The Influence of Cochlear Volume on Temporal Changes of Impedance among Cochlear Implant Patients
Abstract
:1. Introduction
2. Materials and Methods
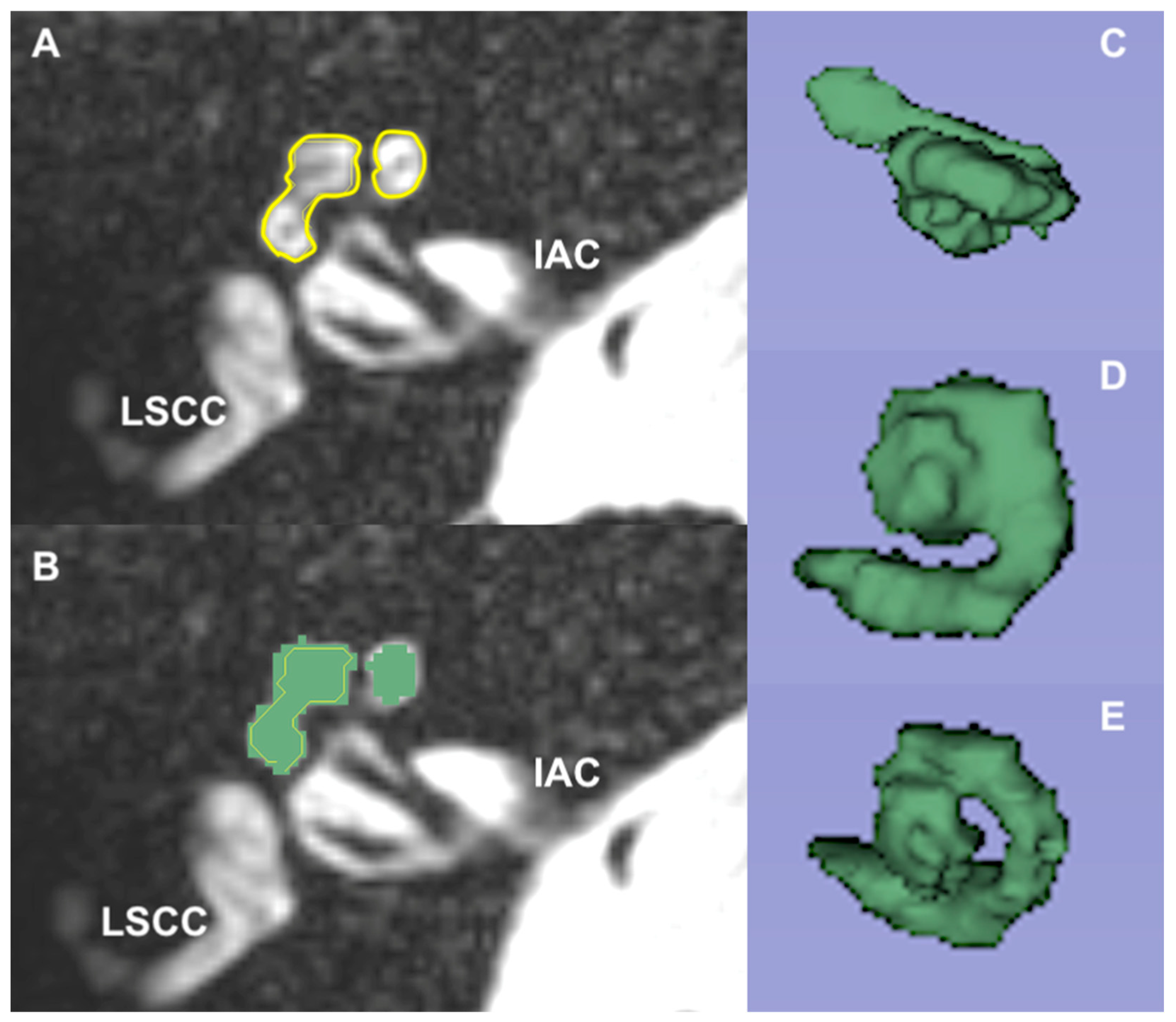
2.1. Magnetic Resonance Imaging
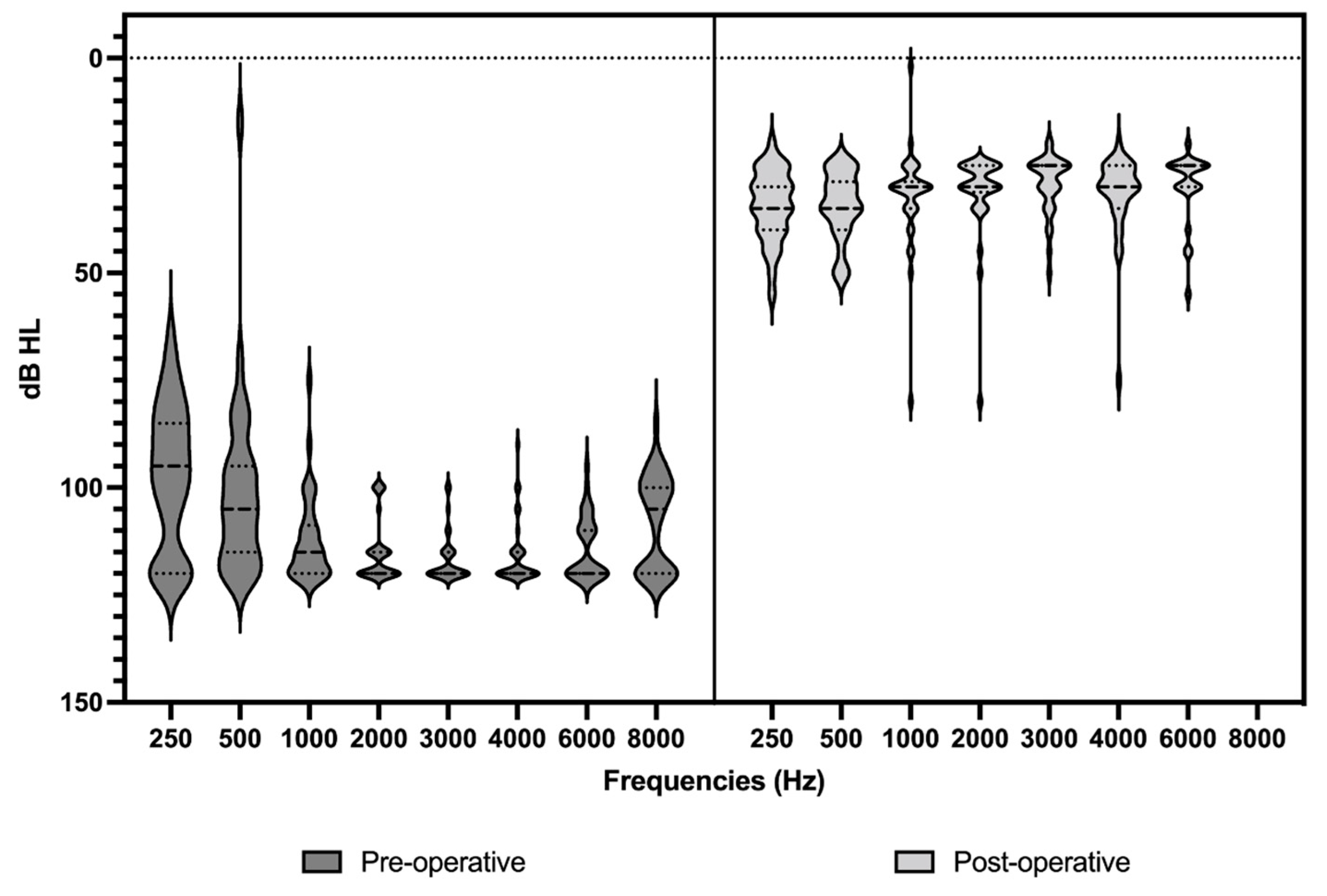
2.2. Audiometric Assessment
2.3. Statistical Analysis
3. Results
3.1. Analysis of Demographic Data
3.2. Magnetic Resonance Imaging
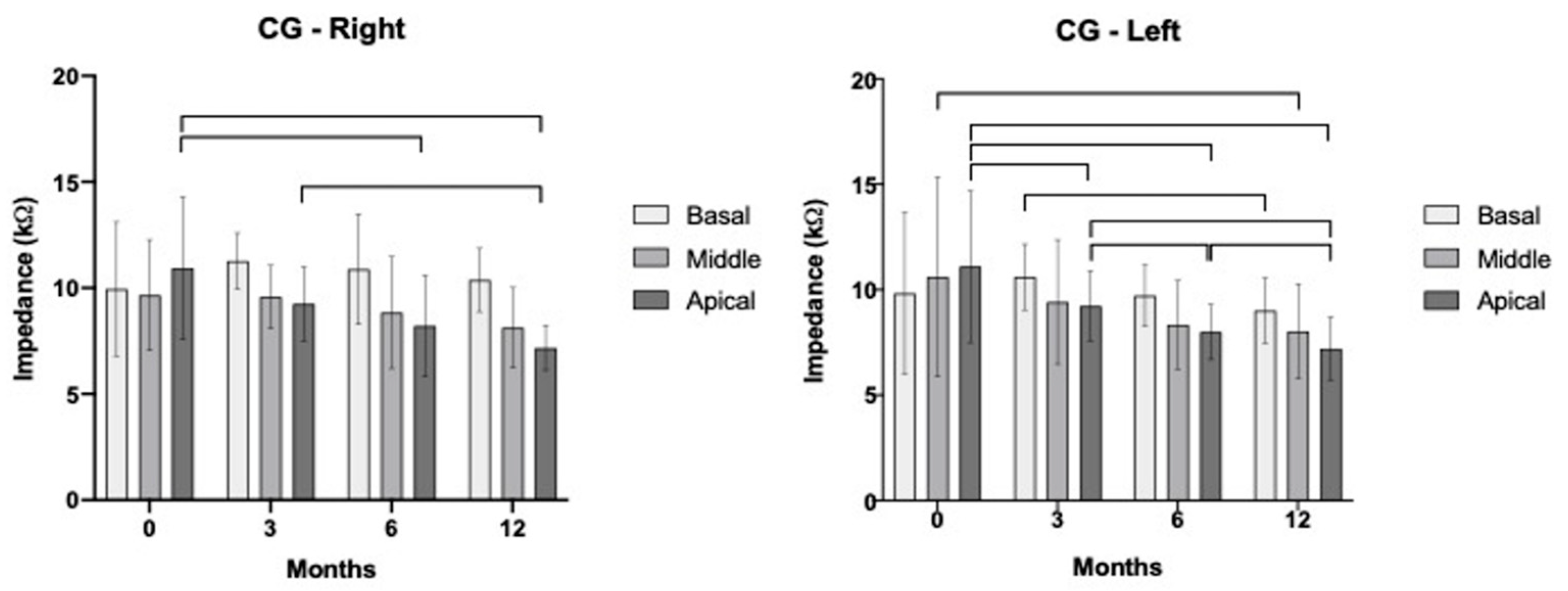
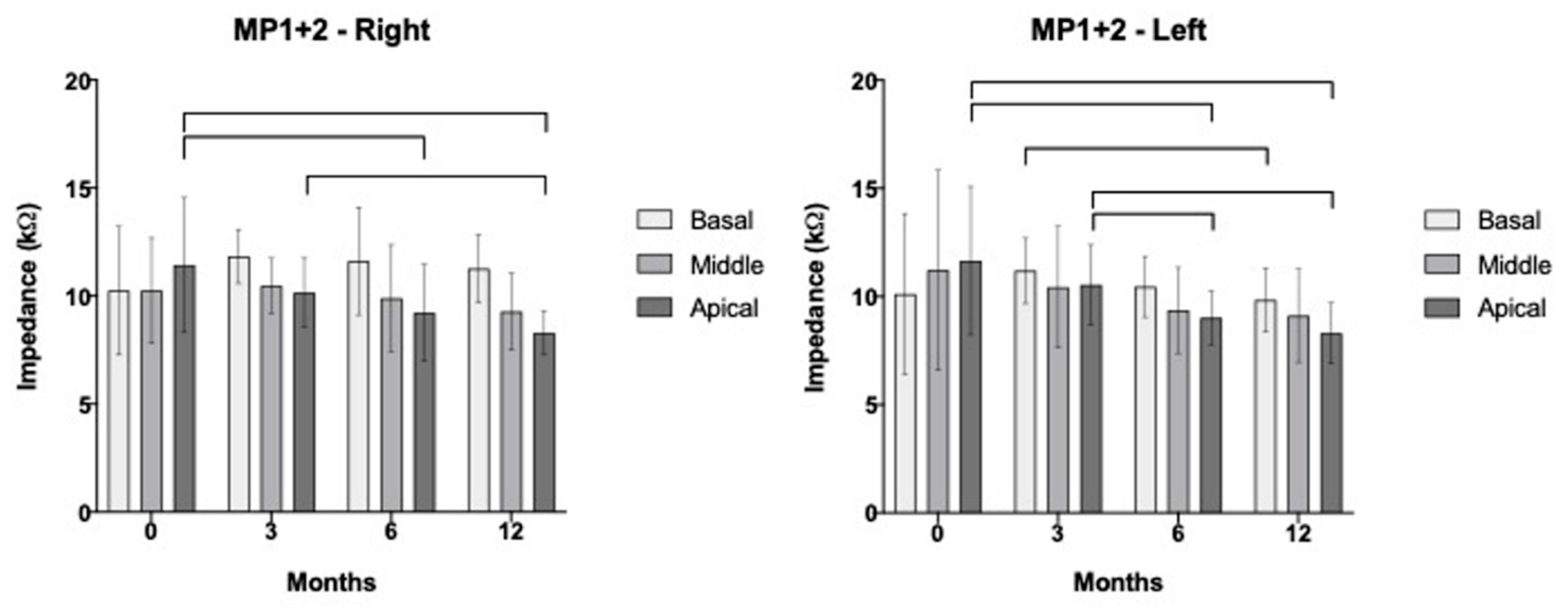
3.3. Segregated Analysis for Apical, Medial, and Basal Electrodes over Time
3.4. Impedance in Relation to Cochlear Volume
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leone, C.A.; Mosca, F.; Grassia, R. Temporal changes in impedance of implanted adults for various cochlear segments. Acta Otorhinolaryngol. Ital. 2017, 37, 312–319. [Google Scholar] [CrossRef]
- Durisin, M.; Büchner, A.; Lesinski-Schiedat, A.; Bartling, S.; Warnecke, A.; Lenarz, T. Cochlear implantation in children with bacterial meningitic deafness: The influence of the degree of ossification and obliteration on impedance and charge of the implant. Cochlear Implant. Int. 2015, 16, 147–158. [Google Scholar] [CrossRef]
- House, W.F. Cochlear Implant: My Perspective; Newport Beach All Hear: Newport Beach, CA, USA, 1995. [Google Scholar]
- Clark, G.M. The multiple-channel cochlear implant: The interface between sound and the central nervous system for hearing, speech, and language in deaf people—A personal perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 791–810. [Google Scholar] [CrossRef]
- Duan, Y.Y.; Clark, G.M.; Cowan, R.S. A study of intra-cochlear electrodes and tissue interface by electrochemical impedance methods in vivo. Biomaterials 2004, 25, 3813–3828. [Google Scholar] [CrossRef]
- Nadol, J.B., Jr. Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation. Otolaryngol. Head. Neck Surg. 1997, 117, 220–228. [Google Scholar] [CrossRef]
