118,120,147,182], but generally did not correlate with nitrification activity and N2O production rates. The reasons are that AOA do not exclusively use ammonia as substrate and that AOA produce N2O with about 30 times lower yields [129]. Therefore, it will likely be useful to distinguish between AOA and AOB amoA when using as traits for N2O production in the future. To predict N2O production by denitrifiers, high levels of their functional genes, mainly nirS + nirK genes of nitrite reductase, low levels of the gene nosZ (N2O reductase), or a high ratio of (nirS + nirK)/nosZ, were found in N hotspots in correlation with high N2O emission [205,218] and could therefore be a suitable microbial trait for N losses. In addition, metatranscriptomic analysis, which detects actively produced transcripts, could be another good tool to detect active microbial N cycling and N losses. This detection method has recently shown a correlation between denitrification activity and N2O production in permafrost-affected soils [136].
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarnocai, C.; Canadell, J.G.; Schuur, E.A.G.; Kuhry, P.; Mazhitova, G.; Zimov, S. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 2009, 23, GB2023. [Google Scholar] [CrossRef]
- Gruber, S. Derivation and analysis of a high-resolution estimate of global permafrost zonation. Cryosphere 2012, 6, 221–233. [Google Scholar] [CrossRef]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.W.; Schuur, E.A.G.; Ping, C.L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D.; et al. Improved estimates show large circumpolar stocks of permafrost carbon while quantifying substantial uncertainty ranges and identifying remaining data gaps. Biogeosci. Discuss. 2014, 11, 4771–4822. [Google Scholar] [CrossRef]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.W.; Schuur, E.A.G.; Ping, C.L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D.; et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 2014, 11, 6573–6593. [Google Scholar] [CrossRef]
- Aalto, J.; Karjalainen, O.; Hjort, J.; Luoto, M. Statistical forecasting of current and future circum-Arctic ground temperatures and active layer thickness. Geophys. Res. Lett. 2018, 45, 4889–4898. [Google Scholar] [CrossRef]
- Obu, J.; Westermanna, S.; Annett, B.; Berdnikovc, N.; Christiansen, H.H.; Dashtseren, A.; Delaloy, R.; Elberling, B.; Etzelmüller, B.; Kholodov, A.; et al. Northern Hemisphere permafrost map based on TTOP modelling for 2000–2016 at 1 km2 scale. Earth-Sci. Rev. 2019, 193, 299–316. [Google Scholar] [CrossRef]
- Marion, G.M.; Bockheim, J.G.; Brown, J. Arctic soils and the ITEX experiment. Glob. Chang. Biol. 1997, 3, 33–43. [Google Scholar] [CrossRef]
- Walker, D.A.; Raynolds, M.K.; Daniëls, F.J.A.; Einarsson, E.; Elvebakk, A.; Gould, W.A.; Katenin, A.E.; Kholod, S.S.; Markon, C.J.; Melnikov, E.S.; et al. The circumpolar Arctic vegetation map. J. Veg. Sci. 2005, 16, 267–282. [Google Scholar] [CrossRef]
- Olefeldt, D.; Turetsky, M.R.; Crill, P.M.; McGuire, A.D. Environmental and physical controls on northern terrestrial methane emissions across permafrost zones. Glob. Chang. Biol. 2013, 19, 589–603. [Google Scholar] [CrossRef]
- Beilman, D.W.; Vitt, D.H.; Halsey, L.A. Localized permafrost Peatlands in Western Canada: Definition, distributions, and degradation. Arct Antarct. Alp. Res. 2001, 33, 70–77. [Google Scholar] [CrossRef]
- van Huissteden, J. Thawing Permafrost-Permafrost Carbon in a Warming Arctic; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Ping, C.L.; Jastrow, J.D.; Jorgenson, M.T.; Michaelson, G.J.; Shur, Y.L. Permafrost soils and carbon cycling. SOIL 2015, 1, 147–171. [Google Scholar] [CrossRef]
- Lipson, D.A.; Zona, D.; Raab, T.K.; Bozzolo, F.; Mauritz, M.; Oechel, W.C. Water-table height and microtopography control biogeochemical cycling in an Arctic coastal tundra ecosystem. Biogeosciences 2012, 9, 577–591. [Google Scholar] [CrossRef]
- Giblin, A.E.; Nadelhoffer, K.J.; Shaver, G.R.; Laundre, J.A.; McKerrow, A.J. Biogeochemical diversity along a riverside toposequence in Arctic Alaska. Ecol. Monogr. 1991, 61, 415–435. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Giblin, A.E.; Shaver, G.R.; Laundre, J.A. Effects of temperature and substrate quality on element mineralization in six Arctic soils. Ecology 1991, 72, 242–253. [Google Scholar] [CrossRef]
- Cheng, W.; Virginia, R.A.; Oberbauer, S.F.; Gillespie, C.T.; Reynolds, J.F.; Tenhunen, J.D. Soil nitrogen, microbial biomass, and respiration along an Arctic toposequence. Soil Sci. Soc. Am. J. 1998, 62, 654–662. [Google Scholar] [CrossRef]
- Björk, R.G.; Klemedtsson, L.; Molau, U.; Harndorf, J.; Ödman, A.M.; Giesler, R. Linkages between N turnover and plant community structure in a tundra landscape. Plant Soil 2007, 294, 247–261. [Google Scholar] [CrossRef]
- Minke, M.; Donner, N.; Karpov, N.S.; de Klerk, P.; Joosten, H. Distribution, diversity, development and dynamics of polygon mires: Examples from Northeast Yakutia (Siberia). Peatl. Int. 2007, 1, 36–40. [Google Scholar]
- Zona, D.; Lipson, D.A.; Zulueta, R.C.; Oberbauer, S.F.; Oechel, W.C. Microtopographic controls on ecosystem functioning in the Arctic Coastal Plain. J. Geophys. Res. Biogeosci. 2011, 116, G00i08. [Google Scholar] [CrossRef]
- Banerjee, S.; Siciliano, S.D. Factors driving potential ammonia oxidation in Canadian Arctic ecosystems: Does spatial scale matter? Appl. Environ. Microbiol. 2012, 78, 346–353. [Google Scholar] [CrossRef]
- Schädel, C.; Schuur, E.A.G.; Bracho, R.; Elberling, B.; Knoblauch, C.; Lee, H.; Luo, Y.; Shaver, G.R.; Turetsky, M.R. Circumpolar assessment of permafrost C quality and its vulnerability over time using long-term incubation data. Glob. Chang. Biol. 2014, 20, 641–652. [Google Scholar] [CrossRef]
- Treat, C.C.; Marushchak, M.E.; Voigt, C.; Zhang, Y.; Tan, Z.; Zhuang, Q.; Virtanen, T.A.; Räsänen, A.; Biasi, C.; Hugelius, G.; et al. Tundra landscape heterogeneity, not interannual variability, controls the decadal regional carbon balance in the Western Russian Arctic. Glob. Chang. Biol. 2018, 24, 5188–5204. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, M.N.; Schimel, J.P. The seasonal dynamics of amino acids and other nutrients in Alaskan Arctic tundra soils. Biogeochemistry 2005, 73, 359–380. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Wal, R.; Jónsdóttir, I.S.; Quirk, H.; Dutton, S. Temporal variability in plant and soil nitrogen pools in a high-Arctic ecosystem. Soil Biol. Biochem. 2007, 39, 2129–2137. [Google Scholar] [CrossRef]
- Lee, H.; Schuur, E.A.G.; Vogel, J.G. Soil CO2 production in upland tundra where permafrost is thawing. J. Geophys. Res. Biogeosci. 2010, 115, G01009. [Google Scholar] [CrossRef]
- Natali, S.M.; Schuur, E.A.G.; Trucco, C.; Pries, C.E.H.; Crummer, K.G.; Lopez, A.F.B. Effects of experimental warming of air, soil and permafrost on carbon balance in Alaskan tundra. Glob. Chang. Biol. 2011, 17, 1394–1407. [Google Scholar] [CrossRef]
- Stewart, K.J.; Coxson, D.; Siciliano, S.D. Small-scale spatial patterns in N2-fixation and nutrient availability in an Arctic hummock–hollow ecosystem. Soil Biol. Biochem. 2011, 43, 133–140. [Google Scholar] [CrossRef]
- Harms, T.K.; Jones, J. Thaw depth determines reaction and transport of inorganic nitrogen in valley bottom permafrost soils: Nitrogen cycling in permafrost soils. Glob. Chang. Biol. 2012, 18, 2958–2968. [Google Scholar] [CrossRef]
- Harms, T.K.; Abbott, B.W.; Jones, J.B. Thermo-erosion gullies increase nitrogen available for hydrologic export. Biogeochemistry 2014, 117, 299–311. [Google Scholar] [CrossRef]
- Norby, R.J.; Sloan, V.L.; Iversen, C.M.; Childs, J. Controls on fine-scale spatial and temporal variability of plant-available inorganic nitrogen in a polygonal tundra landscape. Ecosystems 2019, 22, 528–543. [Google Scholar] [CrossRef]
- Brown, J.; Ferrians, O.J., Jr.; Heginbottom, J.A.; Melnikov, E.S. Circum-Arctic Map of Permafrost and Ground Ice Conditions; National Snow and Ice Data Center/World Data Center for Glaciology: Boulder, CO, USA, 1998; revised February 2001; Available online: https://databasin.org/datasets/1f624a31ab224835a78ad4bf11103419/ (accessed on 30 June 2022).
- Ping, C.L.; Bockheim, J.G.; Kimble, J.M.; Michaelson, G.J.; Walker, D.A. Characteristics of cryogenic soils along a latitudinal transect in Arctic Alaska. J. Geophys. Res. Atmos. 1998, 103, 28917–28928. [Google Scholar] [CrossRef]
- Hugelius, G.; Loisel, J.; Chadburn, S.; Jackson, R.B.; Jones, M.; MacDonald, G.; Marushchak, M.; Olefeldt, D.; Packalen, M.; Siewert, M.B.; et al. Large stocks of peatland carbon and nitrogen are vulnerable to permafrost thaw. Proc. Natl. Acad. Sci. USA 2020, 117, 20438–20446. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.; Hugelius, G.; Shelef, E.; Yang, Y.; Strauss, J.; Lupachev, A.; Harden, J.W.; Jastrow, J.D.; Ping, C.-L.; Riley, W.J.; et al. Spatial heterogeneity and environmental predictors of permafrost region soil organic carbon stocks. Sci. Adv. 2021, 7, eaaz5236. [Google Scholar] [CrossRef] [PubMed]
- Post, W.M.; Zinke, P.J.; Stangenberger, A.G. Global patterns of soil nitrogen storage. Nature 1985, 317, 613–616. [Google Scholar] [CrossRef]
- Rodionov, A.; Flessa, H.; Grabe, M.; Kazansky, O.A.; Shibistova, O.; Guggenberger, G. Organic carbon and total nitrogen variability in permafrost-affected soils in a forest tundra ecotone. Eur. J. Soil Sci. 2007, 58, 1260–1272. [Google Scholar] [CrossRef]
- Harden, J.W.; Koven, C.D.; Ping, C.-L.; Hugelius, G.; David McGuire, A.; Camill, P.; Jorgenson, T.; Kuhry, P.; Michaelson, G.J.; O’Donnell, J.A.; et al. Field information links permafrost carbon to physical vulnerabilities of thawing. Geophys. Res. Lett. 2012, 39, L15704. [Google Scholar] [CrossRef]
- Zubrzycki, S.; Kutzbach, L.; Grosse, G.; Desyatkin, A.; Pfeiffer, E.M. Organic carbon and total nitrogen stocks in soils of the Lena River Delta. Biogeosciences 2013, 10, 3507–3524. [Google Scholar] [CrossRef]
- Fuchs, M.; Grosse, G.; Strauss, J.; Günther, F.; Grigoriev, M.; Maximov, G.M.; Hugelius, G. Carbon and nitrogen pools in thermokarst-affected permafrost landscapes in Arctic Siberia. Biogeosciences 2018, 15, 953–971. [Google Scholar] [CrossRef]
- Weintraub, M.N.; Schimel, J.P. Interactions between carbon and nitrogen mineralization and soil organic matter chemistry in Arctic tundra soils. Ecosystems 2003, 6, 129–143. [Google Scholar] [CrossRef]
- Strauss, J.; Abbott, B.W.; Beermann, F.; Biasi, C.; Fuchs, M.; Grosse, G.; Horn, M.A.; Liebner, S.; Sanders, T.; Schirrmeister, L.; et al. The nitrogen stock of the ice-rich yedoma domain. In Proceedings of the 5th European Conference on Permafrost, Chamonix-Mont-Blanc, France, 23 June–1 July 2018. [Google Scholar]
- Strauss, J.; Biasi, C.; Sanders, T.; Abbott, B.W.; Schneider von Deimling, T.; Voigt, C.; Winkel, M.; Marushchak, M.E.; Kou, D.; Fuchs, M.; et al. A globally relevant stock of soil nitrogen in the Yedoma permafrost domain. Nat. Commun. 2022. in review. [Google Scholar]
- Shaver, G.R.; Chapin, F.S., III. Response to fertilization by various plant growth forms in an Alaskan tundra: Nutrient accumulation and growth. Ecology 1980, 61, 662–675. [Google Scholar] [CrossRef]
- Jonasson, S. Nutrient content and dynamics in north Swedish shrub tundra areas. Ecography 1983, 6, 295–304. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Shaver, G.R.; Giblin, A.E.; Nadelhoffer, K.J.; Laundre, J.A. Responses of Arctic tundra to experimental and observed changes in climate. Ecology 1995, 76, 694–711. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Shaver, G.R. Physiological and growth responses of Arctic plants to a field experiment simulating climatic change. Ecology 1996, 77, 822–840. [Google Scholar] [CrossRef]
