Layer by Layer Deposition of Colloidal SnO2 Nano Particles †
Abstract
:1. Introduction
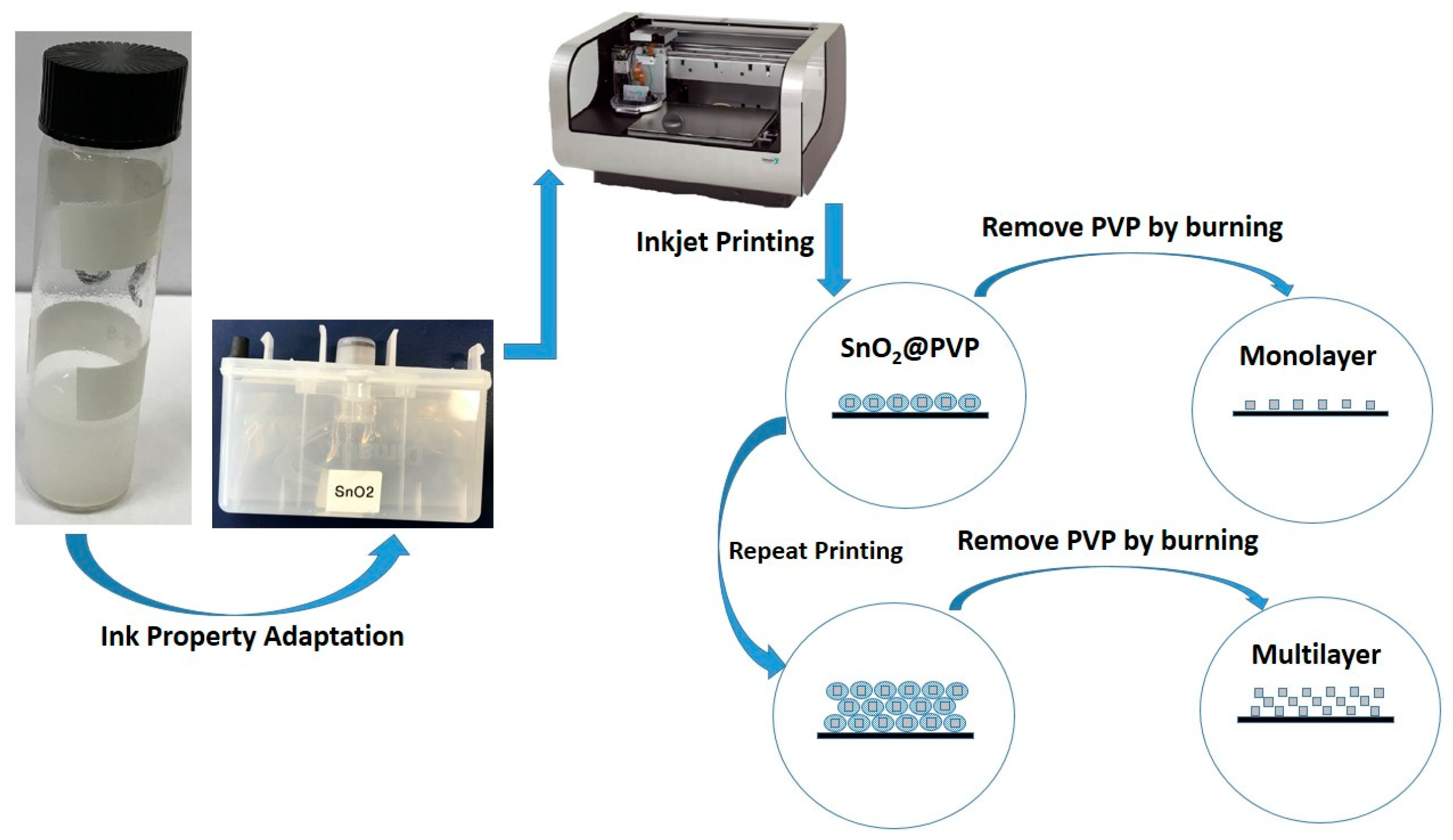
2. Experimental
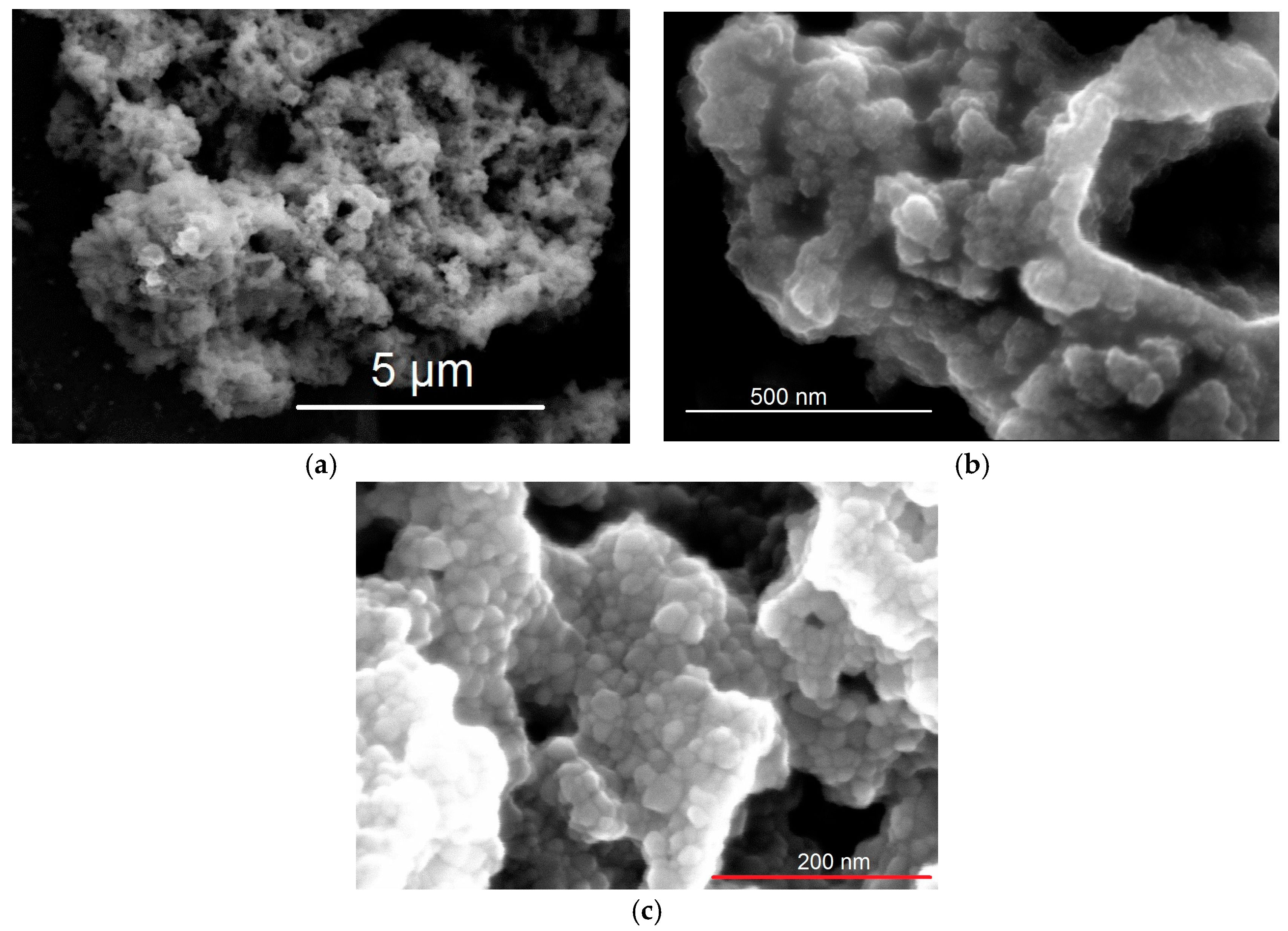
3. Results
Conflicts of Interest
References
- Rieu, M.; Camara, M.; Tournier, G.; Viricelle, J.-P.; Pijolat, C.; de Rooij, N.F.; Briand, D. Fully inkjet printed SnO2 gas sensor on plastic substrate. Sens. Actuator B Chem. 2016, 236, 1091–1097. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.-J.; Lee, I.; Youn, D.-Y.; Park, C.O.; Lee, J.-H.; Tuller, H.L.; Kim, I.-D. Thin-Wall Assembled SnO2 Fibers Functionalized by Catalytic Pt Nanoparticles and their Superior Exhaled-Breath-Sensing Properties for the Diagnosis of Diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Gao, H.; Jia, H.; Bierer, B.; Wöllenstein, J.; Lu, Y.; Palzer, S. Scalable gas sensors fabrication to integrate metal oxide nanoparticles with well-defined shape and size. Sens. Actuator B Chem. 2017, 249, 639–646. [Google Scholar] [CrossRef]