- Fayad, J.N.; Makarem, A.O.; Linthicum, F.H., Jr. Histopathological assessment of fibrosis and new bone formation in implanted human temporal bones using 3D-reconsruction. Otolaryngol. Head Neck Surg. 2009, 141, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Busby, P.A.; Plant, K.L.; Whitford, L.A. Electrode impedance in adults and children using the Nucleus 24 cochlear implant system. Cochlear Implant. Int. 2002, 3, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.M.; Shute, S.A.; Shepherd, R.K.; Carter, T.D. Cochlear implantation: Osteoneogenesis, electrode-tissue impedance, and residual hearing. Ann. Otol. Rhinol. Laryngol. Suppl. 1995, 166, 40–42. [Google Scholar]
- Swanson, B.; Seligman, P.; Carter, P. Impedance measurement of the nucleus 22-electrode array in patients. Ann. Otol. Rhinol. Laryngol. Suppl. 1995, 166, 141–144. [Google Scholar] [PubMed]
- Gleeson, T.G.; Lacy, P.D.; Bresnihan, M.; GaVney, R.; Brennan, P.; Viani, L. High resolution computed tomography and magnetic resonance imaging in the pre-operative assessment of cochlear implant patients. J. Laryngol. Otol. 2003, 117, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Bettman, R.; Beek, E.; Van Olphen, A.; Zonneveld, F.; Huizing, E. MRI versus CT in assessment of cochlear patency in cochlear implant candidates. Acta Otolaryngol. 2004, 124, 577–581. [Google Scholar] [CrossRef]
- Laszig, R.; Chang, S.O.; Kubo, T.; Ramos, M.A.; Frijns, J.H.; Briggs, R.; Haynes, D.S. APSCI panel discussion I: Imaging and surgical issues. Ear Hear. 2007, 28, 119S–123S. [Google Scholar] [CrossRef] [PubMed]
- Beijen, J.; Casselman, J.; Joosten, F.; Stover, T.; Aschendorf, A.; Zarowski, A.; Becker, H.; Mylanus, E. Magnetic resonance in patients with meningitis induced hearing loss. Eur. Arch. Otorhinolaryngol. 2009, 266, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Escudé, B.; James, C.; Deguine, O.; Cochard, N.; Eter, E.; Fraysse, B. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol. Neurotol. 2006, 11 (Suppl. 1), 27–33. [Google Scholar] [CrossRef]