- Shaver, G.R.; Billings, W.D.; Stuart Chapin, F., III; Giblin, A.E.; Nadelhoffer, K.J.; Oechel, W.C.; Rastetter, E.B. Global change and the carbon balance of Arctic ecosystems. BioScience 1992, 42, 433–441. [Google Scholar] [CrossRef]
- Shaver, G.R.; Johnson, L.C.; Cades, D.H.; Murray, G.; Laundre, J.A.; Rastetter, E.B.; Nadelhoffer, K.J.; Giblin, A.E. Biomass and CO2 flux in wet sedge tundras: Responses to nutrients, temperature, and light. Ecol. Monogr. 1998, 68, 75–97. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Nadelhoffer, K.J.; Högberg, P. A synthesis: The role of nutrients as constraints on carbon balances in boreal and Arctic regions. Plant Soil 2002, 242, 163–170. [Google Scholar] [CrossRef]
- Mack, M.C.; Schuur, E.A.G.; Bret-Harte, M.S.; Shaver, G.R.; Chapin, F.S., III. Ecosystem carbon storage in Arctic tundra reduced by long-term nutrient fertilization. Nature 2004, 431, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.; Meyer, H.; Biasi, C.; Rusalimova, O.; Barsukov, P.; Richter, A. Storage and mineralization of carbon and nitrogen in soils of a frost-boil tundra ecosystem in Siberia. Appl. Soil Ecol. 2005, 29, 173–183. [Google Scholar] [CrossRef]
- Sistla, S.A.; Asao, S.; Schimel, J.P. Detecting microbial N-limitation in tussock tundra soil: Implications for Arctic soil organic carbon cycling. Soil Biol. Biochem. 2012, 55, 78–84. [Google Scholar] [CrossRef]
- Stewart, K.J.; Grogan, P.; Coxson, D.S.; Siciliano, S.D. Topography as a key factor driving atmospheric nitrogen exchanges in Arctic terrestrial ecosystems. Soil Biol. Biochem. 2014, 70, 96–112. [Google Scholar] [CrossRef]
- Beermann, F.; Teltewskoi, A.; Fiencke, C.; Pfeiffer, E.M.; Kutzbach, L. Stoichiometric analysis of nutrient availability (N, P, K) within soils of polygonal tundra. Biogeochemistry 2015, 122, 211–227. [Google Scholar] [CrossRef]
- Salmon, V.G.; Schädel, C.; Bracho, R.; Pegoraro, E.; Celis, G.; Mauritz, M.; Mack, M.C.; Schuur, E.A.G. Adding depth to our understanding of nitrogen dynamics in permafrost soils. J. Geophys. Res. Biogeosci. 2018, 123, 2497–2512. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Koba, K.; Koyama, L.A.; Hobbie, S.E.; Weiss, M.S.; Inagaki, Y.; Shaver, G.R.; Giblin, A.E.; Hobara, S.; Nadelhoffer, K.J.; et al. Nitrate is an important nitrogen source for Arctic tundra plants. Proc. Natl. Acad. Sci. USA 2018, 115, 3398–3403. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.A.; Hetz, A. Microbial nitrogen cycling in permafrost soils: Implications for atmospheric chemistry. In Microbial Life in the Cryosphere and Its Feedback on Global Change; Liebner, S., Ganzert, L., Eds.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2021; pp. 53–112. [Google Scholar]
- Ramm, E.; Liu, C.Y.; Ambus, P.; Butterbach-Bahl, K.; Hu, B.; Martikainen, P.J.; Marushchak, M.E.; Mueller, C.W.; Rennenberg, H.; Schloter, M.; et al. A review of the importance of mineral nitrogen cycling in the plant-soil-microbe system of permafrost-affected soils-changing the paradigm. Environ. Res. Lett. 2022, 17, 013004. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Schimel, D.S.; Fisher, H.; Howarth, R.W.; Hedin, L.O.; Perakis, S.S.; Latty, E.F.; Von Fischer, J.C.; Elseroad, A.; et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 1999, 13, 623–645. [Google Scholar] [CrossRef]
- Hobara, S.; McCalley, C.; Koba, K.; Giblin, A.E.; Weiss, M.S.; Gettel, G.M.; Shaver, G.R. Nitrogen fixation in surface soils and vegetation in an Arctic tundra watershed: A key source of atmospheric nitrogen. Arct. Antarct. Alp. Res. 2006, 38, 363–372. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, R.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Soc. Am. 2010, 20, 30–59. [Google Scholar] [CrossRef]
- Ackerman, D.; Millet, D.B.; Chen, X. global estimates of inorganic nitrogen deposition across four decades. Glob. Biogeochem. Cycles 2019, 33, 100–107. [Google Scholar] [CrossRef]
- Van Cleve, K.; Alexander, V. Nitrogen cycling in tundra and boreal ecosystems. Ecol. Bull. 1981, 33, 375–404. [Google Scholar]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Zufelt, E.; Chu, H.Y.; Grogan, P. Soil nitrogen cycling rates in low Arctic shrub tundra are enhanced by litter feedbacks. Plant Soil 2010, 330, 407–421. [Google Scholar] [CrossRef]
- Pearce, A.R.; Rastetter, E.B.; Kwiatkowski, B.L.; Bowden, W.B.; Mack, M.C.; Jiang, Y. Recovery of Arctic tundra from thermal erosion disturbance is constrained by nutrient accumulation: A modeling analysis. Ecol. Appl. 2015, 25, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Wild, B.; Schnecker, J.; Knoltsch, A.; Takriti, M.; Mooshammer, M.; Gentsch, N.; Mikutta, R.; Alves, R.J.; Gittel, A.; Lashchinskiy, N.; et al. Microbial nitrogen dynamics in organic and mineral soil horizons along a latitudinal transect in western Siberia. Glob. Biogeochem. Cycles 2015, 29, 567–582. [Google Scholar] [CrossRef]
- Pastor, A.; Poblador, S.; Skovsholt, L.J.; Riis, T. Microbial carbon and nitrogen processes in high-Arctic riparian soils. Permafr. Periglac. Process. 2020, 31, 223–236. [Google Scholar] [CrossRef]
- Jonasson, S.; Michelsen, A.; Schmidt, I.K. Coupling of nutrient cycling and carbon dynamics in the Arctic, integration of soil microbial and plant processes. Appl. Soil Ecol. 1999, 11, 135–146. [Google Scholar] [CrossRef]
- Wild, B.; Alves, R.J.E.; Bárta, J.; Čapek, P.; Gentsch, N.; Guggenberger, G.; Hugelius, G.; Knoltsch, A.; Kuhry, P.; Lashchinskiy, N.; et al. Amino acid production exceeds plant nitrogen demand in Siberian tundra. Environ. Res. Lett. 2018, 13, 034002. [Google Scholar] [CrossRef]
- Pedersen, E.P.; Elberling, B.; Michelsen, A. Foraging deeply: Depth-specific plant nitrogen uptake in response to climate-induced N-release and permafrost thaw in the High Arctic. Glob. Chang. Biol. 2020, 26, 6523–6536. [Google Scholar] [CrossRef]
- Kielland, K. Amino acid absorption by Arctic plants: Implications for plant nutrition and nitrogen cycling. Ecology 1994, 75, 2373–2383. [Google Scholar] [CrossRef]
- Chu, H.; Grogan, P. Soil microbial biomass, nutrient availability and nitrogen mineralization potential among vegetation-types in a low Arctic tundra landscape. Plant Soil 2010, 329, 411–420. [Google Scholar] [CrossRef]
- Keuper, F.; van Bodegom, P.M.; Dorrepaal, E.; Weedon, J.T.; van Hal, J.; van Logtestijn, R.S.P.; Aerts, R. A frozen feast: Thawing permafrost increases plant-available nitrogen in subarctic peatlands. Glob. Chang. Biol. 2012, 18, 1998–2007. [Google Scholar] [CrossRef]
- Wickland, K.P.; Waldrop, M.P.; Aiken, G.R.; Koch, J.C.; Jorgenson, M.T.; Striegl, R.G. Dissolved organic carbon and nitrogen release from boreal Holocene permafrost and seasonally frozen soils of Alaska. Environ. Res. Lett. 2018, 13, 065011. [Google Scholar] [CrossRef]
- Fouche, J.; Christiansen, C.T.; Lafreniere, M.J.; Grogan, P.; Lamoureux, S.F. Canadian permafrost stores large pools of ammonium and optically distinct dissolved organic matter. Nat. Commun. 2020, 11, 4500. [Google Scholar] [CrossRef]
- Schmidt, I.K.; Jonasson, S.; Shaver, G.R.; Michelsen, A.; Nordin, A. Mineralization and distribution of nutrients in plants and microbes in four Arctic ecosystems: Responses to warming. Plant Soil 2002, 242, 93–106. [Google Scholar] [CrossRef]
- Biasi, C.; Wanek, W.; Rusalimova, O.; Kaiser, C.; Meyer, H.; Barsukov, P.; Richter, A. Microtopography and plant-cover controls on nitrogen dynamics in hummock tundra ecosystems in siberia. Arct. Antarct. Alp. Res. 2005, 37, 435–443. [Google Scholar] [CrossRef]
- Sorensen, P.L.; Jonasson, S.; Michelsen, A. Nitrogen Fixation, Denitrification, and Ecosystem Nitrogen Pools in Relation to VegetationDevelopment in the Subarctic. Arct. Antarct. Alp. Res. 2006, 38, 263–272. [Google Scholar] [CrossRef]
- Kelley, A.M.; Epstein, H.E.; Ping, C.-L.; Walker, D.A. Soil nitrogen transformations associated with small patterned-ground features along a North American Arctic Transect. Permafr. Periglac. Process. 2012, 23, 196–206. [Google Scholar] [CrossRef]
- Voigt, C.; Marushchak, M.E.; Abbott, B.W.; Biasi, C.; Elberling, B.; Siciliano, S.D.; Sonnentag, O.; Stewart, K.J.; Yang, Y.; Martikainen, P.J. Nitrous oxide emissions from permafrost-affected soils. Nat. Rev. Earth Environ. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, S.; Manning, M.; Alley, R.B.; Berntsen, T.; Bindoff, N.L.; Chen, Z.; Chidthaisong, A.; Gregory, J.M.; Hegerl, G.C.; et al. Technical Summary. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, S., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 19–91. [Google Scholar]
- Jones, J.B.; Petrone, K.C.; Finlay, J.C.; Hinzman, L.D.; Bolton, W.R. Nitrogen loss from watersheds of interior Alaska underlain with discontinuous permafrost. Geophys. Res. Lett. 2005, 32, L02401. [Google Scholar] [CrossRef]
- Petrone, K.C.; Jones, J.B.; Hinzman, L.D.; Boone, R.D. Seasonal export of carbon, nitrogen, and major solutes from Alaskan catchments with discontinuous permafrost. J. Geophys. Res. Biogeosci. 2006, 111, e2021GB007242. [Google Scholar] [CrossRef]
- Marushchak, M.E.; Pitkämäki, A.; Koponen, H.; Biasi, C.; Seppälä, M.; Martikainen, P.J. Hot spots for nitrous oxide emissions found in different types of permafrost peatlands. Glob. Chang. Biol. 2011, 17, 2601–2614. [Google Scholar] [CrossRef]
- Marushchak, M.E.; Kerttula, J.; Diáková, K.; Faguet, A.; Gil, J.; Grosse, G.; Knoblauch, C.; Laschinskiy, N.; Nykamb, M.; Martikainen, P.J.; et al. Thawing yedoma permafrost as a neglected nitrous oxide source. Nat. Commun. 2021, 12, 7107. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.W.; Jones, J.B. Permafrost collapse alters soil carbon stocks, respiration, CH4, and N2O in upland tundra. Glob. Chang. Biol. 2015, 21, 4570–4587. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, N.S.; Trumbore, S.E.; Schuur, E.A.G.; Mack, M.C.; Shaver, G.R. Nutrient addition prompts rapid destabilization of organic matter in an Arctic tundra ecosystem. Ecosystems 2008, 11, 16–25. [Google Scholar] [CrossRef]
- Koyama, A.; Wallenstein, M.D.; Simpson, R.T.; Moore, J.C. Carbon-degrading enzyme activities stimulated by increased nutrient availability in Arctic tundra soils. PLoS ONE 2013, 8, e77212. [Google Scholar] [CrossRef]
- Koyama, A.; Wallenstein, M.D.; Simpson, R.T.; Moore, J.C. Soil bacterial community composition altered by increased nutrient availability in Arctic tundra soils. Front. Microbiol. 2014, 5, 516. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Koven, C.D.; Lawrence, D.M.; Riley, W.J. Permafrost carbon−climate feedback is sensitive to deep soil carbon decomposability but not deep soil nitrogen dynamics. Proc. Natl. Acad. Sci. USA 2015, 112, 3752–3757. [Google Scholar] [CrossRef]
- Wieder, W.R.; Allison, S.D.; Davidson, E.A.; Georgiou, K.; Hararuk, O.; He, Y.; Hopkins, F.; Luo, Y.; Smith, M.J.; Sulman, B.; et al. Explicitly representing soil microbial processes in Earth system models. Glob. Biogeochem. Cycles 2015, 29, 1782–1800. [Google Scholar] [CrossRef]
- Kou, D.; Yang, G.; Li, F.; Feng, X.; Zhang, D.; Mao, C.; Zhang, Q.; Peng, Y.; Ji, C.; Zhu, Q.; et al. Progressive nitrogen limitation across the Tibetan alpine permafrost region. Nat. Commun. 2020, 11, 3331. [Google Scholar] [CrossRef]
