Abstract
In today’s third world countries such as Pakistan, there is an ever increasing strain regarding the provision of clean, consumable water. This problem especially arises in rural areas due to the ineffectiveness of the governments and the increasing population in the country. Therefore, this particular project aims to detect and display real-time physiochemical quality of the water in a much more cost effective manner, as opposed to the current methods which involves sampling and laboratory methods, through its wireless, multi-sensor network. It takes into consideration multiple factors and presents this real-time quality through the display of its electrical conductivity, pH, total dissolved solids TDS, turbidity, as well as temperature of water that is being tested. Additionally, this remote control system is specially designed for lakes, reservoir, rivers etc. where we cannot monitor water quality in such complicated scale water environment by just using a stationary system because water parameter vary at every single location. To avoid this, we manufactured a boat which can float and move on the water simply by user controller. This structure is designed as a hull shape which minimize the resistivity of water flow and this shape also maintained the stability of water. This water quality monitoring boat includes an embedded global positioning system GPS which gives the location of the point wherever water quality is varying and radio frequency module for wireless communication. All the results is generated and displayed with their readings and their graphical analogue meters through the graphical user interface GUI technique, along with water’s impurities limitation points and its hazardous level notification. It is proven through various tests conducted in reservoirs, lakes and personal water storage tanks that this project is successfully capable of demonstrating these physiochemical parameters as well as display these readings effectively.
1. Introduction
Quality of drinking water plays a vital role in the health of animals and human beings. Rivers and lakes are the sole sources available to drink water. Irrigation, fishery and energy production organizations are highly relied on water quality. Therefore, the quality of water of rivers and lakes ought to be kept at a certain level. Water supply at homes of urban areas and water sources available in rural regions is however, not fundamentally secure for utilization. Indeed in spite of the fact that its government’s responsibility to guarantee that clean water is conveyed to its citizens. Imperfectly maintained infrastructure and nonstop increment in population causes strain regarding the provision of clean and consumable water. Hence it is necessary to monitor the quality of water which will be utilized for consumption. Monitoring is characterized as the collection of data at set locations and at standard interims in order to provide the information which may be used to direct the current conditions, buildup trends, etc. Conventional water quality checking methods include sampling and laboratory techniques. These strategies are however not cost effective and time consuming which eventually leads to delayed in detection of impurities and reaction to those contaminants in water. So there should be more efficient and productive checking strategies to monitor the quality of water. It can be accomplished through water dependent microbial and physiochemical measurements. Physiochemical parameters of water include turbidity, electrical conductivity EC, total dissolved solids TDS, power of hydrogen pH and temperature etc. These parameters are often analyzed quickly in cost effective manner instead of water sampling and laboratory testing techniques. It also can be measure with online instrumentation. According to the study and research of United States Environmental Protection Agency (USEPA) [] states that the water parameters are influenced by contaminants in specific ways and can be identified, detected and monitored by using suitable or particular water quality sensors. Many products are available commercially for water quality monitoring which are in shape of probes and meters. These products analyze the parameters separately and quite expensive as well. In this paper we have initiated a low-cost, real time, multi-sensor boat system which is specially designed for big complicated scale water environment such as rivers, reservoirs, lakes, etc. for measuring the physicochemical parameters of water. This water quality monitoring boat includes radio frequency module for wireless communication and an embedded global positioning system GPS which gives actual location of sample site []. The implemented system has conductivity, TDS, pH, temperature and turbidity sensors from first principle standards. All the results from the sensors are prepared, analyzed and transmitted to the observer via wireless communication system []. These results are generated and displayed with their readings and their graphical analogue meters through the graphical user interface GUI technique, along with their nominal ranges.
1.1. Water Parameters
1.1.1. Potential of Hydrogen pH
pH is basically an amount of hydrogen ions concentration in the water. pH value defines how acidic or basic water is, which can specifically influence the survival of amphibian life forms. pH has 0 to 14 range. Where 0 means very acidic and 14 very basic and 7 being a neutral. Drinking water range is from 6.5 to 7.5. Changing in pH can affect the chemical dissolved in water. High acidic water is dangerous for aquatic animals whose pH is less than 4.
1.1.2. Temperature
Temperature is the degree of hotness and coldness of anything. Temperature is a basic but the most important water quality parameter since it specifically impacts the measure of dissolved oxygen that is accessible to amphibian living beings. By detecting temperature we can get to know about the particular temperature bearing limit of animals inside the water. All other parameters (TDS, pH, conductivity) are dependent on temperature.
1.1.3. Turbidity
Turbidity is a measure of clarity of water. It measures the number of particles are in the water. For example plant, clay, silt, debris. Overabundance turbidity can lessen the reproduction rates of aquatic life while generating regions and eggs are secured with soil. Unit of turbidity is NTU (Nephelometric Turbidity Units).
