Vertical 3D GaN Nanoarchitectures towards an Integrated Optoelectronic Biosensing Platform in Microbial Fuel Cells †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrochemical Cell Setup
2.2. Sensor for Transmission Measurements
2.3. GaN as Electrode Material
- For A: an n-GaN layer (2.3 µm, donor concentration ND = 1018 cm−3) and a 1.9 µm n-doped layer in the range of 1018–1019 cm−3. This working electrode was cleaned with isopropanol before usage.
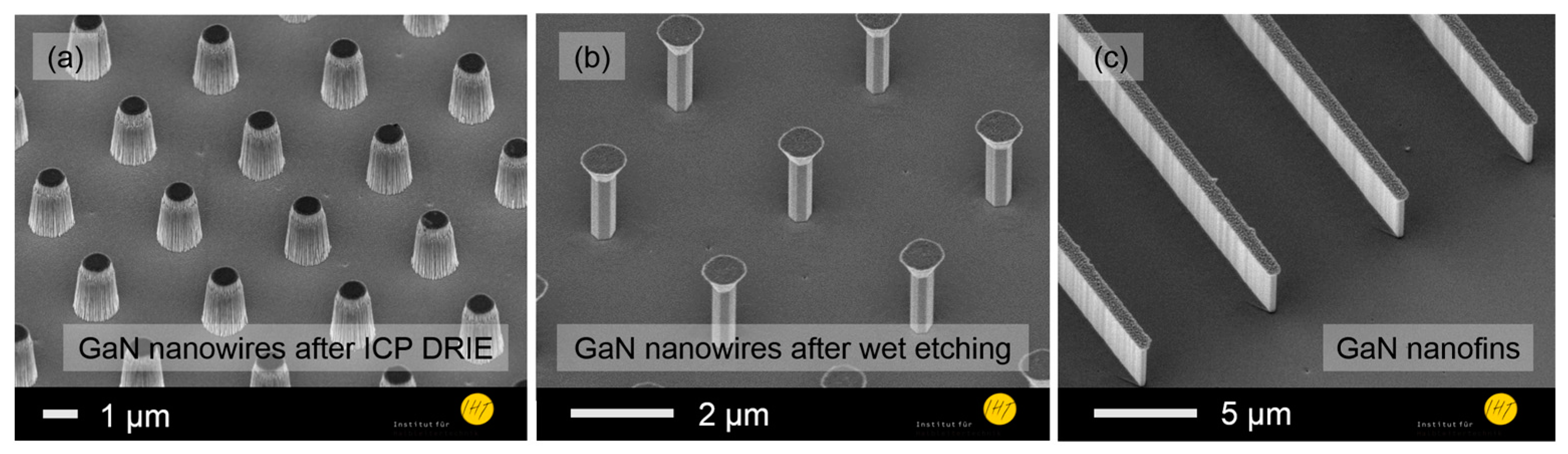
- For B: an n-GaN layer (2.5 µm, ND = 1018 cm−3), a low doped GaN layer (1.5 µm, ND = 1016 cm−3) and an n-GaN layer (1.0 µm, ND = 1018 cm−3). Nanowires and nanofins (Figure 2) were fabricated by inductively coupled plasma deep reactive ion etching (ICP-DRIE), followed by wet etching for sidewall smoothing and wire diameter reduction [6]. The nanowire array had a pitch of ~2 µm, a height of about 1.45 µm and a diameter of 380 nm.
