Monoclinic Zirconium Oxide Nanostructures Having Tunable Band Gap Synthesized under Extremely Non-Equilibrium Plasma Conditions †
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel. Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Wilk, G.D.; Wallace, R.M.; Anthony, J.M. High-κ gate dielectrics: Current status and materials properties considerations. J. Appl. Phys. 2001, 89, 5243–5275. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, J.; Wang, J.; Wu, G.; Chen, L. Sol–gel derived ZrO2–SiO2 highly reflective coatings. Int. J. Inorg. Mater. 2000, 2, 319–323. [Google Scholar] [CrossRef]
- Koch, T.; Ziemann, P. Zr-silicide formation during the epitaxial growth of Y-stabilized zirconia films on Si(100) and its avoidance by ion beam assisted deposition at a reduced temperature. Appl. Surf. Sci. 1996, 99, 51–57. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, B.; Yang, M.; Han, W.; Shao, X. ZrO2/CeO2 nanocomposite: Two step synthesis, microstructure, and visible-light photocatalytic activity. Mater. Lett. 2013, 112, 90–93. [Google Scholar] [CrossRef]
- Gao, P.; Meng, L.J.; dos Santos, M.P.; Teixeira, V.; Andritschky, M. Study of ZrO2–Y2O3 films prepared by rf magnetron reactive sputtering. Thin Solid Films 2000, 377, 32–36. [Google Scholar] [CrossRef]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Weber, E.; Russo, R.; Yang, P. Room-temperature ultraviolet nanowire nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef]