- Takahashi, M.; Arai, Y.; Sakuma, N.; Yabuki, K.; Sano, D.; Nishimura, G.; Oridate, N.; Usami, S.-I. Cochlear volume as a predictive factor for residual-hearing preservation after conventional cochlear implantation. Acta Otolaryngol. 2018, 138, 345–350. [Google Scholar] [CrossRef]
- Lee, J.; Nadol, J.B., Jr.; Eddington, D.K. Depth of electrode insertion and postoperative performance in humans with cochlear implants: A histopathologic study. Audiol. Neurotol. 2010, 15, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Blamey, P. Are spiral ganglion cell numbers important for speech perception with a cochlear implant? Am. J. Otol. 1997, 18, S11–S12. [Google Scholar] [PubMed]
- Fayad, J.N.; Linthicum, F.H., Jr. Multichannel cochlear implants: Relation of histopathology to performance. Laryngoscope. 2006, 116, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Linthicum, F.H., Jr.; Fayad, J.N. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol. Neurotol. 2009, 30, 418–422. [Google Scholar] [CrossRef]
- Henkin, Y.; Kaplan-Neeman, R.; Muchnik, C.; Kronenberg, J.; Hildesheimer, M. Changes over time in electrical stimulation levels and electrode impedance values in children using the Nucleus 24M cochlear implant. Int. J. Ped Otorhinolaryngol. 2003, 67, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Henkin, Y.; Kaplan-Neeman, R.; Muchnik, C.; Kronenberg, J.; Hildesheimer, M. Changes over time in the psycho-electric parameters in children with cochlear implants. Int. J. Audiol. 2003, 42, 274–278. [Google Scholar] [CrossRef]
