A Portable, Optical Scanning System for Large Field of View, High Resolution Imaging of Biological Specimens †
Abstract
:1. Introduction
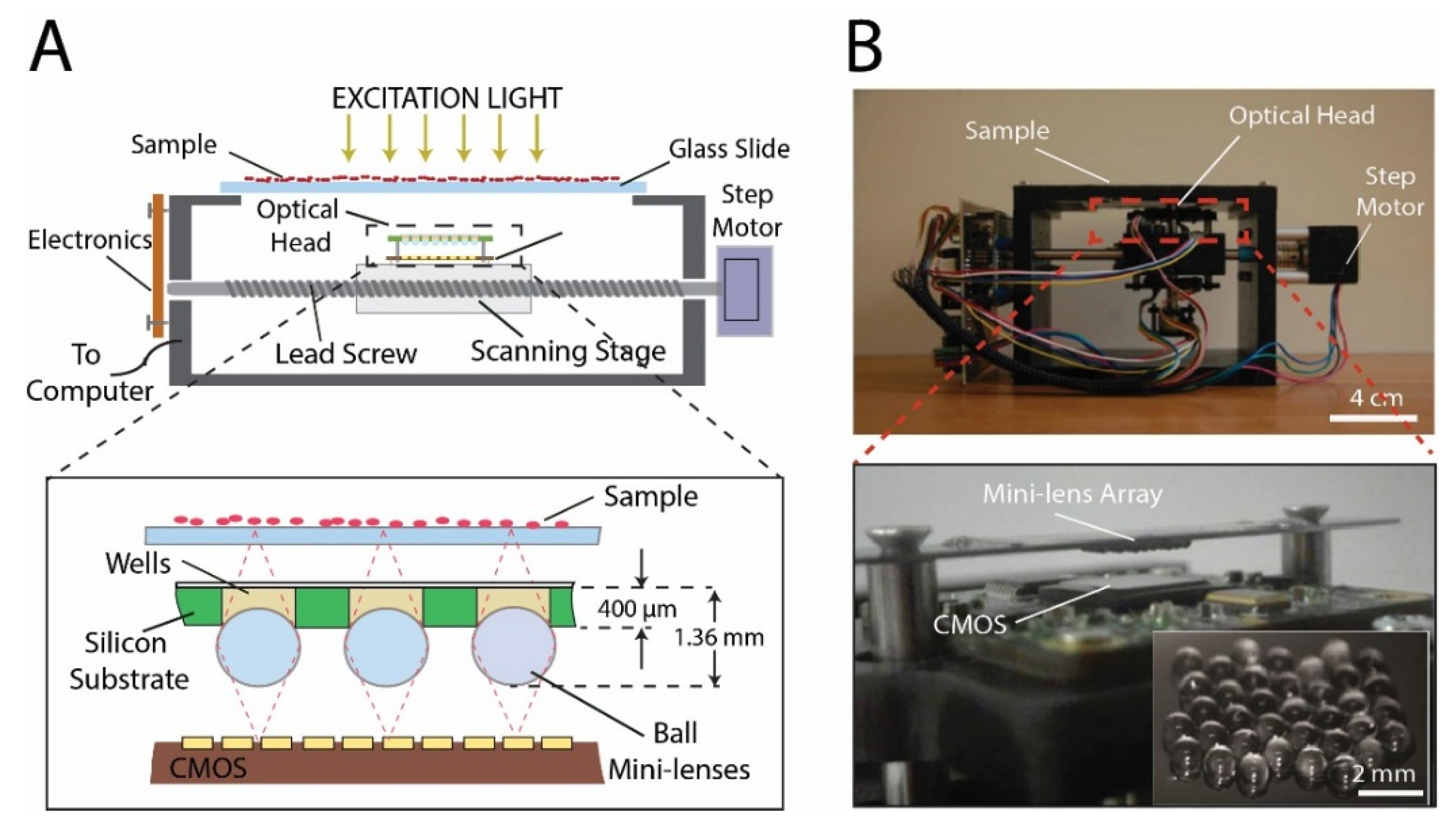
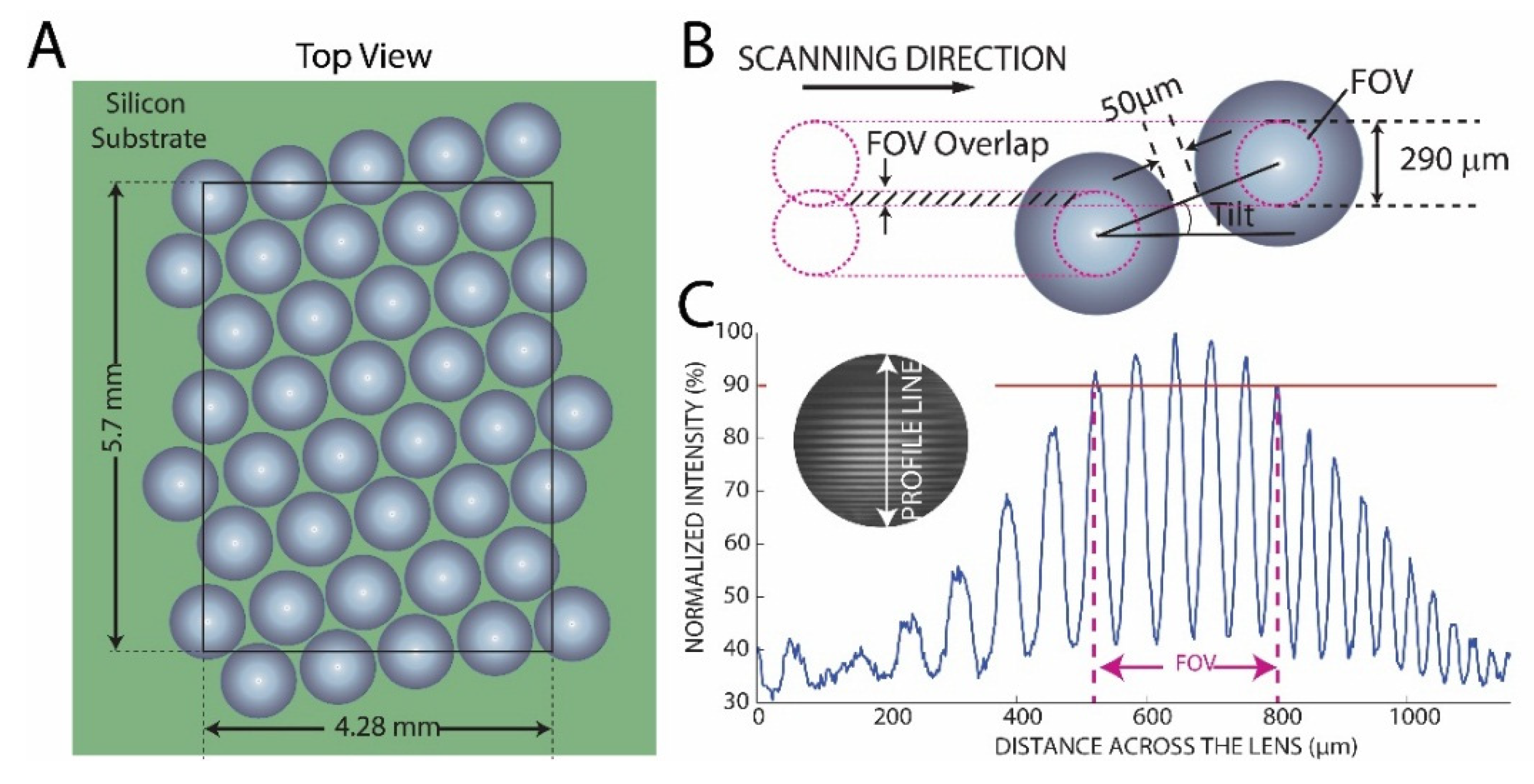
2. Materials and Methods
3. Results
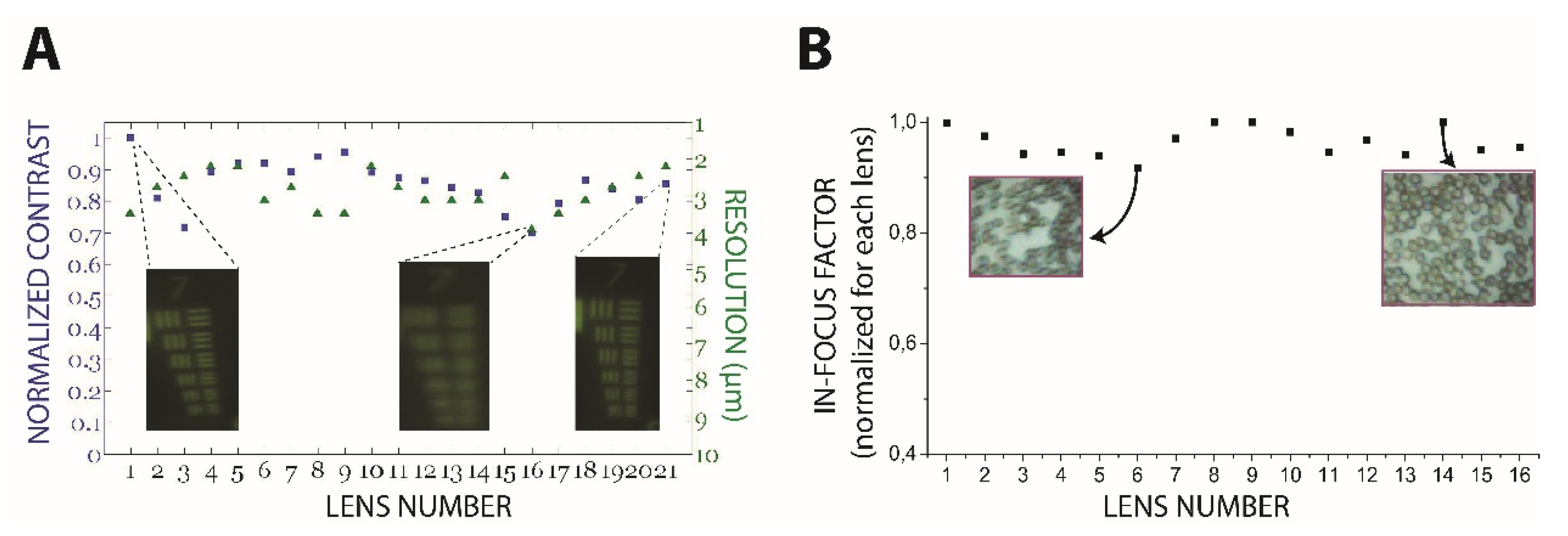
3.1. Optical System Characterization
3.2. Cell Detection
4. Discussion
Acknowledgments
Conflicts of Interest
References
- Boppart, S.A.; Kortum, R. Point-of-care and point-of-procedure optical imaging technologies for primary care and global health. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef]
- Gulari, M.N.; Tripathi, A.; Ghannad-Rezaie, M.; Chronis, N. An optofluidic Lens Array Microchip for High Resolution Stereo Micrscopy. Micromachines 2014, 5, 607–621. [Google Scholar] [CrossRef]