- Terhaar, J.; Lauerwald, R.; Regnier, P.; Gruber, N.; Bopp, L. Around one third of current Arctic Ocean primary production sustained by rivers and coastal erosion. Nat. Commun. 2021, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.E.; Tank, S.E.; Bowden, W.B.; Laurion, I.; Vincent, W.F.; Alekseychik, P.; Amyot, M.; Billet, M.F.; Canario, J.; Cory, R.M.; et al. Reviews and syntheses: Effects of permafrost thaw on Arctic aquatic ecosystems. Biogeosciences 2015, 12, 7129–7167. [Google Scholar] [CrossRef]
- Loranty, M.M.; Abbott, B.W.; Blok, D.; Douglas, T.A.; Epstein, H.E.; Forbes, B.C.; Jones, B.M.; Kholodov, A.L.; Kropp, H.; Malhotra, A.; et al. Reviews and syntheses: Changing ecosystem influences on soil thermal regimes in northern high-latitude permafrost regions. Biogeosciences 2018, 15, 5287–5313. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Abbott, B.W.; Jones, M.C.; Anthony, K.W.; Olefeldt, D.; Schuur, E.A.G.; Grosse, G.; Kuhry, P.; Hugelius, G.; Koven, C.; et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 2020, 13, 138–143. [Google Scholar] [CrossRef]
- Miner, K.R.; Turetsky, M.R.; Malina, E.; Bartsch, A.; Tamminen, J.; McGuire, A.D.; Fix, A.; Sweeney, C.; Elder, C.D.; Miller, C.E. Permafrost carbon emissions in a changing Arctic. Nat. Rev. Earth Environ. 2022, 3, 55–67. [Google Scholar] [CrossRef]
- Jansson, J.K.; Taş, N. The microbial ecology of permafrost. Nat. Rev. Microbiol. 2014, 12, 414–425. [Google Scholar] [CrossRef]
- Salazar, A.; Rousk, K.; Jónsdóttir, I.; Bellenger, J.-P.; Andrésson, Ó. Faster nitrogen cycling and more fungal and root biomass in cold ecosystems under experimental warming: A meta-analysis. Ecol. Soc. Am. 2020, 101, e02938. [Google Scholar] [CrossRef]
- Beermann, F.; Langer, M.; Wetterich, S.; Strauss, J.; Boike, J.; Fiencke, C.; Schirrmeister, L.; Pfeiffer, E.M.; Kutzbach, L. Permafrost thaw and liberation of inorganic nitrogen in eastern siberia. Permafr. Periglac. Process. 2017, 28, 605–618. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bilbrough, C.; Welker, J.M. Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biol. Biochem. 2004, 36, 217–227. [Google Scholar] [CrossRef]
- Mason, R.E.; Craine, J.M.; Lany, N.K.; Jonard, M.; Ollinger, S.V.; Groffman, P.M.; Fulweiler, R.W.; Angerer, J.; Read, Q.D.; Reich, P.B.; et al. Evidence, causes, and consequences of declining nitrogen availability in terrestrial ecosystems. Science 2022, 376, eabh3767. [Google Scholar] [CrossRef]
- Biasi, C.; Meyer, H.; Rusalimova, O.; Hammerle, R.; Kaiser, C.; Baranyi, C.; Daims, H.; Lashchinsky, N.; Barsukov, P.; Richter, A. Initial effects of experimental warming on carbon exchange rates, plant growth and microbial dynamics of a lichen-rich dwarf shrub tundra in Siberia. Plant Soil 2008, 307, 191–205. [Google Scholar] [CrossRef]
- Finger, R.A.; Turetsky, M.R.; Kielland, K.; Ruess, R.W.; Mack, M.C.; Euskirchen, E.S. Effects of permafrost thaw on nitrogen availability and plant–soil interactions in a boreal Alaskan lowland. J. Ecol. 2016, 104, 1542–1554. [Google Scholar] [CrossRef]
- Salmon, V.G.; Soucy, P.; Mauritz, M.; Celis, G.; Natali, S.M.; Mack, M.C.; Schuur, E.A.G. Nitrogen availability increases in a tundra ecosystem during five years of experimental permafrost thaw. Glob. Chang. Biol. 2016, 22, 1927–1941. [Google Scholar] [CrossRef]
- Mao, C.; Kou, D.; Chen, L.; Qin, S.; Zhang, D.; Peng, Y.; Yang, Y. Permafrost nitrogen status and its determinants on the Tibetan Plateau. Glob. Chang. Biol. 2020, 26, 5290–5302. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Kou, D.; Li, F.; Ding, J.Z.; Yang, G.B.; Fang, K.; Yang, Y.H. Linkage of plant and abiotic properties to the abundance and activity of N-cycling microbial communities in Tibetan permafrost-affected regions. Plant Soil 2019, 434, 453–466. [Google Scholar] [CrossRef]
- Ramm, E.; Liu, C.; Mueller, C.; Gschwendtner, S.; Yue, H.; Wang, X.; Bachmann, J.; Bonhoff, J.A.; Ostler, U.; Schloter, M.; et al. Alder-induced stimulation of soil gross nitrogen turnover in a permafrost-affected peatland of Northeast China. Soil Biol. Biochem. 2022, 172, 108787. [Google Scholar] [CrossRef]
- Chapin, D.M. Nitrogen mineralization, nitrification, and denitrification in a high arctic lowland ecosystem, Devon Island, NWT, Canada. Arct. Alp. Res. 1996, 28, 85–92. [Google Scholar] [CrossRef]
- Amundson, R.; Austin, A.T.; Schuur, E.A.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W.T. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 2003, 17, 1031. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Bai, E. Imprint of denitrifying bacteria on the global terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2009, 106, 21713–21716. [Google Scholar] [CrossRef]
- Malone, E.T.; Abbott, B.W.; Klaar, M.J.; Kidd, C.; Sebilo, M.; Milner, A.M.; Pinay, G. Decline in ecosystem δ13C and mid-successional nitrogen loss in a two-century postglacial chronosequence. Ecosystems 2018, 21, 1659–1675. [Google Scholar] [CrossRef]
- Schuur, E.; Crummer, K.; Vogel, J.; Mack, M. Plant species composition and productivity following permafrost thaw and thermokarst in Alaskan tundra. Ecosystems 2007, 10, 280–292. [Google Scholar] [CrossRef]
- Keuper, F.; Dorrepaal, E.; van Bodegom, P.M.; van Logtestijn, R.; Venhuizen, G.; van Hal, J.; Aerts, R. Experimentally increased nutrient availability at the permafrost thaw front selectively enhances biomass production of deep-rooting subarctic peatland species. Glob. Chang. Biol. 2017, 23, 4257–4266. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.J.; Wanek, W.; Zappe, A.; Richter, A.; Svenning, M.M.; Schleper, C.; Urich, T. Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J. 2013, 7, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.; Fiencke, C.; Hüpeden, J.; Pfeiffer, E.-M.; Spieck, E. Cold adapted nitrosospira sp.: A potential crucial contributor of ammonia oxidation in cryosols of permafrost-affected landscapes in northeast Siberia. Microorganisms 2019, 7, 699. [Google Scholar] [CrossRef]
- Siljanen, H.M.P.; Alves, R.J.E.; Ronkainen, J.G.; Lamprecht, R.E.; Bhattarai, H.R.; Bagnoud, A.; Marushchak, M.E.; Martikainen, P.J.; Schleper, C.; Biasi, C. Archaeal nitrification is a key driver of high nitrous oxide emissions from arctic peatlands. Soil Biol. Biochem. 2019, 137, 107539. [Google Scholar] [CrossRef]
- Song, Y.Y.; Liu, C.; Wang, X.W.; Ma, X.Y.; Jiang, L.; Zhu, J.P.; Gao, J.L.; Song, C.C. Microbial abundance as an indicator of soil carbon and nitrogen nutrient in permafrost peatlands. Ecol. Indic. 2020, 115, 106362. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Dannenmann, M. Soil carbon and nitrogen interactions and biosphere-atmosphere exchange of nitrous oxide and methane. In Recarbonization of the Biosphere; Lal, R., Hüttl, R.H., Braun, V.J., Lorenz, K., Schneider, B.U., Eds.; Springer: Dodrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2012; pp. 429–444. [Google Scholar]
- Zhang, S.S.; Zheng, Q.; Noll, L.; Hu, Y.T.; Wanek, W. Environmental effects on soil microbial nitrogen use efficiency are controlled by allocation of organic nitrogen to microbial growth and regulate gross N mineralization. Soil Biol. Biochem. 2018, 135, 304–315. [Google Scholar] [CrossRef]
- Aber, J.D. Nitrogen cycling and nitrogen saturationin in temperate forest ecosystem. Trends Ecol. Evol. 1992, 7, 220–223. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Mao, C.; Qin, S.; Wang, J.; Liu, F.; Blagodatsky, S.; Yang, G.; Zhang, Q.; Zhang, D.; et al. Nitrogen availability regulates topsoil carbon dynamics after permafrost thaw by altering microbial metabolic efficiency. Nat. Commun. 2018, 9, 3951. [Google Scholar] [CrossRef]
- Klemedtsson, L.; Von Arnold, K.; Weslien, P.; Gundersen, P. Soil CN ratio as a scalar parameter to predict nitrous oxide emissions. Glob. Chang. Biol. 2005, 11, 1142–1147. [Google Scholar] [CrossRef]
- Davidson, E.A.; Keller, M.; Erickson, H.E.; Verchot, L.V.; Veldkamp, E. Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 2000, 50, 667–680. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I.; Hink, L.; Gubry-Rangin, C.; Nicol, G.W. Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies. Glob. Chang. Biol. 2020, 26, 103–118. [Google Scholar] [CrossRef]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [CrossRef]
- Banerjee, S.; Si, B.C.; Siciliano, S.D. Evidence of high microbial abundance and spatial dependency in three Arctic soil ecosystems. Soil Sci. Soc. Am. J. 2011, 75, 2227–2232. [Google Scholar] [CrossRef]
- Malard, L.A.; Pearce, D.A. Microbial diversity and biogeography in Arctic soils. Environ. Microbiol. Rep. 2018, 10, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Wilhelm, R.C.; Radtke, K.J.; Mykytczuk, N.C.; Greer, C.W.; Whyte, L.G. Life at the wedge: The activity and diversity of arctic ice wedge microbial communities. Astrobiology 2012, 12, 347–360. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; McFarland, J.; Blazewicz, S.J.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B.; et al. Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature 2015, 521, 208–212. [Google Scholar] [CrossRef]
- Altshuler, I.; Ronholm, J.; Layton, A.; Onstott, T.C.; Greer, C.W.; Whyte, L.G. Denitrifiers, nitrogen-fixing bacteria and N2O soil gas flux in high Arctic ice-wedge polygon cryosols. FEMS Microbiol. Ecol. 2019, 95, fiz049. [Google Scholar] [CrossRef]
- Raymond-Bouchard, I.; Whyte, L.G. From Transcriptomes to metatranscriptomes: Cold adaptation and active metabolisms of psychrophiles from cold environments. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 437–457. [Google Scholar] [CrossRef]
- Margesin, R.; Collins, T. Microbial ecology of the cryosphere (glacial and permafrost habitats): Current knowledge. Appl. Microbiol. Biotechnol. 2019, 103, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Mackelprang, R.; Waldrop, M.P.; DeAngelis, K.M.; David, M.M.; Chavarria, K.L.; Blazewicz, S.J.; Rubin, E.M.; Jansson, J.K. Metagenomic analysis of a permafrost microbial community reveals a rapid response to thaw. Nature 2011, 480, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Taş, N.; Prestat, E.; McFarland, J.W.; Wickland, K.P.; Knight, R.; Berhe, A.A.; Jorgenson, T.; Waldrop, M.P.; Jansson, J.K. Impact of fire on active layer and permafrost microbial communities and metagenomes in an upland Alaskan boreal forest. ISME J. 2014, 8, 1904–1919. [Google Scholar] [CrossRef]
- Taş, N.; Prestat, E.; Wang, S.; Wu, Y.; Ulrich, C.; Kneafsey, T.; Tringe, S.G.; Torn, M.S.; Hubbard, S.S.; Jansson, J.K. Landscape topography structures the soil microbiome in arctic polygonal tundra. Nat. Commun. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Gu, Y.; Zhang, J.; Xue, K.; Qin, Y.; Yuan, M.; Yin, H.; He, Z.; Wu, L.; Schuur, E.A.; et al. Shifts of tundra bacterial and archaeal communities along a permafrost thaw gradient in Alaska. Mol. Ecol. 2015, 24, 222–234. [Google Scholar] [CrossRef]
- Müller, O.; Bang-Andreasen, T.; White, R.A., III; Elberling, B.; Taş, N.; Kneafsey, T.; Jansson, J.K.; Øvreås, L. Disentangling the complexity of permafrost soil by using high resolution profiling of microbial community composition, key functions and respiration rates. Environ. Microbiol. 2018, 20, 4328–4342. [Google Scholar] [CrossRef]
- Rivkina, E.M.; Friedmann, E.I.; McKay, C.P.; Gilichinsky, D.A. Metabolic activity of permafrost bacteria below the freezing point. Appl. Environ. Microbiol. 2000, 66, 3230–3233. [Google Scholar] [CrossRef]