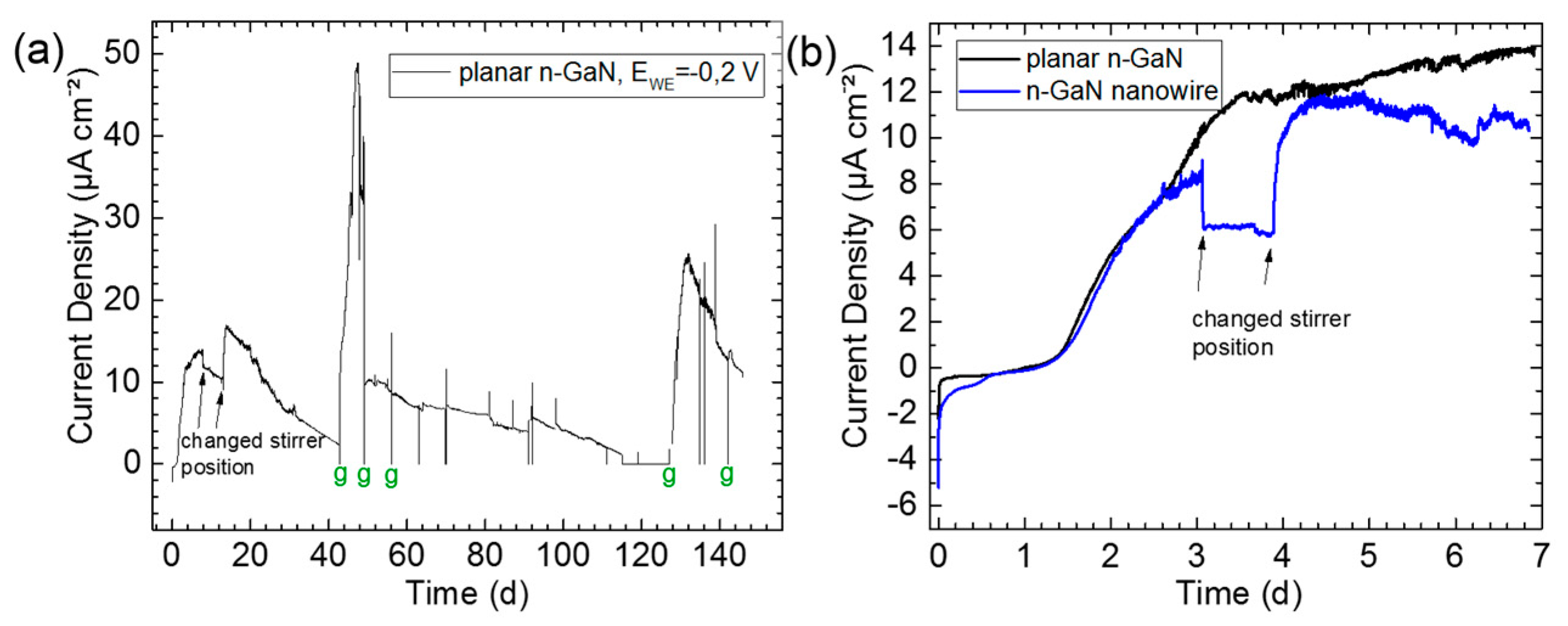
3. Experimental Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Beyenal, H.; Babauta, J.T. Biofilms in Bioelectrochemical Systems: From Laboratory Practice to Data Interpretation, 1st ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Schmidt, I.; Gad, A.; Scholz, G.; Boht, H.; Martens, M.; Schilling, M.; Wasisto, H.S.; Waag, A.; Schröder, U. Gold-modified indium tin oxide as a transparent window in optoelectronic diagnostics of electrochemically active biofilms. Biosens. Bioelectron. 2017, 94, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef]
- Connolly, J.; Jain, A.; Pastorella, G.; Krishnamurthy, S.; Mosnier, J.-P.; Marsili, E. Zinc oxide and indium tin oxide thin films for the growth and characterization of Shewanella loihica PV-4 electroactive biofilms. Virulence 2011, 2, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Waag, A.; Wang, X.; Fündling, S.; Ledig, J.; Erenburg, M.; Neumann, R.; Al Suleiman, M.; Merzsch, S.; Wei, J.; Li, S.; et al. The nanorod approach: GaN NanoLEDs for solid state lighting. Phys. Status Solidi 2011, 8, 2296–2301. [Google Scholar] [CrossRef]
- Yu, F.; Yao, S.; Römer, F.; Witzigmann, B.; Schimpke, T.; Strassburg, M.; Bakin, A.; Schumacher, H.W.; Peiner, E.; Wasisto, H.S.; et al. GaN nanowire arrays with nonpolar sidewalls for vertically integrated field-effect transistors. Nanotechnology 2017, 28, 95206. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boht, H.; Wichmann, H.; Scholz, G.; Yu, F.; Strempel, K.; Mariana, S.; Fatahilah, M.F.; Clavero, I.M.; Prades, J.D.; Schröder, U.; et al. Vertical 3D GaN Nanoarchitectures towards an Integrated Optoelectronic Biosensing Platform in Microbial Fuel Cells. Proceedings 2017, 1, 508. https://doi.org/10.3390/proceedings1040508
Boht H, Wichmann H, Scholz G, Yu F, Strempel K, Mariana S, Fatahilah MF, Clavero IM, Prades JD, Schröder U, et al. Vertical 3D GaN Nanoarchitectures towards an Integrated Optoelectronic Biosensing Platform in Microbial Fuel Cells. Proceedings. 2017; 1(4):508. https://doi.org/10.3390/proceedings1040508
Chicago/Turabian StyleBoht, Heidi, Hilke Wichmann, Gregor Scholz, Feng Yu, Klaas Strempel, Shinta Mariana, Muhammad Fahlesa Fatahilah, Irene Manglano Clavero, Joan Daniel Prades, Uwe Schröder, and et al. 2017. "Vertical 3D GaN Nanoarchitectures towards an Integrated Optoelectronic Biosensing Platform in Microbial Fuel Cells" Proceedings 1, no. 4: 508. https://doi.org/10.3390/proceedings1040508