- Cao, H.Q.; Qiu, X.Q.; Luo, B.; Liang, Y.; Zhang, Y.H.; Tan, R.Q.; Zhao, M.J.; Zhu, Q.M. Synthesis and Room-Temperature Ultraviolet Photoluminescence Properties of Zirconia Nanowires. Adv. Funct. Mater. 2004, 14, 243–246. [Google Scholar] [CrossRef]
- Dwivedi, R.; Maurya, A.; Verma, A.; Prasad, R.; Bartwal, K.S. Microwave assisted sol–gel synthesis of tetragonal zirconia nanoparticles. J. Alloys Compd. 2011, 509, 6848–6851. [Google Scholar] [CrossRef]
- Espinoza-Gonzalez, R.A.; Diaz-Droguett, D.E.; Avila, J.I.; Gonzalez-Fuentes, C.A.; Fuenzalida, V.M. Hydrothermal growth of zirconia nanobars on zirconium oxide. Mater. Lett. 2011, 65, 2121–2123. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, F.; Wu, N.; Liu, X. Up- and Down-Conversion Cubic Zirconia and Hafnia Nanobelts. Adv. Mater. 2008, 20, 4826–4829. [Google Scholar] [CrossRef]
- Dong, W.-S.; Lin, F.-Q.; Liu, C.-L.; Li, M.-Y. Synthesis of ZrO2 nanowires by ionic-liquid route. J. Colloid Interface Sci. 2009, 333, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Zhang, L.; Hou, X.; Li, Y.; Tang, C. Degradation of methyl orange through synergistic effect of zirconia nanotubes and ultrasonic wave. J. Hazard. Mater. 2011, 188, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.; Li, W.Z.; Xu, J.M.; Leblanc, R.M.; Wang, D.Z.; Li, Y.; Guo, H.; Zhang, J. Controlled Hydrothermal Synthesis of Zirconium Oxide Nanostructures and Their Optical Properties. Cryst. Growth Des. 2009, 9, 3874–3880. [Google Scholar] [CrossRef]