- Khmelenina, V.N.; Makutina, V.A.; Khalyuzhnaya, M.G.; Rivkina, E.M.; Gilichinsky, D.A.; Trotsenko, Y.A. Dicovery of viable methanotrophic bacteria in permafrost sediments of Northeast Siberia. Dokl. Biol. Sci. 2002, 384, 235–237. [Google Scholar] [CrossRef]
- Gilichinsky, D.; Rivkina, E.; Shcherbakova, V.; Laurinavichuis, K.; Tiedje, J. Supercooled water brines within permafrost—An unknown ecological niche for microorganisms: A model for astrobiology. Astrobiology 2003, 3, 331–341. [Google Scholar] [CrossRef]
- Frank-Fahle, B.A.; Yergeau, É.; Greer, C.W.; Lantuit, H.; Wagner, D. Microbial functional potential and community composition in permafrost-affected soils of the NW Canadian Arctic. PLoS ONE 2014, 9, e84761. [Google Scholar] [CrossRef]
- Tuorto, S.J.; Darias, P.; McGuinness, L.R.; Panikov, N.; Zhang, T.; Häggblom, M.M.; Kerkhof, L.J. Bacterial genome replication at subzero temperatures in permafrost. ISME J. 2014, 8, 139–149. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, M.J.; Jung, J.Y.; Hwang, C.Y.; Kim, M.; Ro, H.-M.; Chun, J.; Lee, Y.K. Vertical distribution of bacterial community is associated with the degree of soil organic matter decomposition in the active layer of moist acidic tundra. J. Microbiol. 2016, 54, 713–723. [Google Scholar] [CrossRef]
- Altshuler, I.; Goordial, J.; Whyte, L.G. Microbial life in permafrost. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 153–179. [Google Scholar] [CrossRef]
- Mackelprang, R.; Burkert, A.; Haw, M.; Mahendrarajah, T.; Conaway, C.H.; Douglas, T.A.; Waldrop, M.P. Microbial survival strategies in ancient permafrost: Insights from metagenomics. ISME J. 2017, 11, 2305–2318. [Google Scholar] [CrossRef]
- Gilichinsky, D.A.; Vorobyova, E.A.; Erokhina, L.G.; Fyordorov-Davydov, D.G.; Chaikovskaya, N.R.; Fyordorov-Dayvdov, D.G. Long-term preservation of microbial ecosystems in permafrost. Adv. Space Res. 1992, 12, 255–263. [Google Scholar] [CrossRef]
- Gilichinsky, D.A.; Soina, V.S.; Petrova, M.A. Cryoprotective properties of water in the Earth cryolithosphere and its role in exobiology. Orig. Life Evol. Biosph. 1993, 23, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Gilichinsky, D.; Wagener, S. Microbial Life in Permafrost-a Historical Review. Permafr. Periglac. Process. 1995, 6, 243–250. [Google Scholar] [CrossRef]
- Alawi, M.; Lipski, A.; Sanders, T.; Pfeiffer, E.M.; Spieck, E. Cultivation of a novel cold-adapted nitrite oxidizing betaproteobacterium from the Siberian Arctic. ISME J. 2007, 1, 256–264. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; McMahon, S.K.; Schimel, J.P. Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob. Chang. Biol. 2009, 15, 1631–1639. [Google Scholar] [CrossRef]
- McMahon, S.K.; Wallenstein, M.D.; Schimel, J.P. A cross-seasonal comparison of active and total bacterial community composition in Arctic tundra soil using bromodeoxyuridine labeling. Soil Biol. Biochem. 2011, 43, 287–295. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Seasonal patterns of microbial extracellular enzyme activities in an arctic tundra soil: Identifying direct and indirect effects of long-term summer warming. Soil Biol. Biochem. 2013, 66, 119–129. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Banerjee, S.; Siciliano, S.D.; Grogan, P. The seasonal pattern of soil microbial community structure in mesic low arctic tundra. Soil Biol. Biochem. 2013, 65, 338–347. [Google Scholar] [CrossRef]
- Schostag, M.; Stibal, M.; Jacobsen, C.S.; Bælum, J.; Taş, N.; Elberling, B.; Jansson, J.K.; Semenchuk, P.; Priemé, A. Distinct summer and winter bacterial communities in the active layer of Svalbard permafrost revealed by DNA- and RNA-based analyses. Front. Microbiol. 2015, 6, 399. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Saetnan, E.R.; Scullion, J.; Gwynn-Jones, D.; Ostle, N.; Edwards, A. Temporal and spatial influences incur reconfiguration of Arctic heathland soil bacterial community structure. Environ. Microbiol. 2016, 18, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, P. Heterotrophic nitrification—An eternal mystery in the nitrogen cycle. Soil Biol. Biochem. 2022, 168, 108611. [Google Scholar] [CrossRef]
- Ma, W.K.; Schautz, A.; Fishback, L.A.E.; Bedard-Haughn, A.; Farrell, R.E.; Siciliano, S.D. Assessing the potential of ammonia oxidizing bacteria to produce nitrous oxide in soils of a high arctic lowland ecosystem on Devon Island, Canada. Soil Biol. Biochem. 2007, 39, 2001–2013. [Google Scholar] [CrossRef]
- Booth, M.S.; Stark, J.M.; Rastetter, E. Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data. Ecol. Monogr. 2005, 75, 139–157. [Google Scholar] [CrossRef]
- Surey, R.; Lippold, E.; Heilek, S.; Sauheitl, L.; Henjes, S.; Horn, M.A.; Mueller, C.W.; Merbach, I.; Kaiser, K.; Bottcher, J.; et al. Differences in labile soil organic matter explain potential denitrification and denitrifying communities in a long-term fertilization experiment. Appl. Soil Ecol. 2020, 153, 103630. [Google Scholar] [CrossRef]
- Koch, H.; van Kessel, M.A.H.J.; Lucker, S. Complete nitrification: Insights into the ecophysiology of comammox Nitrospira. Appl. Microbiol. Biotechnol. 2019, 103, 177–189. [Google Scholar] [CrossRef]
- Wagner, D.; Spieck, E.; Bock, E.; Pfeiffer, E.-M. Microbial life in terrestrial permafrost: Methanogenesis and nitrification in gelisols as potentials for exobiological processes. In Astrobiology: The Quest for the Conditions of Life; Horneck, G., Baumstark-Khan, C., Eds.; Springer: Berlin, Germany; New York, NY, USA, 2001; pp. 143–159. [Google Scholar]
- Bartosch, S.; Hartwig, C.; Spieck, E.; Bock, E. Immunological detection of nitrospira-like bacteria in various soils. Microb. Ecol. 2002, 43, 26–33. [Google Scholar] [CrossRef]
- Binkley, D.; Stottlemyer, R.; Suarez, F.; Cortina, J. Soil nitrogen availability in some arctic ecosystems in northwest Alaska: Responses to temperature and moisture. Ecoscience 1994, 1, 64–70. [Google Scholar] [CrossRef]
- Atkin, O.K. Reassessing the nitrogen relations of Arctic plants: A mini-review. Plant Cell Environ. 1996, 19, 695–704. [Google Scholar] [CrossRef]
- Wilson, K.; Sprent, J.I.; Hopkins, D.W. Nitrification in Antarctic soils. Nature 1997, 385, 404. [Google Scholar] [CrossRef]
- Hart, S.C.; Gunther, A.J. In situ estimates of annual net nitrogen mineralization and nitrification in a subarctic watershed. Oecologia 1989, 80, 284–288. [Google Scholar] [CrossRef]
- Jones, D.L.; Kielland, K. Soil amino acid turnover dominates the nitrogen flux in permafrost-dominated taiga forest soils. Soil Biol. Biochem. 2002, 34, 209–219. [Google Scholar] [CrossRef]
- Yergeau, E.; Hogues, H.; Whyte, L.G.; Greer, C.W. The functional potential of high Arctic permafrost revealed by metagenomic sequencing, qPCR and microarray analyses. ISME J. 2010, 4, 1206–1214. [Google Scholar] [CrossRef]
- Liebner, S.; Harder, J.; Wagner, D. Bacterial diversity and community structure in polygonal tundra soils from Samoylov Island, Lena Delta, Siberia. Int. Microbiol. 2008, 11, 195–202. [Google Scholar] [CrossRef]
- Pester, M.; Rattei, T.; Flechl, S.; Grongroft, A.; Richter, A.; Overmann, J.; Reinhold-Hurek, B.; Loy, A.; Wagner, M. amoA-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ. Microbiol. 2012, 14, 525–539. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Machado, A.; Frank-Fahle, B.; Lee, C.K.; Cary, S.C. The ecological dichotomy of ammonia-oxidizing archaea and bacteria in the hyper-arid soils of the Antarctic Dry Valleys. Front. Microbiol. 2014, 5, 515. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimomura, Y.; Morimoto, S.; Uchida, M.; Nakatsubo, T.; Hayatsu, M. Characteristics of ammonia oxidation potentials and ammonia oxidizers in mineral soil under Salix polaris–moss vegetation in Ny-Ålesund, Svalbard. Polar Biol. 2016, 39, 725–741. [Google Scholar] [CrossRef]
- Hayashi, K.; Tanabe, Y.; Fujitake, N.; Kida, M.; Wang, Y.; Hayatsu, M.; Kudoh, S. Ammonia oxidation potentials and ammonia oxidizers of lichen-moss vegetated soils at two ice-free areas in east antarctica. Microbes Environ. 2020, 35, ME19126. [Google Scholar] [CrossRef]
- Barnard, S.; Van Goethem, M.W.; de Scally, S.Z.; Cowan, D.A.; van Rensburg, P.J.; Claassens, S.; Makhalanyane, T.P. Increased temperatures alter viable microbial biomass, ammonia oxidizing bacteria and extracellular enzymatic activities in Antarctic soils. FEMS Microbiol. Ecol. 2020, 96, fiaa065. [Google Scholar] [CrossRef] [PubMed]
- Pessi, I.S.; Viitamäki, S.; Virkkala, A.-M.; Eronen-Rasimus, E.; Delmont, T.O.; Marushchak, M.E.; Luoto, M.; Hultman, J. In-depth characterization of denitrifier communities across different soil ecosystems in the tundra. Biorxiv 2022, 17, 30. [Google Scholar] [CrossRef]
- Pessi, I.S.; Rutanen, A.; Hultmanl, J. Candidatus Nitrosopolaris, a genus of putative ammonia oxidizing archaea with a polar/alpine distribution. Biorxiv 2021. preprint. [Google Scholar] [CrossRef]
- Stackhouse, B.T.; Vishnivetskaya, T.A.; Layton, A.; Chauhan, A.; Pfiffner, S.; Mykytczuk, N.C.; Sanders, R.; Whyte, L.G.; Hedin, L.; Saad, N.; et al. Effects of simulated spring thaw of permafrost from mineral cryosol on CO2 emissions and atmospheric CH4 uptake. J. Geophys. Res. Biogeosci. 2015, 120, 1764–1784. [Google Scholar] [CrossRef]
- Xue, Y.; Jonassen, I.; Øvreås, L.; Taş, N. Bacterial and archaeal metagenome-assembled genome sequences from svalbard permafrost. Microbiol. Resour. Announc. 2019, 8, e00516-19. [Google Scholar] [CrossRef] [PubMed]
- Mackelprang, R.; Saleska, S.R.; Jacobsen, C.S.; Jansson, J.K.; Tas, N. Permafrost meta-omics and climate change. Annu Rev. Earth Planet. Sci. 2016, 44, 439–462. [Google Scholar] [CrossRef]
- Monteux, S.; Keuper, F.; Fontaine, S.; Gavazov, K.; Hallin, S.; Juhanson, J.; Krab, E.J.; Revaillot, S.; Verbruggen, E.; Walz, J.; et al. Carbon and nitrogen cycling in Yedoma permafrost controlled by microbial functional limitations. Nat. Geosci. 2020, 13, 794–798. [Google Scholar] [CrossRef]
- Aigle, A.; Prosser, J.I.; Gubry-Rangin, C. The application of high-throughput sequencing technology to analysis of amoA phylogeny and environmental niche specialisation of terrestrial bacterial ammonia-oxidisers. Environ. Microbiome 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, R.; Zheng, Y.; Bao, T.; Hou, L. Effects of sea animal colonization on the coupling between dynamics and activity of soil ammonia-oxidizing bacteria and archaea in maritime Antarctica. Biogeosciences 2019, 16, 4113–4128. [Google Scholar] [CrossRef]
- Dai, H.T.; Zhu, R.B.; Sun, B.W.; Che, C.S.; Hou, L.J. Effects of sea animal activities on tundra soil denitrification and nirS- and nirK-encoding denitrifier community in maritime antarctica. Front. Microbiol. 2020, 11, 573302. [Google Scholar] [CrossRef]
- Alves, R.J.E.; Kerou, M.; Zappe, A.; Bittner, R.; Abby, S.S.; Schmidt, H.A.; Pfeifer, K.; Schleper, C. Ammonia Oxidation by the Arctic Terrestrial Thaumarchaeote Candidatus Nitrosocosmicus arcticus Is Stimulated by Increasing Temperatures. Front. Microbiol. 2019, 10, 1571. [Google Scholar] [CrossRef] [PubMed]
- Kits, K.D.; Jung, M.Y.; Vierheilig, J.; Pjevac, P.; Sedlacek, C.J.; Liu, S.; Herbold, C.; Stein, L.Y.; Richter, A.; Wissel, H.; et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata. Nat. Commun. 2019, 10, 1836. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y. The long-term relationship between microbial metabolism and greenhouse gases. Trends Microbiol. 2020, 28, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Inatomi, M.; Hajima, T.; Ito, A. Fraction of nitrous oxide production in nitrification and its effect on total soil emission: A meta-analysis and global-scale sensitivity analysis using a process-based model. PLoS ONE 2019, 14, e0219159. [Google Scholar] [CrossRef] [PubMed]