- Henkin, Y.; Kaplan-Neeman, R.; Kronenberg, J.; Migirov, L.; Hildesheimer, M.; Muchnik, C. Electrical stimulation levels and electrode impedance values in children using the Med-El Combi 40+ cochlear implant: A one year follow-up. J. Basic Clin. Physiol. Pharmacol. 2005, 16, 127–137. [Google Scholar] [CrossRef]
- Kamakura, T.; Nadol, J.B., Jr. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear. Res. 2016, 339, 132–141. [Google Scholar] [CrossRef]
- van Wermeskerken, G.K.; van Olphen, A.F.; Smoorenburg, G.F. Intra- and postoperative electrode impedance of the straight and Contour arrays of the Nucleus 24 cochlear implant: Relation to T and C levels. Int. J. Audiol. 2006, 45, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, B.; Liu, Y.; Yuan, Y.; Shu, Y.; Chen, B. Comparable electrode impedance and speech perception at 12 months after cochlear implantation using round window versus cochleostomy: An analysis of 40 patients. ORL J. Otorhinolaryngol. Relat. Spec. 2018, 80, 248–258. [Google Scholar] [CrossRef]
- Lehnhardt, E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO 1993, 41, 356–359. [Google Scholar] [PubMed]
- Cohen, N.L. Cochlear implant soft surgery: Fact or fantasy? Otolaryngol. Head Neck Surg. 1997, 117, 214–216. [Google Scholar] [CrossRef]
- Laszig, R. Cochlear implants in children (soft surgery). Adv. Otorhinolaryngol. 2000, 57, 87–89. [Google Scholar] [PubMed]
- James, C.; Albegger, K.; Battmer, R.; Burdo, S.; Deggouj, N.; Deguine, O.; Dillier, N.; Gersdorff, M.; Laszig, R.; Lenarz, T.; et al. Preservation of residual hearing with cochlear implantation: How and why. Acta Otolaryngol. 2005, 125, 481–491. [Google Scholar] [CrossRef]
- Eisenberg, L.S.; Luxford, W.M.; Becker, T.S.; House, W.F. Electrical stimulation of the auditory system in children deafened by meningitis. Otolaryngol. Head Neck Surg. 1984, 92, 700–705. [Google Scholar] [CrossRef]