1.1.4. Conductivity and Total Dissolved Solids TDS
Conductivity is a tendency of water which conduct electricity or electric current. It is an indirect measure of ion particles present in the water. The more particles exhibit, the greater electric current can be conducted by the water. Purity of water can be identified with the minimal of conductivity because pure water cannot conduct electricity. The measure of salt and minerals contaminations in the water is called total dissolved solids (TDS). TDS is approximately half of conductivity. Conductivity is measured in µS/cm (Micro Siemens per centimeter). TDS is expressed in PPM (parts per million). Drinking water ought to be under 500 ppm and agriculture water ought to be under 1200 ppm.
2. System Operation
Mechanical Structure
The main purpose of our project is to monitor the quality of water such as lakes, reservoir, rivers etc. We cannot monitor water quality in such big oceanic rivers by using a stationary system because water parameter vary at every single location. To avoid this, we manufactured a boat which can float and move on the water simply by user controller. Our structure is designed as a hull shape which reduces the resistivity of water flow and maintained the stability in the water.
Our structure is designed using Solid works then to over check the stability of structure. We simulate and calculate the buoyant force by using Archimedes’ principles
Volume were taken from Solid works design after finalizing dimensions.
Constant terms like density of water and gravity were used to check the buoyant force formula. This principle insure us whether our system is floating or sinking.
Mass of body and mass of every component were expected to be.
After adding up all the data for buoyant formula in Equation (1).
Archimedes principle states that buoyant force should be twice or greater than force of gravity.
After putting all the data in Equation (2) to get the force of gravity.
This result proves that this structure will float on the water.
The structure is manufactured by using plastic and PVC sheets. Our boat is consisting of a piped structure on both sides which is used to embed the all sensors together with structure.
3. Results
All the results and outputs are generated via Graphical user interface GUI technique. It is the main component of this project for the monitoring purpose. Different types of buttons, blocks and analogue meters are used to monitor the status of water. There are analogue meters for every water parameter to display the range of the water parameters. Thermometer is used to display the temperature and Gauge meters are used to display pH, Turbidity and Conductivity readings along with their defined ranges as shown in Figure 1.
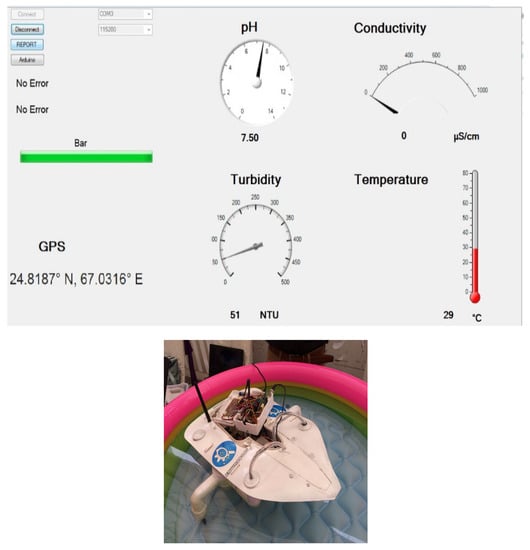
Figure 1.
Graphical user interface and real boat design.
All analogue meters that are displayed on the front panel are taken from the library of measurement studios for visual studios. GUI is also generating report in which absolute values and sensor values are shown in tabular form. User has to click the REPORT button to get the current water quality report about Water’s impurities limitation points and its hazardous level notification with the current GPS location that is also being displayed on the final report. The disconnect button is used to stop the monitoring.
4. Conclusions
To check the quality of water, the current method is to take the water sample manually. These samples were send to the laboratories to test the quality which take extra human effort, cost and time. In our proposed system, it will give the properties of water automatically on screen without any extra human effort. With the help of these properties we could figure out the quality of water for the aquatic animals. This research work provides the design of low-cost, wireless remote controlled mini boat system which indicates the water’s multi parameters along with GPS navigation in real time. This system is useful for the complicated scale water environment. The water parameters are being clearly displayed on the GUI. Warning appears on GUI when any parameter is at insecure or danger level.
References
- Hall, J.; Zaffiro, A.D.; Marx, R.B.; Kefauver, P.C.; Krishnan, E.R.; Haught, R.C.; Herrmann, J.G. On-line water quality parameters as indicators of distribution system contamination. J. Am. Water Works Assoc. 2007, 99, 66–77. [Google Scholar] [CrossRef]
- Chung, W.Y.; Chen, C.L.; Chen, J.B. Design and implementation of low power wireless sensor system for water quality monitoring. In Proceedings of the 2011 5th International Conference on Bioinformatics and Biomedical Engineering, Wuhan, China, 10–12 May 2011. [Google Scholar]
- Lambrou, T.P.; Anastasiou, C.C.; Panayiotou, C.G.; Polycarpou, M.M. A low-cost sensor network for real-time monitoring and contamination detection in drinking water distribution systems. IEEE Sens. J. 2014, 14, 2765–2772. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).