- Pärn, J.; Verhoeven, J.T.A.; Butterbach-Bahl, K.; Dise, N.B.; Ullah, S.; Aasa, A.; Egorov, S.; Espenberg, M.; Järveoja, J.; Jauhiainen, J.; et al. Nitrogen-rich organic soils under warm well-drained conditions are global nitrous oxide emission hotspots. Nat. Commun. 2018, 9, 1135. [Google Scholar] [CrossRef]
- Bouskill, N.; Tang, J.; Riley, W.; Brodie, E. Trait-based representation of biological nitrification: Model development, testing, and predicted community composition. Front. Microbiol. 2012, 3, 364. [Google Scholar] [CrossRef]
- Keuter, S.; Koch, H.; Sass, K.; Wegen, S.; Lee, N.; Lucker, S.; Spieck, E. Some like it cold: The cellular organization and physiological limits of cold-tolerant nitrite-oxidizing Nitrotoga. Environ. Microbiol. 2022, 24, 2059–2077. [Google Scholar] [CrossRef]
- Zumft, W.G.; Kroneck, P.M. Respiratory transformation of nitrous oxide (N2O) to dinitrogen by Bacteria and Archaea. Adv. Microb. Physiol. 2007, 52, 107–227. [Google Scholar] [CrossRef]
- Shoun, H.; Fushinobu, S.; Jiang, L.; Kim, S.W.; Wakagi, T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1186–1194. [Google Scholar] [CrossRef]
- Simek, M.; Cooper, J.E. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Dalal, R.C.; Allen, D.E. Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot. 2008, 56, 369–407. [Google Scholar] [CrossRef]
- Scheer, C.; Fuchs, K.; Pelster, D.E.; Butterbach-Bahl, K. Estimating global terrestrial denitrification from measured N2O: (N2O + N2) product ratios. Curr. Opin. Environ. Sustain. 2020, 47, 72–80. [Google Scholar] [CrossRef]
- Gil, J.; Marushchak, M.E.; Rutting, T.; Baggs, E.M.; Perez, T.; Novakovskiy, A.; Trubnikova, T.; Kaverin, D.; Martikainen, P.J.; Biasi, C. Sources of nitrous oxide and the fate of mineral nitrogen in subarctic permafrost peat soils. Biogeosciences 2022, 19, 2683–2698. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Ma, W.K.; Ferguson, S.; Farrell, R.E. Nitrifier dominance of Arctic soil nitrous oxide emissions arises due to fungal competition with denitrifiers for nitrate. Soil Biol. Biochem. 2009, 41, 1104–1110. [Google Scholar] [CrossRef]
- Christensen, T.R. Potential and actual trace gas fluxes in Arctic terrestrial ecosystems. Polar Res. 1999, 18, 199–206. [Google Scholar] [CrossRef]
- Palmer, K.; Biasi, C.; Horn, M.A. Contrasting denitrifier communities relate to contrasting N2O emission patterns from acidic peat soils in arctic tundra. ISME J. 2012, 6, 1058–1077. [Google Scholar] [CrossRef]
- Yang, S.Z.; Liebner, S.; Walz, J.; Knoblauch, C.; Bornemann, T.L.V.; Probst, A.J.; Wagner, D.; Jetten, M.S.M.; in ‘t Zandt, M.H. Effects of a long-term anoxic warming scenario on microbial community structure and functional potential of permafrost-affected soil. Permafr. Periglac. Process. 2021, 32, 641–656. [Google Scholar] [CrossRef]
- Frey, K.E.; McClelland, J.W.; Holmes, R.M.; Smith, L.C. Impacts of climate warming and permafrost thaw on the riverine transport of nitrogen and phosphorus to the Kara Sea. J. Geophys. Res. Biogeosci. 2007, 112, G04S58. [Google Scholar] [CrossRef]
- Bowden, W.B.; Gooseff, M.N.; Balser, A.; Green, A.; Peterson, B.J.; Bradford, J. Sediment and nutrient delivery from thermokarst features in the foothills of the North Slope, Alaska: Potential impacts on headwater stream ecosystems. J. Geophys. Res. Biogeosci. 2008, 113, G02026. [Google Scholar] [CrossRef]
- McNamara, J.P.; Kane, D.L.; Hobbie, J.E.; Kling, G.W. Hydrologic and biogeochemical controls on the spatial and temporal patterns of nitrogen and phosphorus in the Kuparuk River, arctic Alaska. Hydrol. Process. Int. J. 2008, 22, 3294–3309. [Google Scholar] [CrossRef]
- Abbott, B.W.; Larouche, J.R.; Jones, J.B.; Bowden, W.B.; Balser, A.W. Elevated dissolved organic carbon biodegradability from thawing and collapsing permafrost. J. Geophys. Res. Biogeosci. 2014, 119, 2049–2063. [Google Scholar] [CrossRef]
- Louiseize, N.L.; Lafrenière, M.J.; Hastings, M.G. Stable isotopic evidence of enhanced export of microbially derived NO3-following active layer slope disturbance in the Canadian High Arctic. Biogeochemistry 2014, 121, 565–580. [Google Scholar] [CrossRef]
- Lafreniere, M.J.; Louiseize, N.L.; Lamoureux, S.F. Active layer slope disturbances affect seasonality and composition of dissolved nitrogen export from High Arctic headwater catchments. Arct. Sci. 2017, 3, 429–450. [Google Scholar] [CrossRef]
- Tanski, G.; Lantuit, H.; Ruttor, S.; Knoblauch, C.; Radosavljevic, B.; Strauss, J.; Wolter, J.; Irrgang, A.M.; Ramage, J.; Fritz, M. Transformation of terrestrial organic matter along thermokarst-affected permafrost coasts in the Arctic. Sci. Total Environ. 2017, 581–582, 434–447. [Google Scholar] [CrossRef]
- Repo, M.E.; Susiluoto, S.; Lind, S.E.; Jokinen, S.; Elsakov, V.; Biasi, C.; Virtanen, T.; Martikainen, P.J. Large N2O emissions from cryoturbated peat soil in tundra. Nat. Geosci. 2009, 2, 189–192. [Google Scholar] [CrossRef]
- Marushchak, M.E.; Voigt, C.; Gil, J.; Lamprecht, R.; Trubnikova, T.; Kaverin, D.; Martikainen, P.J.; Biasi, C. Which factors control the interannual variability of nitrous oxide fluxes in subarctic European Russian tundra? In Proceedings of the 20th EGU Gerneral Assembly, Vienna, Austria, 4–13 April 2018. [Google Scholar]
- Diáková, K.; Biasi, C.; Čapek, P.; Martikainen, P.J.; Marushchak, M.E.; Patova, E.N.; Šantrůčková, H. Variation in N2 fixation in subarctic tundra in relation to landscape position and nitrogen pools and fluxes. Arct. Antarct. Alp. Res. 2016, 48, 111–125. [Google Scholar] [CrossRef]
- Gil, J.; Pérez, T.; Boering, K.; Martikainen, P.J.; Biasi, C. Mechanisms responsible for high N2O emissions from subarctic permafrost peatlands studied via stable isotope techniques. Glob. Biogeochem. Cycles 2017, 31, 172–189. [Google Scholar] [CrossRef]
- Yang, G.; Peng, Y.; Marushchak, M.E.; Chen, Y.; Wang, G.; Li, F.; Zhang, D.; Wang, J.; Yu, J.; Liu, L.; et al. Magnitude and pathways of increased nitrous oxide emissions from uplands following permafrost thaw. Environ. Sci. Technol. 2018, 52, 9162–9169. [Google Scholar] [CrossRef]
- Elberling, B.; Christiansen, H.H.; Hansen, B.U. High nitrous oxide production from thawing permafrost. Nat. Geosci. 2010, 3, 332–335. [Google Scholar] [CrossRef]
- Jonasson, S.; Chapin, F.S.; Shaver, G.R. Biogeochemistry in the Arctic: Patterns, processes, and controls. In Global Biogeochemical Cycles in the Climate System; Schulze, E.-D., Heimann, M., Harrison, S., Holland, E., Lloyd, J., Prentice, I.C., Schimel, D., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 139–150. [Google Scholar] [CrossRef]
- Wild, B.; Schnecker, J.; Bárta, J.; Čapek, P.; Guggenberger, G.; Hofhansl, F.; Kaiser, C.; Lashchinsky, N.; Mikutta, R.; Mooshammer, M.; et al. Nitrogen dynamics in turbic cryosols from Siberia and Greenland. Soil Biol. Biochem. 2013, 67, 85–93. [Google Scholar] [CrossRef]
- Heikoop, J.M.; Throckmorton, H.M.; Newman, B.D.; Perkins, G.B.; Iversen, C.M.; Roy Chowdhury, T.; Romanovsky, V.; Graham, D.E.; Norby, R.J.; Wilson, C.J.; et al. Isotopic identification of soil and permafrost nitrate sources in an Arctic tundra ecosystem. J. Geophys. Res. Biogeosci. 2015, 120, 1000–1017. [Google Scholar] [CrossRef]
- Voigt, C.; Marushchak, M.E.; Mastepanov, M.; Lamprecht, R.E.; Christensen, T.R.; Dorodnikov, M.; Jackowicz-Korczyński, M.; Lindgren, A.; Lohila, A.; Nykänen, H.; et al. Ecosystem carbon response of an Arctic peatland to simulated permafrost thaw. Glob. Chang. Biol. 2019, 25, 1746–1764. [Google Scholar] [CrossRef]
- Grogan, P.; Jonasson, S. Controls on annual nitrogen cycling in the understory of a subarctic birch forest. Ecology 2003, 84, 202–218. [Google Scholar] [CrossRef]
- Glanville, H.C.; Hill, P.W.; Maccarone, L.D.; Golyshin, P.N.; Murphy, D.V.; Jones, D.L. Temperature and water controls on vegetation emergence, microbial dynamics, and soil carbon and nitrogen fluxes in a high Arctic tundra ecosystem. Funct. Ecol. 2012, 26, 1366–1380. [Google Scholar] [CrossRef]
- Westergaard-Nielsen, A.; Balstrom, T.; Treier, U.A.; Normand, S.; Elberling, B. Estimating meltwater retention and associated nitrate redistribution during snowmelt in an Arctic tundra landscape. Environ. Res. Lett. 2020, 15, 034025. [Google Scholar] [CrossRef]
- Rasmussen, L.H.; Zhang, W.; Ambus, P.; Jansson, P.-E.; Kitzler, B.; Elberling, B. Nitrogen transport in a tundra landscape: The effects of early and late growing season lateral N inputs on arctic soil and plant N pools and N2O Fluxes. Biogeochemistry 2021, 157, 69–84. [Google Scholar] [CrossRef]
- Treat, C.C.; Wollheim, W.M.; Varner, R.K.; Bowden, W.B. Longer thaw seasons increase nitrogen availability for leaching during fall in tundra soils. Environ. Res. Lett. 2016, 11, 064013. [Google Scholar] [CrossRef]
- Shogren, A.J.; Zarnetske, J.P.; Abbott, B.W.; Iannucci, F.; Bowden, W.B. We cannot shrug off the shoulder seasons: Addressing knowledge and data gaps in an Arctic headwater. Environ. Res. Lett. 2020, 15, 104027. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Chapin, F.S. Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 1996, 35, 327–338. [Google Scholar] [CrossRef]
- Schimel, J.P.; Clein, J.S. Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biol. Biochem. 1996, 28, 1061–1066. [Google Scholar] [CrossRef]
- Herrmann, A.; Witter, E. Sources of C and N contributing to the flush in mineralization upon freeze-thaw cycles in soils. Soil Biol. Biochem. 2002, 34, 1495–1505. [Google Scholar] [CrossRef]
- Edwards, K.A.; McCulloch, J.; Kershaw, G.P.; Jefferies, R.L. Soil microbial and nutrient dynamics in a wet Arctic sedge meadow in late winter and early spring. Soil Biol. Biochem. 2006, 38, 2843–2851. [Google Scholar] [CrossRef]
- Hayashi, K.; Tanabe, Y.; Ono, K.; Loonen, M.J.J.E.; Asano, M.; Fujitani, H.; Tokida, T.; Uchida, M.; Hayatsu, M. Seabird-affected taluses are denitrification hotspots and potential N2O emitters in the High Arctic. Sci. Rep. 2018, 8, 17261. [Google Scholar] [CrossRef]
- Zhu, R.B.; Liu, Y.H.; Xu, H.; Ma, J.; Zhao, S.P.; Sun, L.G. Nitrous oxide emissions from sea animal colonies in the maritime Antarctic. Geophys. Res. Lett. 2008, 35, L09807. [Google Scholar] [CrossRef]
- Zhu, R.B.; Liu, Y.S.; Xu, H.; Ma, D.W.; Jiang, S. Marine animals significantly increase tundra N2O and CH4 emissions in maritime Antarctica. J. Geophys. Res. Biogeosci. 2013, 118, 1773–1792. [Google Scholar] [CrossRef]
- Sun, L.G.; Zhu, R.B.; Xie, Z.Q.; Xing, G.X. Emissions of nitrous oxide and methane from Antarctic Tundra: Role of penguin dropping deposition. Atmos. Environ. 2002, 36, 4977–4982. [Google Scholar] [CrossRef]
- Abbott, B.W.; Rocha, A.V.; Shogren, A.; Zarnetske, J.P.; Iannucci, F.; Bowden, W.B.; Bratsman, S.P.; Patch, L.; Watts, R.; Fulweber, R.; et al. Tundra wildfire triggers sustained lateral nutrient loss in Alaskan Arctic. Glob. Chang. Biol. 2021, 27, 1408–1430. [Google Scholar] [CrossRef]
- Schirrmeister, L.; Kunitsky, V.; Grosse, G.; Wetterich, S.; Meyer, H.; Schwamborn, G.; Babiy, O.; Derevyagin, A.; Siegert, C. Sedimentary characteristics and origin of the Late Pleistocene Ice Complex on north-east Siberian Arctic coastal lowlands and islands A review. Quart. Int. 2011, 241, 3–25. [Google Scholar] [CrossRef]
