Demonstration of Intracortical Chronic Recording and Acute Microstimulation Using Novel Floating Neural Probes †
Abstract
:1. Introduction
2. Microfabrication of Floating Neural Probes
3. Functional Characterization In Vitro and In Vivo
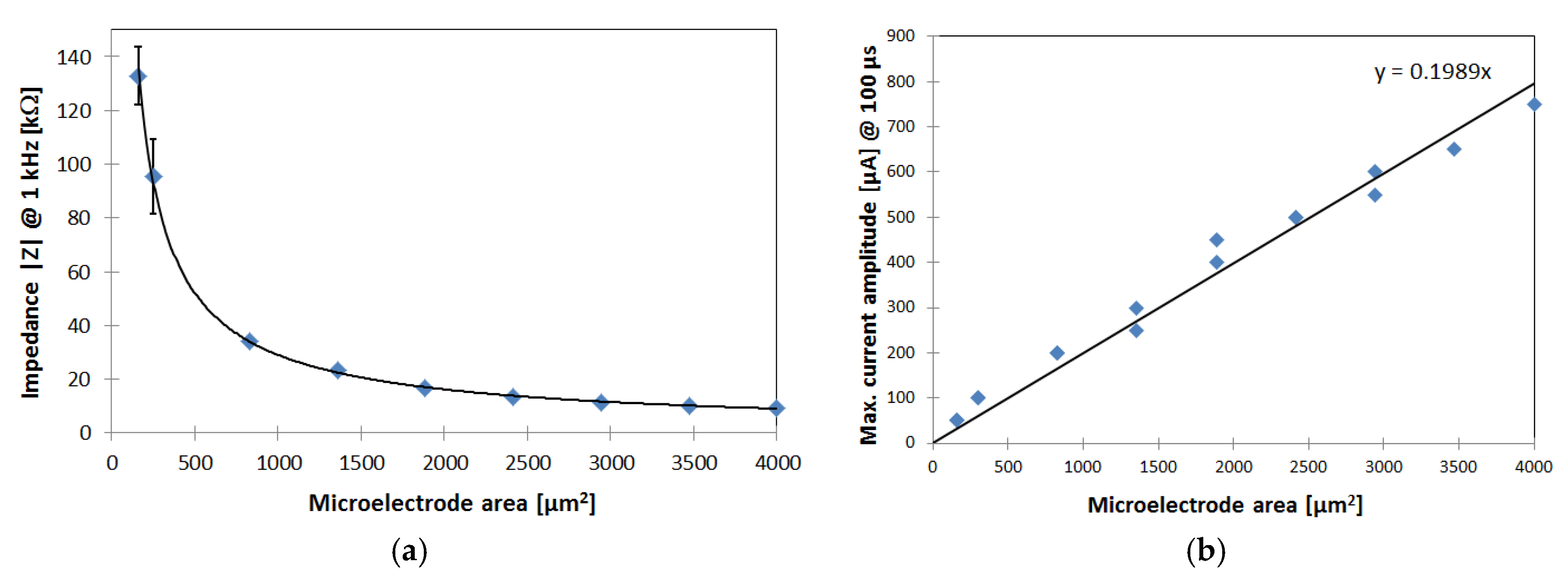
3.1. In Vitro Characterization
3.2. In Vivo Characterization
4. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Kook, G.; Lee, S.W.; Lee, H.C.; Cho, I.J.; Lee, H.J. Neural probes for chronic applications. Micromachines 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.C.; Sy, J.C.; Falcón-Banchs, R.; Cima, M.J. A three dimensional in vitro glial scar model to investigate the local strain effects from micromotion around neural implants. Lab Chip 2017, 17, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 2017, 3, e1601966. [Google Scholar] [CrossRef] [PubMed]
- Schander, A.; Stemmann, H.; Tolstosheeva, E.; Roese, R.; Biefeld, V.; Kempen, L.; Kreiter, A.K.; Lang, W. Design and fabrication of novel multi-channel floating neural probes for intracortical chronic recording. Sens. Actuators A Phys. 2016, 247, 125–135. [Google Scholar] [CrossRef]
- Schander, A.; Teßmann, T.; Strokov, S.; Stemmann, H.; Kreiter, A.K.; Lang, W. In Vitro evaluation of the long-term stability of PEDOT: PSS coated microelectrodes for chronic recording and electrical stimulation of neurons. In Proceedings of the 2016 IEEE 38th Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 6174–6177. [Google Scholar]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Venkatraman, S.; Hendricks, J.; King, Z.A.; Sereno, A.J.; Richardson-Burns, S.; Martin, D.; Carmena, J.M. In Vitro and In Vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schander, A.; Stemmann, H.; Kreiter, A.K.; Lang, W. Demonstration of Intracortical Chronic Recording and Acute Microstimulation Using Novel Floating Neural Probes. Proceedings 2017, 1, 511. https://doi.org/10.3390/proceedings1040511
Schander A, Stemmann H, Kreiter AK, Lang W. Demonstration of Intracortical Chronic Recording and Acute Microstimulation Using Novel Floating Neural Probes. Proceedings. 2017; 1(4):511. https://doi.org/10.3390/proceedings1040511
Chicago/Turabian StyleSchander, Andreas, Heiko Stemmann, Andreas K. Kreiter, and Walter Lang. 2017. "Demonstration of Intracortical Chronic Recording and Acute Microstimulation Using Novel Floating Neural Probes" Proceedings 1, no. 4: 511. https://doi.org/10.3390/proceedings1040511