- Peli, E. Contrast in complex images. J. Opt. Soc. Am. 1990, 7, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Ortiz de Solorzano, C.; Vaquero, J.J.; Rena, J.M.; Malpica, N.; del Pozo, F. Evaluation of autofocus functions in molecular cytogenetic analysis. J. Microsc. 1997, 188, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duthaler, S.; Nelson, B.J. Autofocusing in Computer Microscopy: Selecting the Optimal Focus Algorithm. Microsc. Res. Tech. 2004, 65, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Buggethin, F.; Marr, C.; Schwarzfischer, M.; Hoppe, P.S.; Hilsenbeck, O.; Schroeder, T.; Theis, F.J. An automatic method for robust and fast cell detection in bright field images from high-throughput microscopy. BMC Bioinform. 2013, 14. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korompili, G.; Kanakaris, G.; Ampatis, C.; Chronis, N. A Portable, Optical Scanning System for Large Field of View, High Resolution Imaging of Biological Specimens. Proceedings 2017, 1, 548. https://doi.org/10.3390/proceedings1040548
Korompili G, Kanakaris G, Ampatis C, Chronis N. A Portable, Optical Scanning System for Large Field of View, High Resolution Imaging of Biological Specimens. Proceedings. 2017; 1(4):548. https://doi.org/10.3390/proceedings1040548
Chicago/Turabian StyleKorompili, Georgia, Georgios Kanakaris, Christos Ampatis, and Nikos Chronis. 2017. "A Portable, Optical Scanning System for Large Field of View, High Resolution Imaging of Biological Specimens" Proceedings 1, no. 4: 548. https://doi.org/10.3390/proceedings1040548