- Ling, X.; Li, S.; Zhou, M.; Liu, X.; Zhao, Y.; Shao, J.; Fan, Z. Annealing effect on the laser-induced damage resistance of ZrO𝟤 films in vacuum. Appl. Opt. 2009, 48, 5459–5463. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Dadkhan, M.; Davar, F. Pure cubic ZrO2 nanoparticles by thermolysis of a new precursor. Polyhedron 2009, 28, 3005–3009. [Google Scholar] [CrossRef]
- Liang, J.; Deng, Z.; Jiang, X.; Li, F.; Li, Y. Photoluminescence of Tetragonal ZrO2 Nanoparticles Synthesized by Microwave Irradiation. Inorg. Chem. 2002, 41, 3602–3604. [Google Scholar] [CrossRef]
- Kumari, L.; Du, G.H.; Li, W.Z.; Vennila, R.S.; Saxena, S.K.; Wang, D.Z. Synthesis, microstructure and optical characterization of zirconium oxide nanostructures. Ceram. Int. 2009, 35, 2401–2408. [Google Scholar] [CrossRef]
- Lai, L.-J.; Lu, H.-C.; Chen, H.-K.; Cheng, B.-M.; Lin, M.-I.; Chu, T.-C. Photoluminescence of zirconia films with VUV excitation. J. Electron Spectrosc. Relat. Phenom. 2005, 144, 865–868. [Google Scholar] [CrossRef]
- Kumari, L.; Li, W.Z.; Wang, D.Z. Monoclinic zirconium oxide nanostructures synthesized by a hydrothermal route. Nanotechnol. 2008, 19, 195602. [Google Scholar] [CrossRef]
- Mikhailov, M.M.; Verevkin, A.C. The Variation of Band Gap Width in Zirconium Oxide Powders on Grinding. Russ. Phys. J. 2004, 47, 600–604. [Google Scholar] [CrossRef]
- Mangla, O.; Srivastava, M.P. GaN nanostructures by hot dense and extremely non-equilibrium plasma and their characterizations. J. Mater. Sci. 2013, 48, 304–310. [Google Scholar] [CrossRef]