- Seppala, M. Synthesis of studies of palsa formation underlining the importance of local environmental and physical characteristics. Quat. Res. 2011, 75, 366–370. [Google Scholar] [CrossRef]
- Kaverin, D.A.; Pastukhov, A.V.; Lapteva, E.M.; Biasi, C.; Marushchak, M.; Martikainen, P. Morphology and properties of the soils of permafrost peatlands in the southeast of the Bol’shezemel’skaya tundra. Eurasian Soil Sci. 2016, 49, 498–511. [Google Scholar] [CrossRef]
- Yakushev, A.V.; Matyshak, G.V.; Tarkhov, M.O.; Kachalkin, A.V.; Sefilyan, A.R.; Petrov, D.G. Microbiological characteristics of bare peat circles on flat-topped peat mounds in the north of Western Siberia. Eurasian Soil Sci. 2019, 52, 1081–1090. [Google Scholar] [CrossRef]
- Valiranta, M.; Marushchak, M.E.; Tuovinen, J.P.; Lohila, A.; Biasi, C.; Voigt, C.; Zhang, H.; Piilo, S.; Virtanen, T.; Rasanen, A.; et al. Warming climate forcing impact from a sub-arctic peatland as a result of late Holocene permafrost aggradation and initiation of bare peat surfaces. Quat. Sci. Rev. 2021, 264, 107022. [Google Scholar] [CrossRef]
- Biasi, C.; Jokinen, S.; Marushchak, M.E.; Hämäläinen, K.; Trubnikova, T.; Oinonen, M.; Martikainen, P.J. microbial respiration in arctic upland and peat soils as a source of atmospheric carbon dioxide. Ecosystems 2014, 17, 112–126. [Google Scholar] [CrossRef]
- Sannel, A.B.K.; Kuhry, P. Long-term stability of permafrost in subarctic peat plateaus, west-central Canada. Holocene 2008, 18, 589–601. [Google Scholar] [CrossRef]
- Hugelius, G. Spatial upscaling using thematic maps: An analysis of uncertainties in permafrost soil carbon estimates. Glob. Biogeochem. Cycles 2012, 26, 1–15. [Google Scholar] [CrossRef]
- Voigt, C.; Lamprecht, R.E.; Marushchak, M.E.; Lind, S.E.; Novakovskiy, A.; Aurela, M.; Martikainen, P.J.; Biasi, C. Warming of subarctic tundra increases emissions of all three important greenhouse gases–carbon dioxide, methane, and nitrous oxide. Glob. Chang. Biol. 2017, 23, 3121–3138. [Google Scholar] [CrossRef]
- Voigt, C.; Marushchak, M.E.; Lamprecht, R.E.; Jackowicz-Korczyński, M.; Lindgren, A.; Mastepanov, M.; Granlund, L.; Christensen, T.R.; Tahvanainen, T.; Martikainen, P.J.; et al. Increased nitrous oxide emissions from Arctic peatlands after permafrost thaw. Proc. Natl. Acad. Sci. USA 2017, 114, 6238–6243. [Google Scholar] [CrossRef]
- Hugelius, G.; Virtanen, T.; Kaverin, D.; Pastukhov, A.; Rivkin, F.; Marchenko, S.; Romanovsky, V.; Kuhry, P. High-resolution mapping of ecosystem carbon storage and potential effects of permafrost thaw in periglacial terrain, European Russian Arctic. J. Geophys. Res. Biogeosci. 2011, 116, G03024. [Google Scholar] [CrossRef]
- Marushchak, M.E.; Kiepe, I.; Biasi, C.; Elsakov, V.; Friborg, T.; Johansson, T.; Soegaard, H.; Virtanen, T.; Martikainen, P.J. Carbon dioxide balance of subarctic tundra from plot to regional scales. Biogeosciences 2013, 10, 437–452. [Google Scholar] [CrossRef]
- Marushchak, M.E.; Friborg, T.; Biasi, C.; Herbst, M.; Johansson, T.; Kiepe, I.; Liimatainen, M.; Lind, S.E.; Martikainen, P.J.; Virtanen, T.; et al. Methane dynamics in the subarctic tundra: Combining stable isotope analyses, plot- and ecosystem-scale flux measurements. Biogeosciences 2016, 13, 597–608. [Google Scholar] [CrossRef]
- Flessa, H.; Rodionov, A.; Guggenberger, G.; Fuchs, H.; Magdon, P.; Shibistova, O.; Zrazhevskaya, G.; Mikheyeva, N.; Kasansky, O.A.; Blodau, C. Landscape controls of CH4 fluxes in a catchment of the forest tundra ecotone in northern Siberia. Glob. Chang. Biol. 2008, 14, 2040–2056. [Google Scholar] [CrossRef]
- Hetz, S.A.; Horn, M.A. Burkholderiaceae are key acetate assimilators during complete denitrification in acidic cryoturbated peat circles of the Arctic tundra. Front. Microbiol. 2021, 12, 628269. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Horn, M.A. Actinobacterial nitrate reducers and proteobacterial denitrifiers are abundant in N2O-metabolizing palsa peat. Appl. Environ. Microbiol. 2012, 78, 5584–5596. [Google Scholar] [CrossRef]
- Bhattarai, H.R.; Marushchak, M.E.; Ronkainen, J.; Lamprecht, R.E.; Siljanen, H.M.P.; Martikainen, P.J.; Biasi, C.; Maljanen, M. Emissions of atmospherically reactive gases nitrous acid and nitric oxide from Arctic permafrost peatlands. Environ. Res. Lett. 2022, 17, 024034. [Google Scholar] [CrossRef]
- Wegner, R.; Fiencke, C.; Knoblauch, C.; Sauerland, L.; Beer, C. Rapid permafrost thaw removes nitrogen limitation and rises the potential of N2O emissions. Nitrogen 2022. (submitted). [Google Scholar]
- Melchert, J.O.; Wischhöfer, P.; Knoblauch, C.; Eckhard, T.; Liebner, S.; Rethemeyer, J. Sources of CO2 produced in freshly thawed Pleistocene-age Yedoma permafrost. Front. Earth Sci. 2022, 9, 737237. [Google Scholar] [CrossRef]
- Hetz, S.A.; Poehlein, A.; Horn, M.A. Whole-genome sequences of two new caballeronia strains isolated from cryoturbated peat circles of the permafrost-affected eastern european tundra. Microbiol Resour. Announc. 2020, 9, e00731-20. [Google Scholar] [CrossRef] [PubMed]
- Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. Archaea rather than bacteria control nitrifcation in two agricultural acidic soils. FEMS Microbiol. Ecol. 2010, 74, 566–574. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Wang, H.; Bagnoud, A.; Ponce-Toledo, R.I.; Kerou, M.; Weil, M.; Schleper, C.; Urich, T. Linking 16S rRNA gene classification to amoA Gene taxonomy reveals environmental distribution of ammonia-oxidizing archaeal clades in peatland soils. Msystems 2021, 6, e0054621. [Google Scholar] [CrossRef]
- Pautler, B.G.; Simpson, A.J.; Mcnally, D.J.; Lamoureux, S.F.; Simpson, M.J. Arctic permafrost active layer detachments stimulate microbial activity and degradation of soil organic matter. Env. Sci. Technol. 2010, 44, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.W.; Jones, J.B.; Godsey, S.E.; Larouche, J.R.; Bowden, W.B. Patterns and persistence of hydrologic carbon and nutrient export from collapsing upland permafrost. Biogeosci. Discuss. 2015, 12, 3725–3740. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Peng, Y.; Olefeldt, D.; Chen, Y.; Wang, G.; Li, F.; Zhang, D.; Wang, J.; Yu, J.; Liu, L.; et al. Changes in methane flux along a permafrost thaw sequence on the tibetan plateau. Environ. Sci. Technol. 2018, 52, 1244–1252. [Google Scholar] [CrossRef]
- Verdonen, M.; Berner, L.T.; Forbes, B.C.; Kumpula, T. Periglacial vegetation dynamics in Arctic Russia: Decadal analysis of tundra regeneration on landslides with time series satellite imagery. Environ. Res. Lett. 2020, 15, 105020. [Google Scholar] [CrossRef]
- Kokelj, S.V.; Jorgenson, M.T. Advances in thermokarst research. Permafr. Periglac. Process. 2013, 24, 108–119. [Google Scholar] [CrossRef]
- Lantuit, H.; Pollard, W.H.; Couture, N.; Fritz, M.; Schirrmeister, L.; Meyer, H.; Hubberten, H.W. Modern and late holocene retrogressive thaw slump activity on the yukon coastal plain and Herschel Island, Yukon Territory, Canada. Permafr. Periglac. Process. 2012, 23, 39–51. [Google Scholar] [CrossRef]
- Olefeldt, D.; Goswami, S.; Grosse, G.; Hayes, D.; Hugelius, G.; Kuhry, P.; McGuire, A.D.; Romanovsky, V.E.; Sannel, A.B.; Schuur, E.A.; et al. Circumpolar distribution and carbon storage of thermokarst landscapes. Nat. Commun. 2016, 7, 13043. [Google Scholar] [CrossRef]
- Zhang, T.; Heginbottom, J.A.; Barry, R.G.; Brown, J. Further statistics on the distribution of permafrost and ground ice in the Northern Hemisphere1. Polar Geogr. 2000, 24, 126–131. [Google Scholar] [CrossRef]
- Grosse, G.; Harden, J.; Turetsky, M.; McGuire, A.D.; Camill, P.; Tarnocai, C.; Frolking, S.; Schuur, E.A.G.; Jorgenson, T.; Marchenko, S.; et al. Vulnerability of high-latitude soil organic carbon in North America to disturbance. J. Geophys. Res. Biogeosci. 2011, 116, G00k06. [Google Scholar] [CrossRef]
- Slater, A.G.; Lawrence, D.M. Diagnosing present and future permafrost from climate models. J. Clim. 2013, 26, 5608–5623. [Google Scholar] [CrossRef]
- Lacelle, D.; Bjornson, J.; Lauriol, B. Climatic and geomorphic factors affecting contemporary (1950–2004) activity of retrogressive thaw slumps on the Aklavik Plateau, Richardson Mountains, NWT, Canada. Permafr. Periglac. Process. 2010, 21, 1–15. [Google Scholar] [CrossRef]
- Lacelle, D.; Brooker, A.; Fraser, R.H.; Kokelj, S.V. Distribution and growth of thaw slumps in the Richardson Mountains-Peel Plateau region, northwestern Canada. Geomorphology 2015, 235, 40–51. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, T.; Zhang, X.; Li, L.; Guo, H.; Zhao, Q.; Cao, L.; Wu, Q.; Cheng, G. Carbon loss and chemical changes from permafrost collapse in the northern Tibetan Plateau. J. Geophys. Res. Biogeosci. 2016, 121, 1781–1791. [Google Scholar] [CrossRef]
- Mu, C.C.; Abbott, B.W.; Zhao, Q.; Su, H.; Wang, S.F.; Wu, Q.B.; Zhang, T.J.; Wu, X.D. Permafrost collapse shifts alpine tundra to a carbon source but reduces N2O and CH4 release on the northern Qinghai-Tibetan Plateau. Geophys. Res. Lett. 2017, 44, 8945–8952. [Google Scholar] [CrossRef]
- Luo, J.; Niu, F.J.; Lin, Z.J.; Liu, M.H.; Yin, G.A. Recent acceleration of thaw slumping in permafrost terrain of Qinghai-Tibet Plateau: An example from the Beiluhe Region. Geomorphology 2019, 341, 79–85. [Google Scholar] [CrossRef]
- Lantuit, H.; Atkinson, D.; Paul Overduin, P.; Grigoriev, M.; Rachold, V.; Grosse, G.; Hubberten, H.-W. Coastal erosion dynamics on the permafrost-dominated Bykovsky Peninsula, north Siberia, 1951–2006. Polar Res. 2011, 30, 7341. [Google Scholar] [CrossRef]
- Stettner, S.; Beamish, A.; Bartsch, A.; Heim, B.; Grosse, G.; Roth, A.; Lantuit, H. Monitoring inter-and intra-seasonal dynamics of rapidly degrading ice-rich permafrost riverbanks in the Lena Delta with TerraSAR-X time series. Remote Sens. 2017, 10, 51. [Google Scholar] [CrossRef]
- Fuchs, M.; Nitze, I.; Strauss, J.; Gunther, F.; Wetterich, S.; Kizyakov, A.; Fritz, M.; Opel, T.; Grigoriev, M.N.; Maksimov, G.T.; et al. Rapid fluvio-thermal erosion of a yedoma permafrost cliff in the lena river delta. Front. Earth Sci. 2020, 8, 336. [Google Scholar] [CrossRef]
- Czudek, T.; Demek, J. Thermokarst in Siberia and its influence on the development of lowland relief. Quart. Res. 1970, 1, 103–120. [Google Scholar] [CrossRef]
- Strauss, J.; Schirrmeister, L.; Mangelsdorf, K.; Eichhorn, L.; Wetterich, S.; Herzschuh, U. Organic-matter quality of deep permafrost carbon–A study from Arctic Siberia. Biogeosciences 2015, 12, 2227–2245. [Google Scholar] [CrossRef]
- Strauss, J.; Schirrmeister, L.; Grosse, G.; Fortier, D.; Hugelius, G.; Knoblauch, C.; Romanovsky, V.; Schädel, C.; Schneider von Deimling, T.; Schuur, E.A.G.; et al. Deep Yedoma permafrost: A synthesis of depositional characteristics and carbon vulnerability. Earth-Sci. Rev. 2017, 172, 75–86. [Google Scholar] [CrossRef]
- Zimov, S.A.; Schuur, E.A.G.; Chapin, F.S. Permafrost and the global carbon budget. Science 2006, 312, 1612–1613. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.E.; Lohse, K.A.; Crosby, B.T.; Mora, C.I. Variations in soil carbon dioxide efflux across a thaw slump chronosequence in northwestern Alaska. Environ. Res. Lett. 2014, 9, 025001. [Google Scholar] [CrossRef]
- Knoblauch, C.; Beer, C.; Schuett, A.; Sauerland, L.; Liebner, S.; Steinhof, A.; Rethemeyer, J.; Grigoriev, M.N.; Faguet, A.; Pfeiffer, E.M. Carbon dioxide and methane release following abrupt thaw of pleistocene permafrost deposits in Arctic siberia. J. Geophys. Res. Biogeosci. 2021, 126, e2021JG006543. [Google Scholar] [CrossRef]
