Effect of the Chemical Composition of Simulated Body Fluids on Aerogel-Based Bioactive Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silica Aerogel-Based Composites
2.3. Characterization
2.4. Simulated Body Fluid Compositions
2.5. In Vitro Study of Bioactivity
3. Results
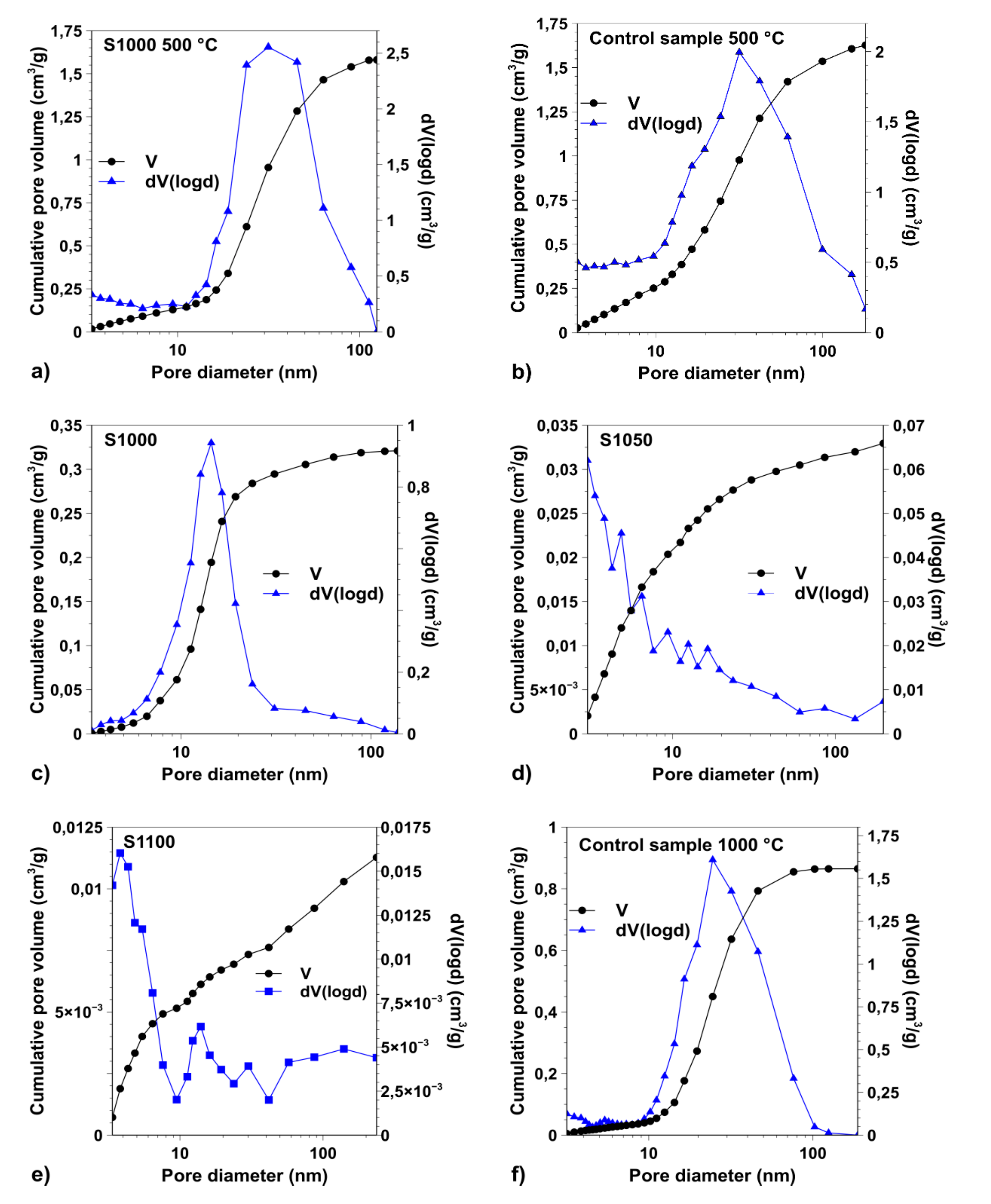
3.1. Porosity of the Samples
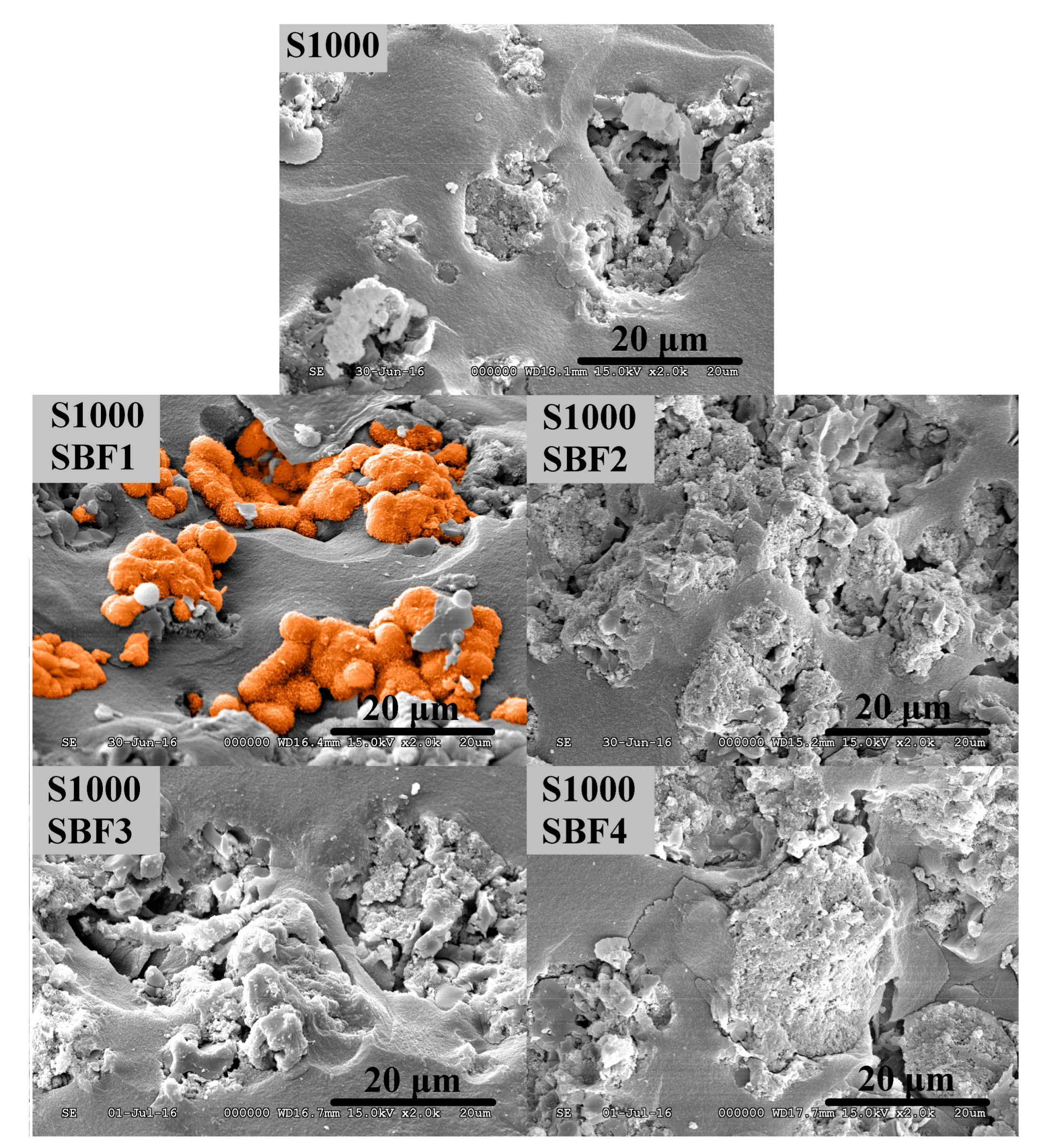
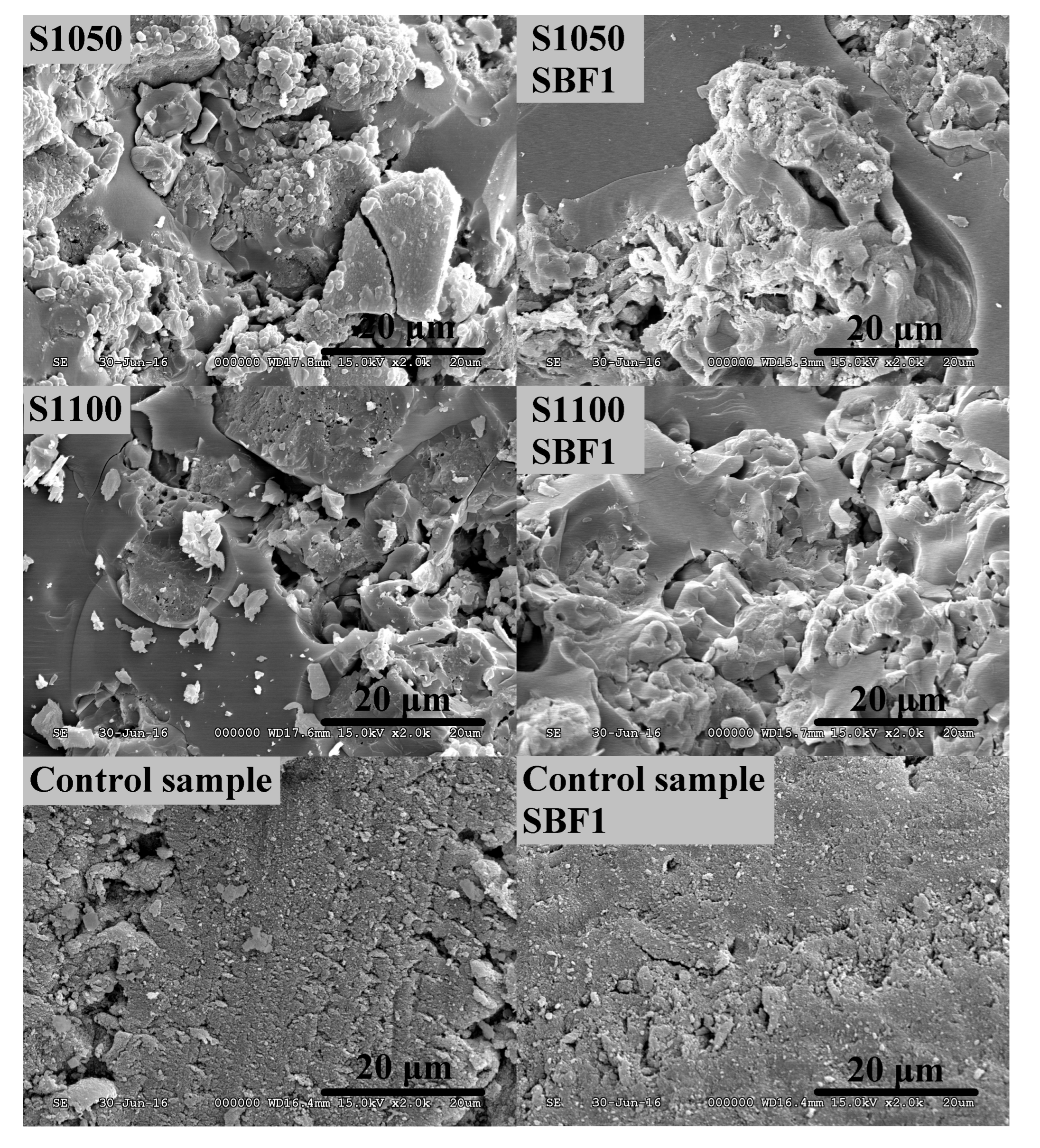
3.2. Study of Biocompatibility
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Smith, A.A.; Liu, B.; Li, J.; Hunter, D.J.; Dhamdhere, G.; Salmon, B.; Jiang, J.; Cheng, D.; Johnson, C.A.; et al. Reengineering autologous bone grafts with the stem cell activator WNT3A. Biomaterials 2015, 47, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Tamimi, I.; Cabrejos-Azama, J.; Tresguerres, I.; Alkhraisat, M.; López-Cabarcos, E.; Hernández, G.; Tamimi, F. Monetite granules versus particulate autologous bone in bone regeneration. Ann. Anat. Anat. Anz. 2015, 200, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. New trends in bioactive scaffolds: The importance of nanostructure. J. Eur. Ceram. Soc. 2009, 29, 1275–1281. [Google Scholar] [CrossRef]
- Wei, C.-K.; Ding, S.-J. Acid-resistant calcium silicate-based composite implants with high-strength as load-bearing bone graft substitutes and fracture fixation devices. J. Mech. Behav. Biomed. Mater. 