- Rotteveel, L.J.; Snik, A.F.; Vermeulen, A.M.; Mylanus, E.A. Three-year follow-up of children with postmeningitic deafness and partial cochlear implant insertion. Clin. Otolaryngol. 2005, 30, 242–248. [Google Scholar] [PubMed]
- Henkin, Y.; Kaplan-Neeman, R.; Kronenberg, J.; Migirov, L.; Hildesheimer, M.; Muchnik, C. A longitudinal study of electrical stimulation levels and electrode impedance in children using the Clarion cochlear implant. Acta Otolaryngol. 2006, 126, 581–586. [Google Scholar] [CrossRef]
- Hughes, M.L.; Vander Werff, K.R.; Brown, C.J.; Abbas, P.J.; Kelsay, D.M.R.; Teagle, H.F.B.; Lowder, M.W. A longitudinal study of electrode impedance, the electrically evoked compound action potential, and behavioral measures in Nucleus 24 cochlear implant users. Ear Hear. 2001, 22, 471–486. [Google Scholar] [CrossRef]
- Kumar, G.; Chokshi, M.; Richter, C.P. P. Electrical impedance measurements of cochlear structures using the four-electrode reflection-coefficient technique. Hear. Res. 2010, 259, 86–94. [Google Scholar]
- Molisz, A.; Zarowski, A.; Vermeiren, A.; Theunen, T.; De Coninck, L.; Siebert, J.; Offeciers, E.F. Postimplantation changes of electrophysiological parameters in patients with cochlear implants. Audiol. Neurotol. 2015, 20, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Nadol, J.B., Jr.; Shiao, J.Y.; Burgess, B.J.; Gantz, B.J.; Coker, N.J.; Ketten, D.R.; Kos, I.; Roland, T., Jr.; Shiao, J.Y.; Eddington, D.K.; et al. Histopathology of cochlear implants in humans. Ann. Otol. Rhinol. Laryngol. 2001, 110, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Tykocinski, M.; Cohen, L.T.; Cowan, R.S. Measurement and analysis of access resistance and polarization impedance in cochlear implant recipients. Otol. Neurotol. 2005, 26, 948–956. [Google Scholar]
- Brummer, S.B.; Turner, M.J. Electrical stimulation with Pt electrodes: I- A method for determination of ‘real’ electrode areas. IEEE Trans. Biomed. Eng. 1977, 24, 436–439. [Google Scholar] [CrossRef]