- Mangla, O.; Roy, S.; Ostrikov, K. Dense Plasma Focus-Based Nanofabrication of III–V Semiconductors: Unique Features and Recent Advances. Nanomater. 2016, 6, 4. [Google Scholar] [CrossRef] [PubMed]

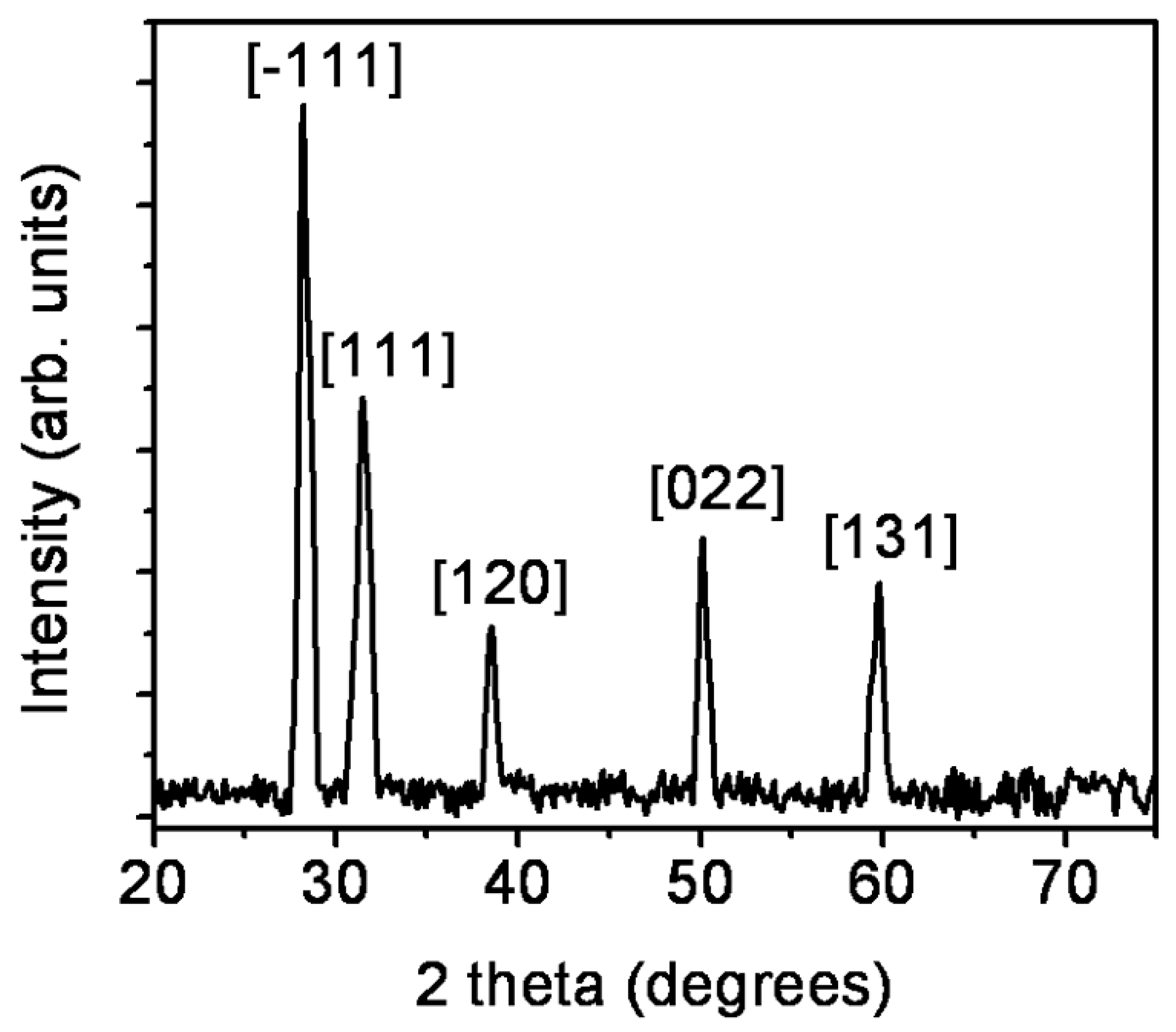
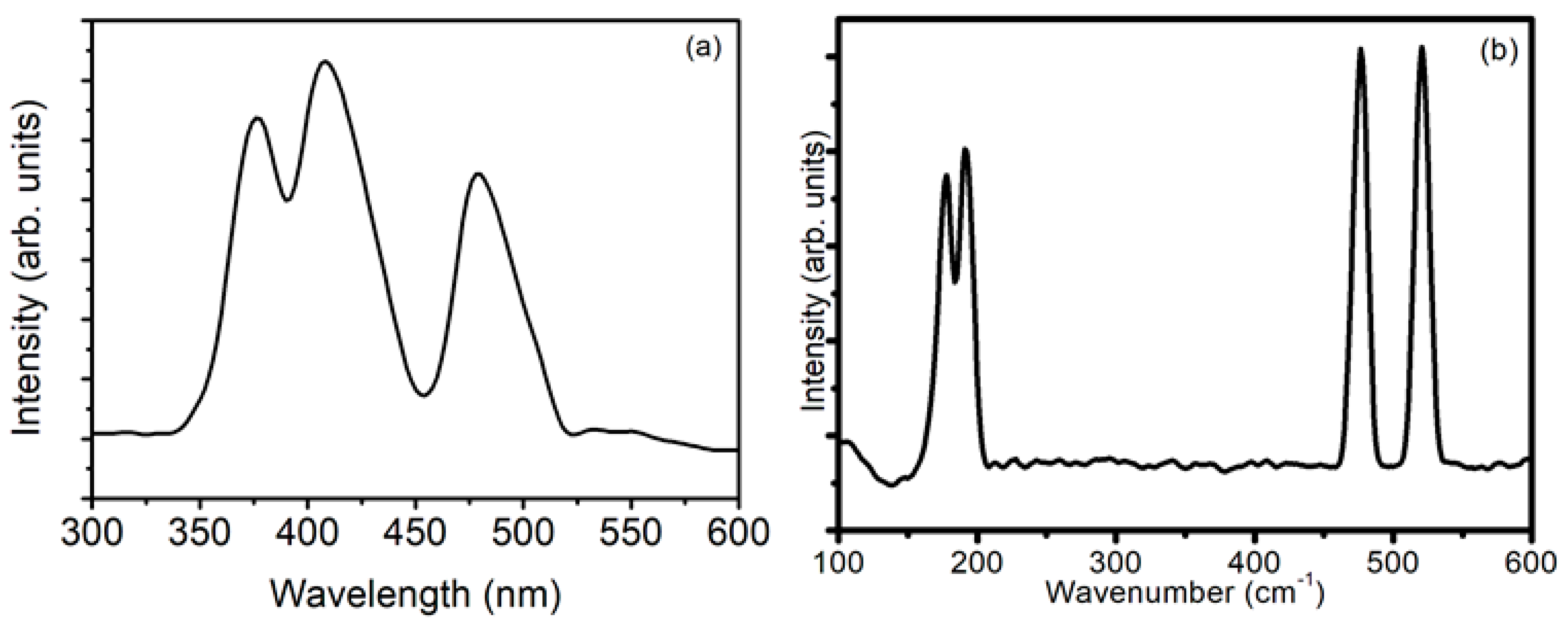
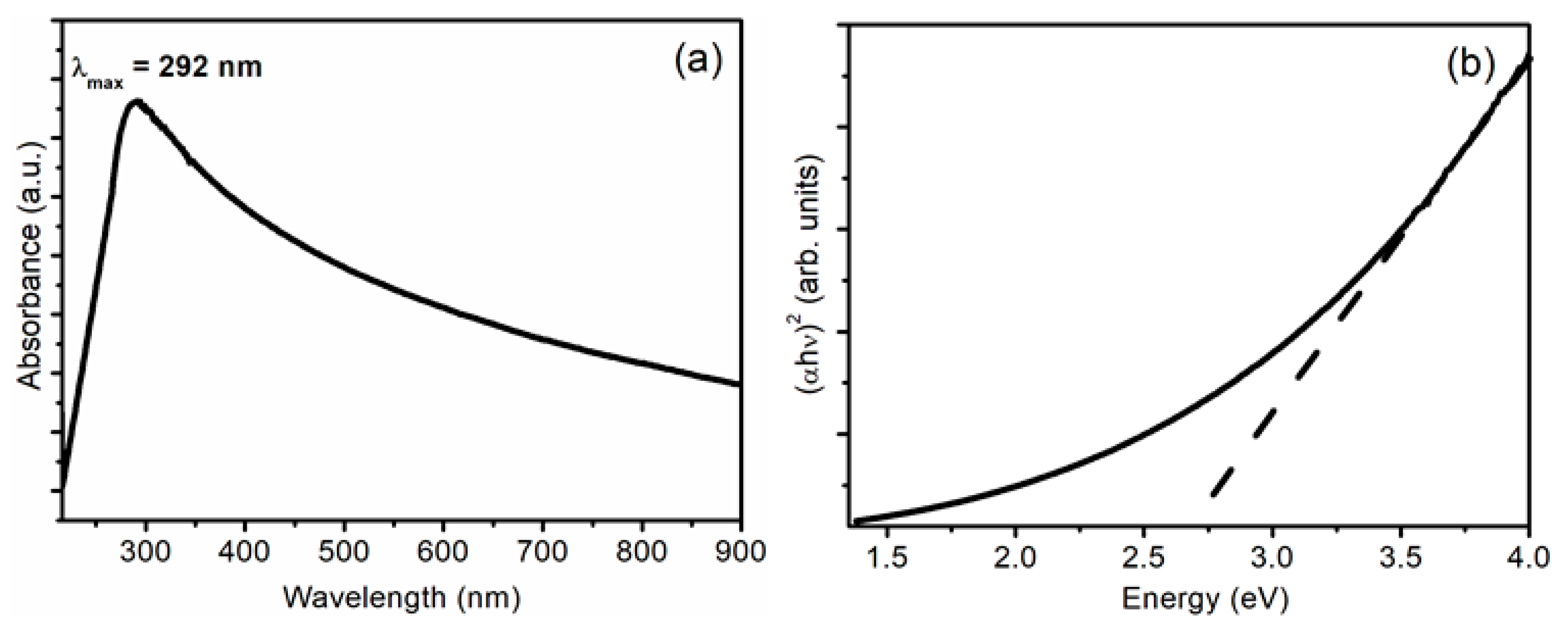
| 2θ (°) | θ (°) | β (Radians) | D (nm) | δ (×10−3 nm−2) | ε (×10−3) |
|---|---|---|---|---|---|
| 28.2 | 14.1 | 0.01166 | 12 | 6.94 | 2.83 |
| 31.5 | 15.75 | 0.01307 | 11 | 8.26 | 3.14 |
| 38.5 | 19.25 | 0.00865 | 17 | 3.46 | 2.04 |
| 50.1 | 25.05 | 0.00936 | 16 | 3.91 | 2.12 |
| 59.8 | 29.9 | 0.01089 | 15 | 4.44 | 2.36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangla, O.; Roy, S. Monoclinic Zirconium Oxide Nanostructures Having Tunable Band Gap Synthesized under Extremely Non-Equilibrium Plasma Conditions. Proceedings 2019, 3, 10. https://doi.org/10.3390/IOCN_2018-1-05486
Mangla O, Roy S. Monoclinic Zirconium Oxide Nanostructures Having Tunable Band Gap Synthesized under Extremely Non-Equilibrium Plasma Conditions. Proceedings. 2019; 3(1):10. https://doi.org/10.3390/IOCN_2018-1-05486
Chicago/Turabian StyleMangla, Onkar, and Savita Roy. 2019. "Monoclinic Zirconium Oxide Nanostructures Having Tunable Band Gap Synthesized under Extremely Non-Equilibrium Plasma Conditions" Proceedings 3, no. 1: 10. https://doi.org/10.3390/IOCN_2018-1-05486
APA StyleMangla, O., & Roy, S. (2019). Monoclinic Zirconium Oxide Nanostructures Having Tunable Band Gap Synthesized under Extremely Non-Equilibrium Plasma Conditions. Proceedings, 3(1), 10. https://doi.org/10.3390/IOCN_2018-1-05486