- Walden, P.; Kneer, J.; Knobelspies, S.; Kronast, W.; Mescheder, U.; Palzer, S. Micromachined Hotplate Platform for the Investigation of Ink-Jet Printed, Functionalized Metal Oxide Nanoparticles. J. Microelectromech. Syst. 2015, 24, 1384–1390. [Google Scholar] [CrossRef]
- Liu, R.; Yang, S.; Wang, F.; Lu, X.; Yang, Z.; Ding, B. Sodium Chloride Template Synthesis of Cubic Tin Oxide Dioxide Hollow Particles for Lithium Ion Battery Applications. ACS Appl. Mater. Interface 2015, 4, 15–27. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Lyu, X.; Wöllenstein, J.; Palzer, S. Layer by Layer Deposition of Colloidal SnO2 Nano Particles. Proceedings 2017, 1, 318. https://doi.org/10.3390/proceedings1040318
Gao H, Lyu X, Wöllenstein J, Palzer S. Layer by Layer Deposition of Colloidal SnO2 Nano Particles. Proceedings. 2017; 1(4):318. https://doi.org/10.3390/proceedings1040318
Chicago/Turabian StyleGao, Haitao, Xuemeng Lyu, Jürgen Wöllenstein, and Stefan Palzer. 2017. "Layer by Layer Deposition of Colloidal SnO2 Nano Particles" Proceedings 1, no. 4: 318. https://doi.org/10.3390/proceedings1040318