2016, 62, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Van der Stok, J.; Van Lieshout, E.M.M.; El-Massoudi, Y.; Van Kralingen, G.H.; Patka, P. Bone substitutes in the Netherlands—A systematic literature review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Abdal-hay, A.; Hamdy, A.S.; Khalil, K.A.; Lim, J.H. A novel simple in situ biomimetic and simultaneous deposition of bonelike apatite within biopolymer matrix as bone graft substitutes. Mater. Lett. 2014, 137, 260–264. [Google Scholar] [CrossRef]
- Liu, C.; Wan, P.; Tan, L.L.; Wang, K.; Yang, K. Preclinical investigation of an innovative magnesium-based bone graft substitute for potential orthopaedic applications. J. Orthop. Transl. 2014, 2, 139–148. [Google Scholar] [CrossRef]
- Blokhuis, T.J. Bioresorbable bone graft substitutes. In Bone Substitute Biomaterials; Woodhead Publishing: Amsterdam, The Netherlands, 2014; pp. 80–92. [Google Scholar]
- Lacroix, J.; Jallot, E.; Lao, J. Gelatin-bioactive glass composites scaffolds with controlled macroporosity. Chem. Eng. J. 2014, 256, 9–13. [Google Scholar] [CrossRef]
- Vernè, E.; Ferraris, S.; Vitale-Brovarone, C.; Cochis, A.; Rimondini, L. Bioactive glass functionalized with alkaline phosphatase stimulates bone extracellular matrix deposition and calcification in vitro. Appl. Surf. Sci. 2014, 313, 372–381. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. Sol–gel derived bioactive glasses with low tendency to crystallize: Synthesis, post-sintering bioactivity and possible application for the production of porous scaffolds. Mater. Sci. Eng. C 2014, 43, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Chen, X.; Brauer, D.S.; Wilson, R.M.; Hill, R.G.; Karpukhina, N. Novel alkali free bioactive fluorapatite glass ceramics. J. Non-Cryst. Solids 2014, 402, 172–177. [Google Scholar] [CrossRef]