- Lenarz, T.; Stover, T.; Buechner, A.; Paasche, G.; Briggs, R.; Risi, F.; Pesch, J.; Battmer, R.D. Temporal bone results and hearing preservation with a new straight electrode. Audiol. Neurotol. 2006, 11 (Suppl. 1), 34–41. [Google Scholar]
- Nadol, J.B., Jr.; Eddington, D.K. Histologic evaluation of the tissue seal and biologic response around cochlear implant electrodes in the human. Otol. Neurotol. 2004, 252, 57–62. [Google Scholar]
- Neuburger, J.; Lenarz, T.; Lesinski-Schiedat, A.; Büchner, A. Spontaneous increases in impedance following cochlear implantation: Suspected causes and management. Int. J. Audiol. 2009, 48, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.L.; Stille, L.J. Effect of stimulus and recording parameters on spatial spread of excitation and masking patterns obtained with the electrically evoked compound action potential in cochlear implants. Ear Hear. 2010, 31, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Runge-Samuelson, C.; Firszt, J.B.; Gaggl, W.; Wackym, P.A. Electrically evoked auditory brain-stem responses in adults and children: Effects of lateral to medial placement of the nucleus 24 contour electrode array. Otol. Neurotol. 2009, 30, 464–470. [Google Scholar] [CrossRef]
- Xi, X.; Ji, F.; Han, D.; Hong, M.; Chen, A. Electrode interaction in cochlear implant recipients: Comparison of straight and contour electrode arrays. ORL J. Otorhinolaryngol. Relat. Spec. 2009, 71, 228–237. [Google Scholar] [PubMed]
- Finley, C.C.; Holden, T.A.; Holden, L.K.; Whiting, B.R.; Chole, R.A.; Neely, G.J.; Hullar, T.E.; Skinner, M.W. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol. Neurotol. 2008, 29, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Esquia Medina, G.N.; Borel, S.; Nguyen, Y.; Ambert-Dahan, E.; Ferrary, E.; Sterkers, O.; Grayeli, A.B. Is electrode-modiolus distance a prognostic factor for hearing performances after cochlear implant surgery? Audiol. Neurotol. 2013, 18, 406–413. [Google Scholar] [CrossRef]