- Kanevskiy, M.; Shur, Y.; Strauss, J.; Jorgenson, T.; Fortier, D.; Stephani, E.; Vasiliev, A. Patterns and rates of riverbank erosion involving ice-rich permafrost (yedoma) in northern Alaska. Geomorphology 2016, 253, 370–384. [Google Scholar] [CrossRef]
- Pizano, C.; Barón, A.F.; Schuur, E.A.G.; Crummer, K.G.; Mack, M.C. Effects of thermo-erosional disturbance on surface soil carbon and nitrogen dynamics in upland arctic tundra. Environ. Res. Lett. 2014, 9, 075006. [Google Scholar] [CrossRef]
- Burn, C.R.; Friele, P.A. Geomorphology, vegetation succession, soil characteristics and permafrost in retrogressive thaw slumps near mayo, Yukon Territory. Arctic 1989, 42, 31–40. [Google Scholar] [CrossRef]
- Lantz, T.C.; Kokelj, S.V.; Gergel, S.E.; Henry, G.H.R. Relative impacts of disturbance and temperature: Persistent changes in microenvironment and vegetation in retrogressive thaw slumps. Glob. Chang. Biol. 2009, 15, 1664–1675. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Schaeffer, S.M.; Schimel, J.P. Vegetation leachate during Arctic thaw enhances soil microbial phosphorus. Ecosystems 2016, 19, 477–489. [Google Scholar] [CrossRef]
- Lantuit, H.; Pollard, W.H. Fifty years of coastal erosion and retrogressive thaw slump activity on Herschel Island, southern Beaufort Sea, Yukon Territory, Canada. Geomorphology 2008, 95, 84–102. [Google Scholar] [CrossRef]
- Cray, H.A.; Pollard, W.H. Vegetation recovery patterns following permafrost disturbance in a Low Arctic setting: Case study of Herschel Island, Yukon, Canada. Arct. Antarct. Alp. Res. 2015, 47, 99–113. [Google Scholar] [CrossRef]
- Vonk, J.E.; Mann, P.J.; Davydov, S.; Davydova, A.; Spencer, R.G.M.; Schade, J.; Sobczak, W.V.; Zimov, N.; Zimov, S.; Bulygina, E.; et al. High biolability of ancient permafrost carbon upon thaw. Geophys. Res. Lett. 2013, 40, 2689–2693. [Google Scholar] [CrossRef]
- Knoblauch, C.; Beer, C.; Liebner, S.; Grigoriev, M.N.; Pfeiffer, E.-M. Methane production as key to the greenhouse gas budget of thawing permafrost. Nat. Clim. Chang. 2018, 8, 309–312. [Google Scholar] [CrossRef]
- Tanski, G.; Wagner, D.; Knoblauch, C.; Fritz, M.; Sachs, T.; Lantuit, H. Rapid CO2 Release from eroding permafrost in seawater. Geophys. Res. Lett. 2019, 46, 11244–11252. [Google Scholar] [CrossRef]
- Turner, K.W.; Pearce, M.D.; Hughes, D.D. detailed characterization and monitoring of a retrogressive thaw slump from remotely piloted aircraft systems and identifying associated influence on carbon and nitrogen export. Remote Sens. 2021, 13, 171. [Google Scholar] [CrossRef]
- Everett, K.R. Evolution of the soil landscape in the sand region of the Arctic Coastal Plain as exemplified at Atkasook, Alaska. Arctic. Arctic 1979, 32, 207–223. [Google Scholar] [CrossRef]
- Schickhoff, U.; Walker, M.D.; Walker, D.A. Riparian willow communities on the Arctic Slope of Alaska and their environmental relationships: A classification and ordination analysis. Phytocoenologia 2002, 32, 145–204. [Google Scholar] [CrossRef]
- Dobrovol’ski, G.V.; Balabko, P.N.; Stasjuk, N.V.; Bykova, E.P. Alluvial soils of river floodplains and deltas and their zonal differences. Arid Ecosyst. 2011, 1, 119–124. [Google Scholar] [CrossRef]
- Sanders, T.; Fiencke, C.; Pfeiffer, E.-M. Small-scale variability of dissolved inorganic nitrogen (DIN), C/N ratios and ammonia oxidizing capacities in various permafrost affected soils of Samoylov Island, Lena River Delta, Northeast Siberia. Polarforschung 2010, 80, 23–35. [Google Scholar]
- Polyakov, V.; Orlova, K.; Abakumov, E. Soils of the lena river delta, Yakutia, Russia: Diversity, characteristics and humic acids molecular composition. Polarforschung 2019, 88, 135–150. [Google Scholar] [CrossRef]
- Sanders, T.; Fiencke, C.; Fuchs, M.; Haugk, C.; Juhls, B.; Mollenhauer, G.; Ogneva, O.; Overduin, P.; Palmtag, J.; Povazhniy, V.; et al. Seasonal nitrogen fluxes of the Lena River Delta. Ambio 2021, 51, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Pinay, G.; Bernal, S.; Abbott, B.W.; Lupon, A.; Marti, E.; Sabater, F.; Krause, S. Riparian corridors: A new conceptual framework for assessing nitrogen buffering across biomes. Front. Environ. Sci. 2018, 6, 47. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Hopkins, D.W.; Elberling, B.; Sparrow, A.D.; Novis, P.; Greenfield, L.G.; Rochette, P. Emission of CO2, CH4 and N2O from lakeshore soils in an Antarctic dry valley. Soil Biol. Biochem. 2006, 38, 3120–3129. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Sparrow, A.D.; Elberling, B.; Gregorich, E.G.; Novis, P.M.; Greenfiled, L.G.; Tilston, E.L. Carbon, nitrogen and temperature controls on microbial activity in soils from an Antarctic dry valley. Soil Biol. Biochem. 2006, 38, 3120–3140. [Google Scholar] [CrossRef]
- Rocha, A.V.; Loranty, M.M.; Higuera, P.E.; Mack, M.C.; Hu, F.S.; Jones, B.M.; Breen, A.L.; Rastetter, E.B.; Goetz, S.J.; Shaver, G.R. The footprint of Alaskan tundra fires during the past half-century: Implications for surface properties and radiative forcing. Environ. Res. Lett. 2012, 7, 044039. [Google Scholar] [CrossRef]
- Hu, F.S.; Higuera, P.E.; Duffy, P.; Chipman, M.L.; Rocha, A.V.; Young, A.M.; Kelly, R.; Dietze, M.C. Arctic tundra fires: Natural variability and responses to climate change. Front. Ecol. Environ. 2015, 13, 369–377. [Google Scholar] [CrossRef]
- Young, A.M.; Higuera, P.E.; Duffy, P.A.; Hu, F.S. Climatic thresholds shape northern high-latitude fire regimes and imply vulnerability to future climate change. Ecography 2017, 40, 606–617. [Google Scholar] [CrossRef]
- Koster, E.; Koster, K.; Berninger, F.; Aaltonen, H.; Zhou, X.; Pumpanen, J. Carbon dioxide, methane and nitrous oxide fluxes from a fire chronosequence in subarctic boreal forests of Canada. Sci. Total Environ. 2017, 601–602, 895–905. [Google Scholar] [CrossRef]
- Rodríguez-Cardona, B.M.; Coble, A.A.; Wymore, A.S.; Kolosov, R.; Podgorski, D.C.; Zito, P.; Spencer, R.G.M.; Prokushkin, A.S.; McDowell, W.H. Wildfres lead to decreased carbon and increased nitrogen concentrations in upland arctic streams. Nature 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Mack, M.C.; Bret-Harte, M.S.; Hollingsworth, T.N.; Jandt, R.R.; Schuur, E.A.; Shaver, G.R.; Verbyla, D.L. Carbon loss from an unprecedented Arctic tundra wildfire. Nature 2011, 475, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Higuera, P.E.; Chipman, M.L.; Barnes, J.L.; Urban, M.A.; Hu, F.S. Variability of tundra fire regimes in Arctic Alaska: Millennial-scale patterns and ecological implications. Ecol. Appl. 2011, 21, 3211–3226. [Google Scholar] [CrossRef]
- Jones, B.M.; Grosse, G.; Arp, C.D.; Miller, E.; Liu, L.; Hayes, D.J.; Larsen, C.F. Recent Arctic tundra fire initiates widespread thermokarst development. Sci. Rep. 2015, 5, 15865. [Google Scholar] [CrossRef]
- Bret-Harte, M.S.; Mack, M.C.; Shaver, G.R.; Huebner, D.C.; Johnston, M.; Mojica, C.A.; Pizano, C.; Reiskind, J.A. The response of Arctic vegetation and soils following an unusually severe tundra fire. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120490. [Google Scholar] [CrossRef]
- Jiang, Y.; Rastetter, E.B.; Rocha, A.V.; Pearce, A.R.; Kwiatkowski, B.L.; Shaver, G.R. Modeling carbon-nutrient interactions during the early recovery of tundra after fire. Ecol. Appl. 2015, 25, 1640–1652. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lambaek, A.; Holm, S.S.; Furbo-Halken, A.; Elberling, B.; Ambus, P.L. Effects of experimental fire in combination with climate warming on greenhouse gas fluxes in Arctic tundra soils. Sci. Total Environ. 2021, 795, 148847. [Google Scholar] [CrossRef]
- Larouche, J.R.; Abbott, B.W.; Bowden, W.B.; Jones, J.B. The role of watershed characteristics, permafrost thaw, and wildfire on dissolved organic carbon biodegradability and water chemistry in Arctic headwater streams. Biogeosciences 2015, 12, 4221–4233. [Google Scholar] [CrossRef]
- Xu, W.Y.; Elberling, B.; Ambus, P.L. Fire increases soil nitrogen retention and alters nitrogen uptake patterns among dominant shrub species in an Arctic dry heath tundra. Sci. Total Environ. 2022, 807, 150990. [Google Scholar] [CrossRef]
- Jiang, Y.; Rastetter, E.B.; Shaver, G.R.; Rocha, A.V.; Zhuang, Q.; Kwiatkowski, B.L. Modeling long-term changes in tundra carbon balance following wildfire, climate change, and potential nutrient addition. Ecol. Appl. 2016, 27, 105–117. [Google Scholar] [CrossRef]
- Lacroix, F.; Zaehle, S.; Caldararu, S.; Schaller, J.; Stimmler, P.; Holl, D.; Kutzbach, L.; Göckede, M. Decoupling of permafrost thaw and vegetation growth could mean both ongoing nutrient limitation and an emergent source of N2O in high latitudes. Earth Space Sci. Open Arch. 2022, preprint. [Google Scholar] [CrossRef]
- Tian, H.; Yang, J.; Lu, C.; Xu, R.; Canadell, J.G.; Jackson, R.B.; Arneth, A.; Chang, J.; Chen, G.; Ciais, P.; et al. The Global N2O Model Intercomparison Project. Bull. Am. Meteorol. Soc. 2018, 99, 1231–1251. [Google Scholar] [CrossRef]
- Syakila, A.; Kroeze, C. The global nitrous oxide budget revisited. Greenh. Gas. Meas. Manag. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Wild, B.; Schnecker, J.; Alves, R.J.E.; Barsukov, P.; Bárta, J.; Čapek, P.; Gentsch, N.; Gittel, A.; Guggenberger, G.; Lashchinskiy, N.; et al. Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in arctic permafrost soil. Soil Biol. Biochem. 2014, 75, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wologo, E.; Shakil, S.; Zolkos, S.; Textor, S.; Ewing, S.; Klassen, J.; Spencer, R.G.M.; Podgorski, D.C.; Tank, S.E.; Baker, M.A.; et al. Stream dissolved organic matter in permafrost regions shows surprising compositional similarities but negative priming and nutrient effects. Glob. Biogeochem. Cycles 2021, 35, e2020GB006719. [Google Scholar] [CrossRef]
- Lavoie, M.; Mack, M.C.; Schuur, E.A.G. Effects of elevated nitrogen and temperature on carbon and nitrogen dynamics in Alaskan arctic and boreal soils. J. Geophys. Res. Biogeosci. 2011, 116, G03013. [Google Scholar] [CrossRef]
- Wild, B.; Gentsch, N.; Čapek, P.; Diáková, K.; Alves, R.J.E.; Bárta, J.; Gittel, A.; Hugelius, G.; Knoltsch, A.; Kuhry, P.; et al. Plant-derived compounds stimulate the decomposition of organic matter in arctic permafrost soils. Sci. Rep. 2016, 6, 25607. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Kicklighter, D.W.; Melillo, J.M.; Monier, E.; Sokolov, A.P.; Zhuang, Q. Future nitrogen availability and its effect on carbon sequestration in Northern Eurasia. Nat. Commun. 2019, 10, 3024. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Jung, J.Y.; Tripathi, B.M.; Gockede, M.; Lee, Y.K.; Kim, M. Dynamics of microbial communities and CO2 and CH4 fluxes in the tundra ecosystems of the changing Arctic. J. Microbiol. 2019, 57, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Burke, E.; Chadburn, S.; Huntingford, C. Thawing permafrost as a nitrogen fertiliser: Implications for climate feedbacks. Nitrogen 2022, 3, 353–375. [Google Scholar] [CrossRef]
- Lamb, E.G.; Han, S.; Lanoil, B.D.; Henry, G.H.R.; Brummell, M.E.; Banerjee, S.; Siciliano, S.D. A High Arctic soil ecosystem resists long-term environmental manipulations. Glob. Chang. Biol. 2011, 17, 3187–3194. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Zhang, T.; Mao, T.; Wei, D.; Hu, Z.; Song, C. Effects of warming and nitrogen fertilization on GHG flux in the permafrost region of an alpine meadow. Atmos Environ. 2017, 157, 111–124. [Google Scholar] [CrossRef]
