Sensitive and Selective Ammonia Gas Sensor Based on Molecularly Modified SnO2 †
Abstract
:1. Introduction
2. Materials and Methods
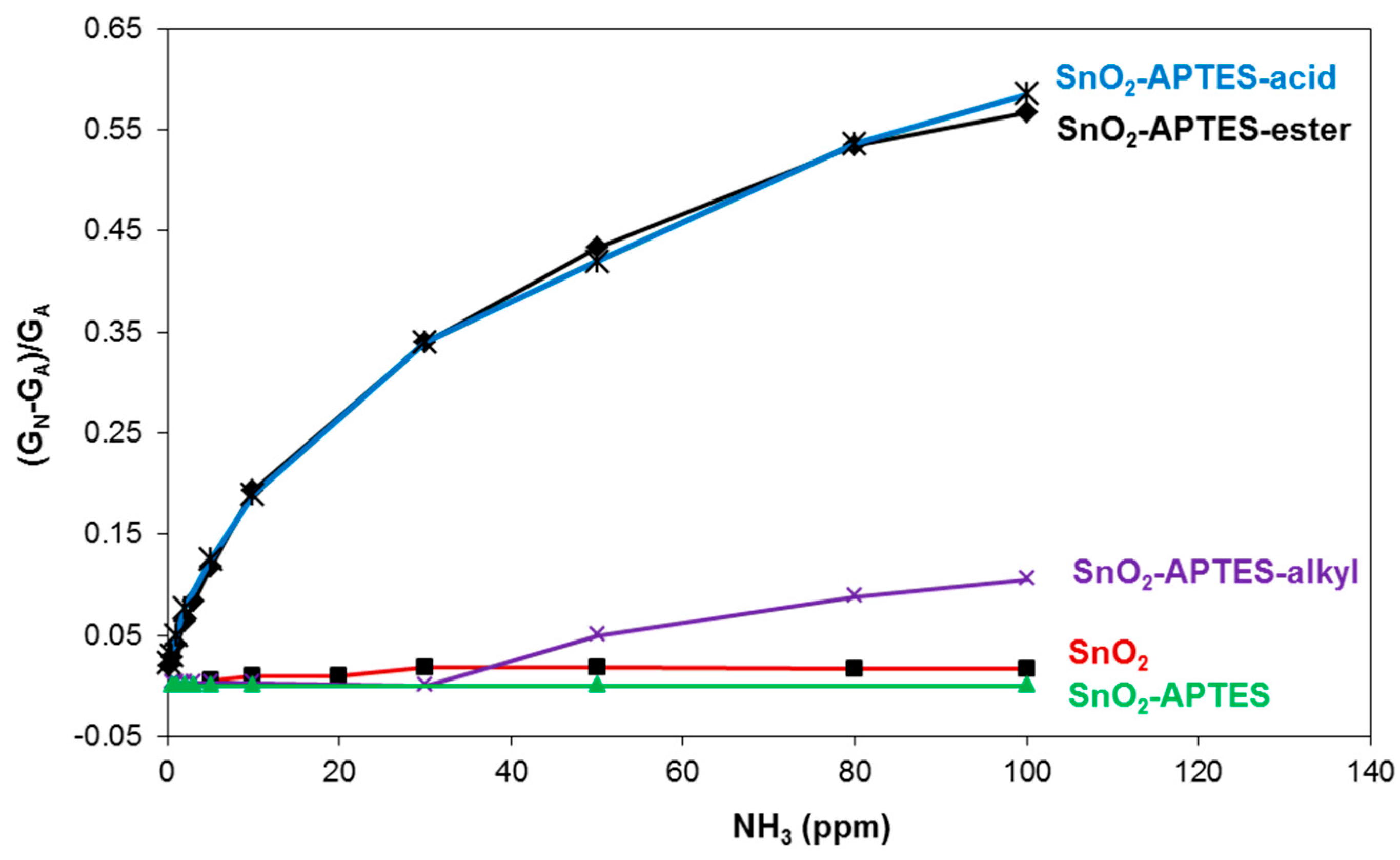
3. Results and Discussion
4. Conclusions
Conflicts of Interest
References
- Kahn, N.; Lavie, O.; Paz, M.; Segev, Y.; Haick, H. Dynamic Nanoparticle-Based Flexible Sensors: Diagnosis of Ovarian Carcinoma from Exhaled Breath. Nano Lett. 2015, 15, 7023–7028. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Haick, H. Effect of Functional Groups on the Sensing Properties of Silicon Nanowires toward Volatile Compounds. ACS Appl. Mater. Interfaces 2013, 5, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Tournier, G.; Pijolat, C. Selective filter for SnO2-based gas sensor: Application to hydrogen trace detection. Sens. Actuators B Chem. 2005, 106, 553–562. [Google Scholar] [CrossRef]
- Le, M.; Jimenez, C.; Chainet, E.; Stambouli, V. A Label-Free Impedimetric DNA Sensor Based on a Nanoporous SnO2 Film: Fabrication and Detection Performance. Sensors 2015, 15, 10686–10704. [Google Scholar] [CrossRef] [PubMed]
- Khun Khun, K.; Mahajan, A.; Bedi, R.K. SnO2 thick films for room temperature gas sensing applications. J. Appl. Phys. 2009, 106. [Google Scholar] [CrossRef]
- Hoft, R.C.; Ford, M.J.; Cortie, M.B. Effect of dipole moment on current-voltage characteristics of single molecules. In Proceedings of the International Conference on Nanoscience and Nanotechnology (ICONN), Brisbane, Australia, 3–7 July 2006. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijazi, M.; Stambouli, V.; Rieu, M.; Tournier, G.; Pijolat, C.; Viricelle, J.-P. Sensitive and Selective Ammonia Gas Sensor Based on Molecularly Modified SnO2. Proceedings 2017, 1, 399. https://doi.org/10.3390/proceedings1040399
Hijazi M, Stambouli V, Rieu M, Tournier G, Pijolat C, Viricelle J-P. Sensitive and Selective Ammonia Gas Sensor Based on Molecularly Modified SnO2. Proceedings. 2017; 1(4):399. https://doi.org/10.3390/proceedings1040399
Chicago/Turabian StyleHijazi, Mohamad, Valérie Stambouli, Mathilde Rieu, Guy Tournier, Christophe Pijolat, and Jean-Paul Viricelle. 2017. "Sensitive and Selective Ammonia Gas Sensor Based on Molecularly Modified SnO2" Proceedings 1, no. 4: 399. https://doi.org/10.3390/proceedings1040399