| Case | Sex | Volume | Age at Surgery (Years) | Pre/Post | Time of HL (Years) |
|---|---|---|---|---|---|
| 1 | M | 50.04 | 3 | Pre | 2 |
| 2 | F | 90.98 | 4 | Pre | 2.5 |
| 3 | F | 72.03 | 6 | Pre | 5 |
| 4 | F | 78.63 | 51 | Post | 48 |
| 5 | F | 72.87 | 5 | Pre | 3 |
| 6 | F | 57.51 | 17 | Post | 9 |
| 7 | M | 60.23 | 6 | Pre | 5 |
| 8 | F | 56.25 | 41 | Post | 28 |
| 9 | F | 75.41 | 5 | Post | 2 |
| 10 | M | 69.8 | 6 | Pre | 5 |
| 11 | M | 64.39 | 11 | Pre | 9.5 |
| 12 | M | 68.3 | 1 | Pre | 1 |
| 13 | F | 69.67 | 6 | Pre | 5 |
| 14 | F | 56.5 | 4 | Pre | 4 |
| 15 | F | 52.36 | 42 | Post | 38 |
| 16 | F | 60.58 | 5 | Pre | 5 |
| 17 | M | 38.35 | 5 | Pre | 3 |
| 18 | M | 69.28 | 5 | Pre | 4 |
| 19 | F | 60.77 | 4 | Pre | 3 |
| 20 | F | 13.68 | 4 | Pre | 3 |
| 21 | F | 41.34 | 10 | Pre | 8 |
| 22 | F | 57.86 | 4 | Pre | 3 |
| 23 | M | 68.32 | 4 | Pre | 3 |
| 24 | F | 54.2 | 8 | Pre | 2 |
| 25 | M | 63.04 | 5 | Pre | 3 |
| 26 | F | 65.09 | 39 | Post | 5 |
| 27 | F | 63.62 | 68 | Post | 17 |
| 28 | M | 81.11 | 5 | Pre | 3 |
| 29 | F | 57.35 | 47 | Post | 13 |
| 30 | F | 46.96 | 4 | Pre | 3 |
| 31 | F | 54.03 | 3 | Pre | 3 |
| 32 | F | 53.98 | 16 | Pre | 15 |
| 33 | F | 61.1 | 6 | Pre | 6 |
| 34 | F | 67.94 | 4 | Pre | 3 |
| Mean | - | 60.93 ± 13.76 | 5.00 ± 17.11 | - | 4.00 ± 10.45 |
| T0 | T3 | T6 | T12 | ||
|---|---|---|---|---|---|
| CG | Basal | p = 0.553 r = 0.166 | p < 0.001 * r = 0.811 | p = 0.006 * r = 0.670 | p = 0.004 * r = 0.694 |
| Middle | p = 0.098 r = 0.442 | p = 0.001 * r = 0.741 | p = 0.021 * r = 0.588 | p = 0.054 r = 0.505 | |
| Apical | p = 0.083 r = 0.462 | p = 0.001 * r = 0.739 | p = 0.016 * r = 0.609 | p = 0.002 * r = 0.727 |
| T0 | T3 | T6 | T12 | ||