- Durgalakshmi, D.; Subhathirai, S.P.; Balakumar, S. Nano-bioglass: A Versatile Antidote for Bone Tissue Engineering Problems. Procedia Eng. 2014, 92, 2–8. [Google Scholar] [CrossRef]
- Mutlu, I. Sinter-coating method for the production of TiN-coated titanium foam for biomedical implant applications. Surf. Coat. Technol. 2013, 232, 396–402. [Google Scholar] [CrossRef]
- Rivard, J.; Brailovski, V.; Dubinskiy, S.; Prokoshkin, S. Fabrication, morphology and mechanical properties of Ti and metastable Ti-based alloy foams for biomedical applications. Mater. Sci. Eng. C 2014, 45, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Butev, E.; Esen, Z.; Bor, S. In vitro bioactivity investigation of alkali treated Ti6Al7Nb alloy foams. Appl. Surf. Sci. 2015, 327, 437–443. [Google Scholar] [CrossRef]
- Fidancevska, E.; Ruseska, G.; Bossert, J.; Lin, Y.-M.; Boccaccini, A.R. Fabrication and characterization of porous bioceramic composites based on hydroxyapatite and titania. Mater. Chem. Phys. 2007, 103, 95–100. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.-C.; Dascălu, C.A.; Maidaniuc, A.; Batalu, D.; Berbecaru, A.; Voicu, S.I.; Miculescu, M.; Thakur, V.K.; Ciocan, L.T. Facile synthesis and characterization of hydroxyapatite particles for high value nanocomposites and biomaterials. Vacuum 2017, 146, 614–622. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L.T. Progress in Hydroxyapatite–Starch Based Sustainable Biomaterials for Biomedical Bone Substitution Applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Colilla, M.; Vallet-Regí, M. Nanostructured Mesoporous Silicas for Bone Tissue Regeneration. J. Nanomater. 2008, 2008, e106970. [Google Scholar] [CrossRef]
