Tin Dioxide-Graphene Based Chemi-Device for NO2 Detection in the Sub ppm Range †
Abstract
:1. Introduction
2. Materials and Methods
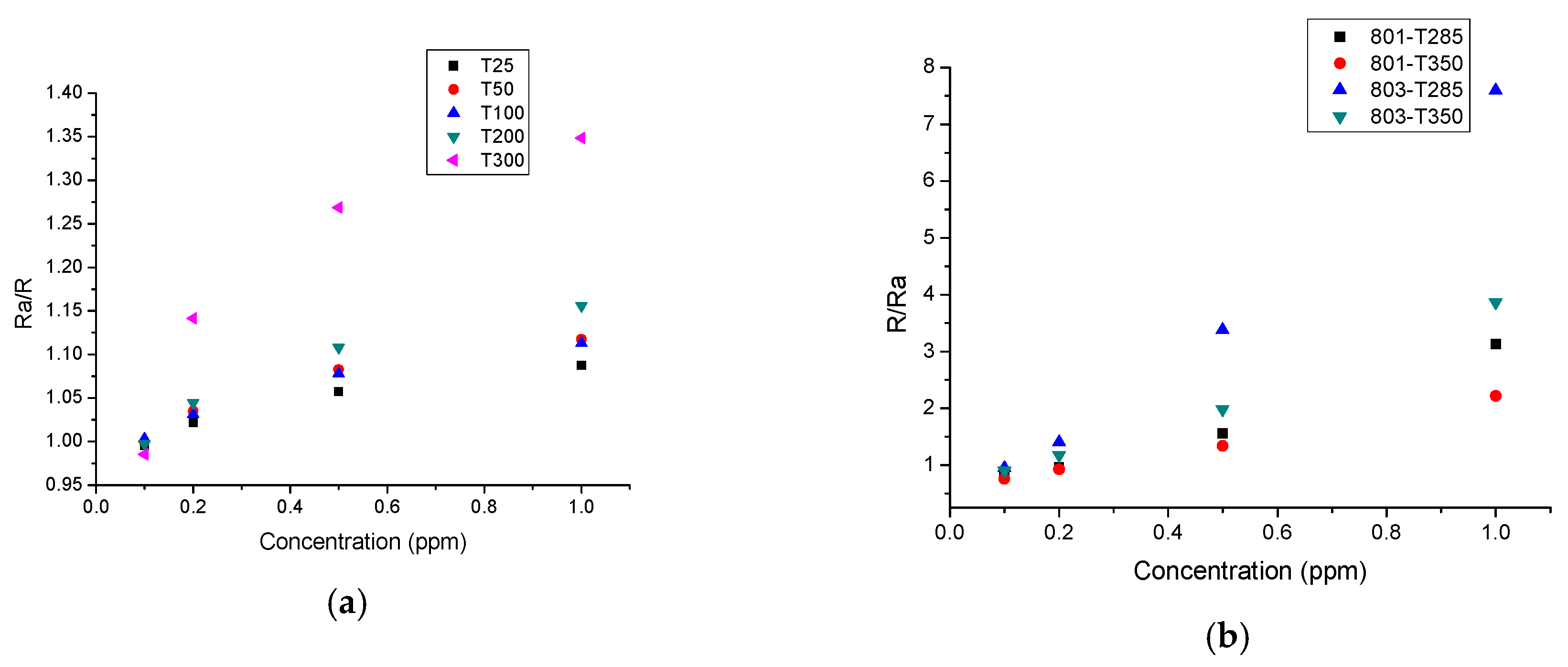
3. Results and Discussion
Acknowledgments
Conflicts of Interest
References
- Air Quality in Europe 2016; EEA Report No 28/2016. Publications Office of the European Union: Luxembourg, Luxemburg, 2016; ISSN 1977-8449. [CrossRef]
- Miglietta, M.L.; Massera, E.; Romano, S.; Polichetti, T.; Nasti, I.; Ricciardella, F.; Fattoruso, G. Chemically exfoliated graphene detects NO2 at the ppb level. Procedia Eng. 2011, 25, 1145–1148. [Google Scholar] [CrossRef]
- Fedi, F.; Miglietta, M.L.; Polichetti, T.; Ricciardella, F.; Massera, E.; Ninno, D.; di Francia, G. A study on the physicochemical properties of hydro-alcoholic solutions to improve the direct exfoliation of natural graphite down to few-layers graphene. Mater. Res. Express 2015, 2, 35601–35608. [Google Scholar] [CrossRef]
- Santos, J.P.; Fernández, M.J.; Fontecha, J.L.; Matatagui, D.; Sayago, I.; Horrillo, M.C.; Gracia, I. Nanocrystalline tin oxide nanofibers deposited by a novel focused electrospinning method. Application to the detection of TATP precursors. Sensors 2014, 14, 24231–24243. [Google Scholar] [CrossRef] [PubMed]
- AMS.com. Available online: http://ams.com/eng/Products/Environmental-Sensors/Gas-Sensors/CCS801 (accessed on 5 March 2017).



| Solution | Needle Tip 1-Substrate Distance (cm) | Inter-Electrode Voltage (kV) | Flow Rate (μL/min) | Deposition Time (min) |
|---|---|---|---|---|
| SnCl4-PVA | 8 | 19 | 4 | 25 |
| Gr1-SnCl4-PVA | 5 | 19 | 1 | 20 |
| Gr2-SnCl4-PVA | 5 | 19 | 1 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.P.; Polichetti, T.; Aleixandre, M.; Hontañón, E.; Sayago, I.; Alfano, B.; Miglietta, M.L.; Francia, G.D. Tin Dioxide-Graphene Based Chemi-Device for NO2 Detection in the Sub ppm Range. Proceedings 2017, 1, 442. https://doi.org/10.3390/proceedings1040442
Santos JP, Polichetti T, Aleixandre M, Hontañón E, Sayago I, Alfano B, Miglietta ML, Francia GD. Tin Dioxide-Graphene Based Chemi-Device for NO2 Detection in the Sub ppm Range. Proceedings. 2017; 1(4):442. https://doi.org/10.3390/proceedings1040442
Chicago/Turabian StyleSantos, José Pedro, Tiziana Polichetti, Manuel Aleixandre, Esther Hontañón, Isabel Sayago, Brigida Alfano, Maria Lucia Miglietta, and Girolamo Di Francia. 2017. "Tin Dioxide-Graphene Based Chemi-Device for NO2 Detection in the Sub ppm Range" Proceedings 1, no. 4: 442. https://doi.org/10.3390/proceedings1040442