|---|---|---|---|---|---|
| MP1+2 | Basal | p = 0.450 r = 0.211 | p < 0.001 * r = 0.816 | p = 0.004 * r = 0.695 | p = 0.004 * r = 0.694 |
| Middle | p = 0.062 r = 0.491 | p < 0.001 * r = 0.787 | p = 0.009 * r = 0.646 | p = 0.002 * r = 0.568 | |
| Apical | p = 0.050 r = 0.513 | p < 0.001 * r = 0.782 | p = 0.006 * r = 0.668 | p = 0.001 * r = 0.759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauna, H.F.; Do Amaral, M.S.A.; Fonseca, D.S.; Pessini, R.; Garcia, D.M.; Echeverria, J.; Guimarães, A.C.; Da Silva, V.A.R.; Hyppolito, M.A. The Influence of Cochlear Volume on Temporal Changes of Impedance among Cochlear Implant Patients. J. Otorhinolaryngol. Hear. Balance Med. 2023, 4, 3. https://doi.org/10.3390/ohbm4010003
Pauna HF, Do Amaral MSA, Fonseca DS, Pessini R, Garcia DM, Echeverria J, Guimarães AC, Da Silva VAR, Hyppolito MA. The Influence of Cochlear Volume on Temporal Changes of Impedance among Cochlear Implant Patients. Journal of Otorhinolaryngology, Hearing and Balance Medicine. 2023; 4(1):3. https://doi.org/10.3390/ohbm4010003
Chicago/Turabian StylePauna, Henrique F., Maria Stella A. Do Amaral, Daniela S. Fonseca, Rodrigo Pessini, Denny M. Garcia, Jéssica Echeverria, Alexandre C. Guimarães, Vagner A. R. Da Silva, and Miguel A. Hyppolito. 2023. "The Influence of Cochlear Volume on Temporal Changes of Impedance among Cochlear Implant Patients" Journal of Otorhinolaryngology, Hearing and Balance Medicine 4, no. 1: 3. https://doi.org/10.3390/ohbm4010003
APA StylePauna, H. F., Do Amaral, M. S. A., Fonseca, D. S., Pessini, R., Garcia, D. M., Echeverria, J., Guimarães, A. C., Da Silva, V. A. R., & Hyppolito, M. A. (2023). The Influence of Cochlear Volume on Temporal Changes of Impedance among Cochlear Implant Patients. Journal of Otorhinolaryngology, Hearing and Balance Medicine, 4(1), 3. https://doi.org/10.3390/ohbm4010003