- Parekh, B.; Joshi, M.; Vaidya, A. Characterization and inhibitive study of gel-grown hydroxyapatite crystals at physiological temperature. J. Cryst. Growth 2008, 310, 1749–1753. [Google Scholar] [CrossRef]
- Walschus, U.; Hoene, A.; Neumann, H.-G.; Wilhelm, L.; Lucke, S.; Lüthen, F.; Rychly, J.; Schlosser, M. Morphometric immunohistochemical examination of the inflammatory tissue reaction after implantation of calcium phosphate-coated titanium plates in rats. Acta Biomater. 2009, 5, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42 (Suppl. S2), S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cao, G.; Kale, R.P.; Prabakar, S.; López, G.P.; Brinker, C.J. Microporous Silica Prepared by Organic Templating: Relationship between the Molecular Template and Pore Structure. Chem. Mater. 1999, 11, 1223–1229. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Müller, F.A. Preparation of SBF with different content and its influence on the composition of biomimetic apatites. Acta Biomater. 2006, 2, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xu, X.; Yin, G.; Chen, A.; Liao, L.; Yao, Y.; Huang, Z.; Liao, X. A comparative study of the in vitro degradation of poly(l-lactic acid)/β-tricalcium phosphate scaffold in static and dynamic simulated body fluid. Eur. Polym. J. 2007, 43, 1768–1778. [Google Scholar] [CrossRef]