- Kashi, N.N.; Hobbie, E.A.; Varner, R.K.; Wymore, A.S.; Ernakovich, J.G.; Giesler, R. Nutrients alter methane production and oxidation in a thawing permafrost mire. Ecosystems 2022. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Laanbroek, H.J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- Bodelier, P.L.E. Interactions between nitrogenous fertilizers and methane cycling in wetland and upland soils. Curr. Opin. Environ. Sustain. 2011, 3, 379–388. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Steenbergh, A.K. Interactions between methane and the nitrogen cycle in light of climate change. Curr. Opin. Environ. Sustain. 2014, 9–10, 26–36. [Google Scholar] [CrossRef]
- Klüber, H.D.; Conrad, R. Effects of nitrate, nitrite, NO and N2O on methanogenesis and other redox processes in anoxic rice field soil. FEMS Microbiol. Ecol. 1998, 25, 301–318. [Google Scholar] [CrossRef]
- Klueber, H.D.; Conrad, R. Inhibitory effects of nitrate, nitrite, NO and N2O on methanogenesis by Methanosarcina barkeri and Methanobacterium bryantii. FEMS Microbiol. Ecol. 1998, 25, 331–339. [Google Scholar] [CrossRef]
- Conrad, R. Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: A mini review. Pedosphere 2020, 30, 25–39. [Google Scholar] [CrossRef]
- King, G.M. Ecological aspects of methane oxidation, a key determinant of global methane dynamics. Adv. Microb. Ecol. 1992, 12, 431–468. [Google Scholar] [CrossRef]
- Stein, L.Y.; Roy, R.; Dunfield, P.F. Aerobic methanotrophy and nitrification: Processes and connections. In Encyclopedia of Life Sciences (eLS); Battista, J., Bynum, W., Cooper, D., Delves, P.J., Harper, D., Jansson, R., Kehrer-Sawatzki, H., Levitan, I., Melino, G., Phillips, G., et al., Eds.; John Wiley and Sons: Chichester, UK, 2012; pp. 1–11. [Google Scholar] [CrossRef]
- Lee, E.Y. Methanotrophs-Microbiology Fundamentals and Biotechnological Applications; Springer: Münster, Germany, 2019; Volume 32. [Google Scholar]
- Yonemura, S.; Uchida, M.; Iwahana, G.; Kim, Y.; Yoshikawa, K. Technical advances in measuring greenhouse gas emissions from thawing permafrost soils in the laboratory. Polar Sci. 2019, 19, 137–145. [Google Scholar] [CrossRef]
- Box, J.E.; Colgan, W.T.; Christensen, T.R.; Schmidt, N.M.; Lund, M.; Parmentier, F.J.W.; Brown, R.; Bhatt, U.S.; Euskirchen, E.S.; Romanovsky, V.E.; et al. Key indicators of Arctic climate change: 1971–2017. Environ. Res. Lett. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Smith, D.M.; Screen, J.A.; Deser, C.; Cohen, J.; Fyfe, J.C.; Garcia-Serrano, J.; Jung, T.; Kattsov, V.; Matei, D.; Msadek, R.; et al. The Polar Amplification Model Intercomparison Project (PAMIP) contribution to CMIP6: Investigating the causes and consequences of polar amplification. Geosci. Model. Dev. 2019, 12, 1139–1164. [Google Scholar] [CrossRef]
- Irrgang, A.M.; Bendixen, M.; Farquharson, L.M.; Baranskaya, A.V.; Erikson, L.H.; Gibbs, A.E.; Ogorodov, S.A.; Overduin, P.P.; Lantuit, H.; Grigoriev, M.; et al. Drivers, dynamics and impacts of changing Arctic coasts. Nat. Rev. Earth Environ. 2022, 3, 39–54. [Google Scholar] [CrossRef]
- McGuire, A.D.; Lawrence, D.M.; Koven, C.; Clein, J.S.; Burke, E.; Chen, G.; Jafarov, E.; MacDougall, A.H.; Marchenko, S.; Nicolsky, D.; et al. Dependence of the evolution of carbon dynamics in the northern permafrost region on the trajectory of climate change. Proc. Natl. Acad. Sci. USA 2018, 115, 3882–3887. [Google Scholar] [CrossRef]
- Biskaborn, B.K.; Smith, S.L.; Noetzli, J.; Matthes, H.; Vieira, G.; Streletskiy, D.A.; Schoeneich, P.; Romanovsky, V.E.; Lewkowicz, A.G.; Abramov, A.; et al. Permafrost is warming at a global scale. Nat. Commun. 2019, 10, 264. [Google Scholar] [CrossRef]
- Koven, C.D.; Ringeval, B.; Friedlingstein, P.; Ciais, P.; Cadule, P.; Khvorostyanov, D.; Krinner, G.; Tarnocai, C. Permafrost carbon-climate feedbacks accelerate global warming. Proc. Natl. Acad. Sci. USA 2011, 108, 14769–14774. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Phillips, S.J.; Loranty, M.M.; Beck, P.S.A.; Damoulas, T.; Knight, S.J.; Goetz, S.J. Shifts in Arctic vegetation and associated feedbacks under climate change. Nat. Clim. Chang. 2013, 3, 673–677. [Google Scholar] [CrossRef]
- Panikov, N.S. Understanding and prediction of soil microbial community dynamics under global change. Appl. Soil Ecol. 1999, 11, 161–176. [Google Scholar] [CrossRef]
- Meyer, H.; Kaiser, C.; Biasi, C.; Hämmerle, R.; Rusalimova, O.; Lashchinsky, N.; Baranyi, C.; Daims, H.; Barsukov, P.; Richter, A. Soil carbon and nitrogen dynamics along a latitudinal transect in Western Siberia, Russia. Biogeochemistry 2006, 81, 239–252. [Google Scholar] [CrossRef]
- Walker, T.W.N.; Kaiser, C.; Strasser, F.; Herbold, C.W.; Leblans, N.I.W.; Woebken, D.; Janssens, I.A.; Sigurdsson, B.D.; Richter, A. Microbial temperature sensitivity and biomass change explain soil carbon loss with warming. Nat. Clim. Chang. 2018, 8, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Ernakovich, J.G.; Barbato, R.A.; Rich, V.I.; Schadel, C.; Hewitt, R.E.; Doherty, S.J.; Whalen, E.D.; Abbott, B.W.; Barta, J.; Biasi, C.; et al. Microbiome assembly in thawing permafrost and its feedbacks to climate. Glob. Chang. Biol. 2022, 28, 5007–5026. [Google Scholar] [CrossRef]
- McGuire, A.D.; Anderson, L.G.; Christensen, T.R.; Dallimore, S.; Guo, L.D.; Hayes, D.J.; Heimann, M.; Lorenson, T.D.; Macdonald, R.W.; Roulet, N. Sensitivity of the carbon cycle in the Arctic to climate change. Ecol. Monogr. 2009, 79, 523–555. [Google Scholar] [CrossRef]
- McGuire, A.D.; Christensen, T.R.; Hayes, D.; Heroult, A.; Euskirchen, E.; Kimball, J.S.; Koven, C.; Lafleur, P.; Miller, P.A.; Oechel, W.; et al. An assessment of the carbon balance of Arctic tundra: Comparisons among observations, process models, and atmospheric inversions. Biogeosciences 2012, 9, 3185–3204. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Abbott, B.W.; Bowden, W.B.; Brovkin, V.; Camill, P.; Canadell, J.G.; Chanton, J.P.; Chapin, F.S.; Christensen, T.R.; Ciais, P.; et al. Expert assessment of vulnerability of permafrost carbon to climate change. Clim. Chang. 2013, 119, 359–374. [Google Scholar] [CrossRef]
- Treat, C.C.; Natali, S.M.; Ernakovich, J.; Iversen, C.M.; Lupascu, M.; McGuire, A.D.; Norby, R.J.; Roy Chowdhury, T.; Richter, A.; Šantrůčková, H.; et al. A pan-Arctic synthesis of CH4 and CO2 production from anoxic soil incubations. Glob. Chang. Biol. 2015, 21, 2787–2803. [Google Scholar] [CrossRef]
- Dutta, K.; Schuur, E.A.G.; Neff, J.C.; Zimov, S.A. Potential carbon release from permafrost soils of Northeastern Siberia. Glob. Chang. Biol. 2006, 12, 2336–2351. [Google Scholar] [CrossRef]
- Schaefer, K.; Lantuit, H.; Romanovsky, V.E.; Schuur, E.A.G.; Witt, R. The impact of the permafrost carbon feedback on global climate. Environ. Res. Lett. 2014, 9, 085003. [Google Scholar] [CrossRef]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.H.C.; Gurevitch, J.; Gcte-News. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, S.M.; Sharp, E.; Schimel, J.P.; Welker, J.M. Soil-plant N processes in a High Arctic ecosystem, NW Greenland are altered by long-term experimental warming and higher rainfall. Glob. Chang. Biol. 2013, 19, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Elmore, A.J.; Wang, L.; Aranibar, J.; Bauters, M.; Boeckx, P.; Crowley, B.E.; Dawes, M.A.; Delzon, S.; Fajardo, A.; et al. Isotopic evidence for oligotrophication of terrestrial ecosystems. Nat. Ecol. Evol. 2018, 2, 1735–1744. [Google Scholar] [CrossRef]
- Holtgrieve, G.W.; Schindler, D.E.; Hobbs, W.O.; Leavitt, P.R.; Ward, E.J.; Bunting, L.; Chen, G.; Finney, B.P.; Gregory-Eaves, I.; Holmgren, S.; et al. A coherent signature of anthropogenic nitrogen deposition to remote watersheds of the Northern Hemisphere. Science 2011, 334, 1545–1548. [Google Scholar] [CrossRef]
- Martin, A.C.; Macias-Fauria, M.; Bonsall, M.B.; Forbes, B.C.; Zetterberg, P.; Jeffers, E.S. Common mechanisms explain nitrogen-dependent growth of Arctic shrubs over three decades despite heterogeneous trends and declines in soil nitrogen availability. New Phytol. 2021, 233, 670–686. [Google Scholar] [CrossRef]
- Borge, A.F.; Westermann, S.; Solheim, I.; Etzelmuller, B. Strong degradation of palsas and peat plateaus in northern Norway during the last 60 years. Cryosphere 2017, 11, 1–16. [Google Scholar] [CrossRef]
- Fewster, R.E.; Morris, P.J.; Ivanovic, R.F.; Swindles, G.T.; Peregon, A.M.; Smith, C.J. Imminent loss of climate space for permafrost peatlands in Europe and Western Siberia. Nat. Clim. Chang. 2022, 12, 373–379. [Google Scholar] [CrossRef]
- Liljedahl, A.K.; Boike, J.; Daanen, R.P.; Fedorov, A.N.; Frost, G.V.; Grosse, G.; Hinzman, L.D.; Iijma, Y.; Jorgenson, J.C.; Matveyeva, N.; et al. Pan-Arctic ice-wedge degradation in warming permafrost and its influence on tundra hydrology. Nat. Geosci. 2016, 9, 312–318. [Google Scholar] [CrossRef]
- Runge, A.; Nitze, I.; Grosse, G. Remote sensing annual dynamics of rapid permafrost thaw disturbances with LandTrendr. Remote Sens. Environ. 2022, 268, 112752. [Google Scholar] [CrossRef]
- Abbott, B.W.; Jones, J.B.; Schuur, E.A.G.; Chapin, F.S.; Bowden, W.B.; Bret-Harte, M.S.; Epstein, H.E.; Flannigan, M.D.; Harms, T.K.; Hollingsworth, T.N.; et al. Biomass offsets little or none of permafrost carbon release from soils, streams, and wildfire: An expert assessment. Environ. Res. Lett. 2016, 11, 034014. [Google Scholar] [CrossRef]
- Hu, F.S.; Higuera, P.E.; Walsh, J.E.; Chapman, W.L.; Duffy, P.A.; Brubaker, L.B.; Chipman, M.L. Tundra burning in Alaska: Linkages to climatic change and sea ice retreat. J. Geophys. Res. Biogeosci. 2010, 115, G04002. [Google Scholar] [CrossRef]
- Luoto, M.; Fronzek, S.; Zuidhoff, F.S. Spatial modelling of palsa mires in relation to climate in northern Europe. Earth Surf. Process. Landf. J. Br. Geomorphol. Res. Group 2004, 29, 1373–1387. [Google Scholar] [CrossRef]
- Fronzek, S.; Luoto, M.; Carter, T.R. Potential effect of climate change on the distribution of palsa mires in subarctic Fennoscandia. Clim. Res. 2006, 32, 1–12. [Google Scholar] [CrossRef]
- Hazard, C.; Prosser, J.I.; Nicol, G.W. Use and abuse of potential rates in soil microbiology. Soil Biol. Biochem. 2021, 157, 108242. [Google Scholar] [CrossRef]
- Shogren, A.J.; Zarnetske, J.P.; Abbott, B.W.; Iannucci, F.; Medvedeff, A.; Cairns, S.; Duda, M.J.; Bowden, W.B. Arctic concentration–discharge relationships for dissolved organic carbon and nitrate vary with landscape and season. Limnol. Oceanogr. 2020, 66, S197–S215. [Google Scholar] [CrossRef]
- Wegner, R.; (Universität Hamburg, Hamburg, Germany). Personal communications, 2022.
- Marushchak, M.; (University of Eastern Finland, Kuopio, Finland). Personal communications, 2022.

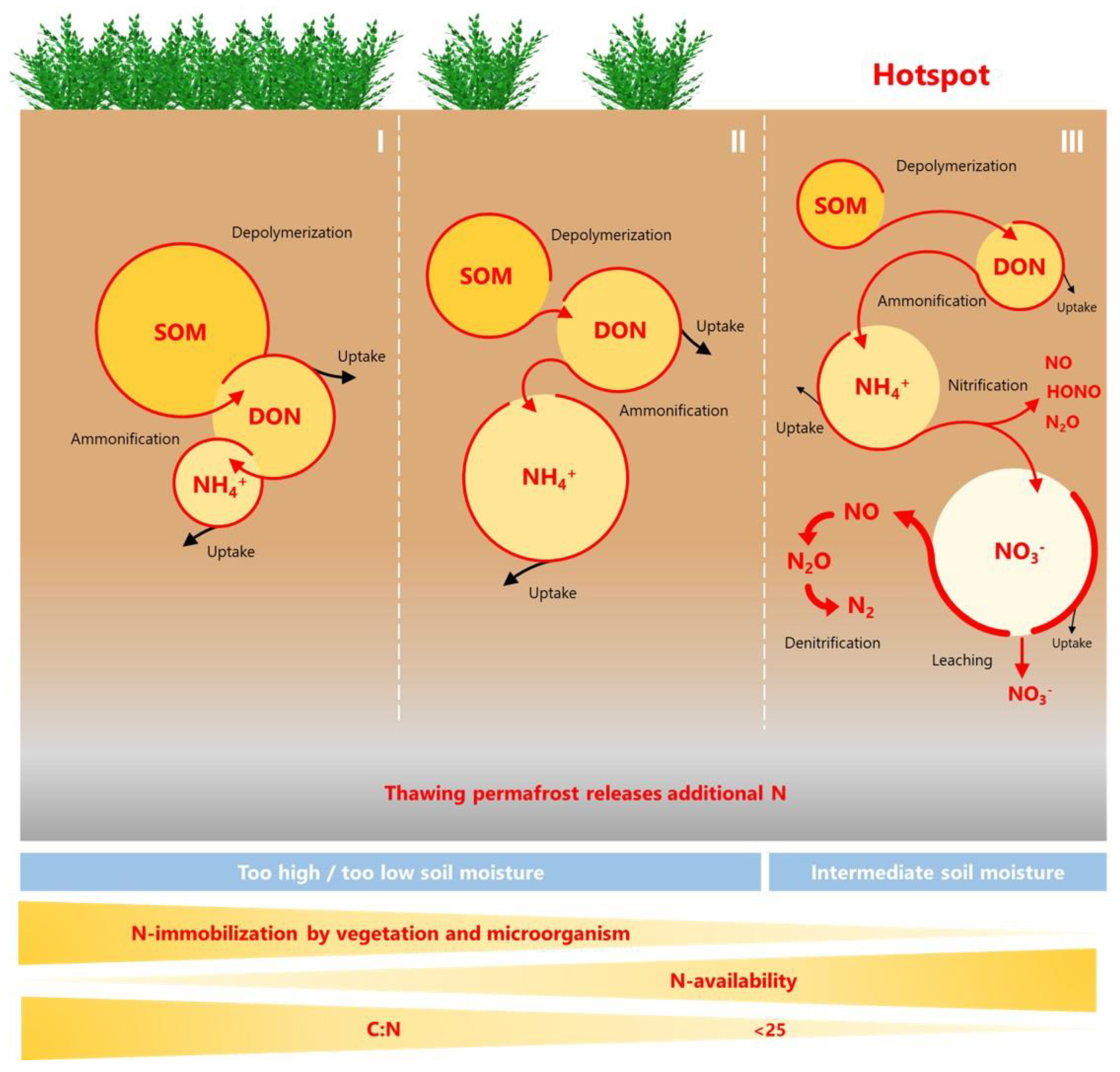

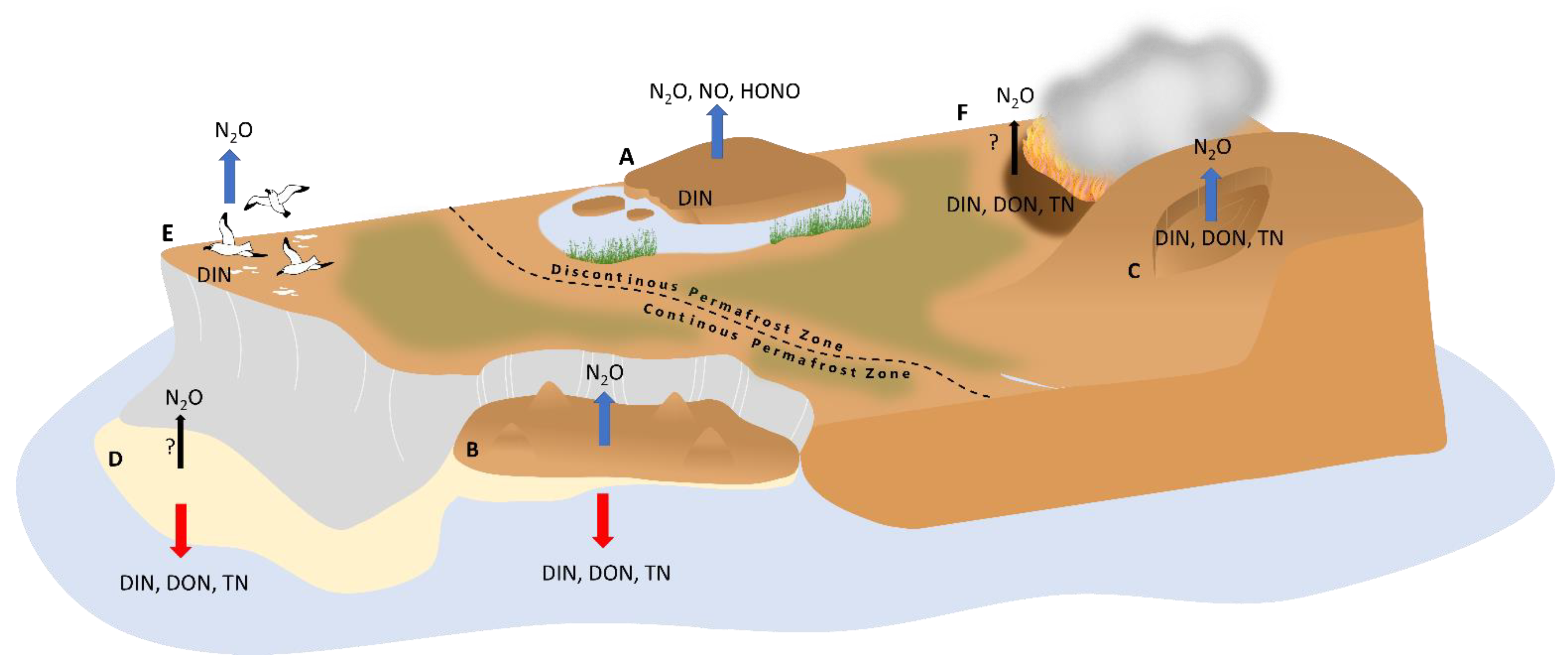
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiencke, C.; Marushchak, M.E.; Sanders, T.; Wegner, R.; Beer, C. Microbiogeochemical Traits to Identify Nitrogen Hotspots in Permafrost Regions. Nitrogen 2022, 3, 458-501. https://doi.org/10.3390/nitrogen3030031
Fiencke C, Marushchak ME, Sanders T, Wegner R, Beer C. Microbiogeochemical Traits to Identify Nitrogen Hotspots in Permafrost Regions. Nitrogen. 2022; 3(3):458-501. https://doi.org/10.3390/nitrogen3030031
Chicago/Turabian StyleFiencke, Claudia, Maija E. Marushchak, Tina Sanders, Rica Wegner, and Christian Beer. 2022. "Microbiogeochemical Traits to Identify Nitrogen Hotspots in Permafrost Regions" Nitrogen 3, no. 3: 458-501. https://doi.org/10.3390/nitrogen3030031