- Towler, M.R.; Boyd, D.; Freeman, C.; Brook, I.M.; Farthing, P. Comparison of in vitro and in vivo Bioactivity of SrO–CaO–ZnO–SiO2 Glass Grafts. J. Biomatrial Appl. 2009, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Y.; Fan, W.; Ke, X.; Hu, X.; Zhou, Y.; Xiao, Y. CaSiO3 microstructure modulating the in vitro and in vivo bioactivity of poly(lactide-co-glycolide) microspheres. J. Biomed. Mater. Res. A 2011, 98A, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Neel, E.A.; Mizoguchi, T.; Ito, M.; Bitar, M.; Salih, V.; Knowles, J.C. In vitro bioactivity and gene expression by cells cultured on titanium dioxide doped phosphate-based glasses. Biomaterials 2007, 28, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.; Zhao, L.; Weir, M.D.; Sun, J.; Chen, W.; Man, Y.; Xu, H.H.K. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015, 18, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Teoh, S.-H.; Hui, J.H.P.; Fisk, N.M.; Choolani, M.; Chan, J.K.Y. The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials 2012, 33, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Wittenburg, G.; Flade, V.; Garbe, A.I.; Lauer, G.; Labudde, D. Scaffold preferences of mesenchymal stromal cells and adipose-derived stem cells from green fluorescent protein transgenic mice influence the tissue engineering of bone. Br. J. Oral Maxillofac. Surg. 2014, 52, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Gu, W.; Cui, J.; Yu, M.; Zhang, Y.; Tang, C.; Yang, P.; Xu, X. Platelet-rich plasma enhanced umbilical cord mesenchymal stem cells-based bone tissue regeneration. Arch. Oral Biol. 2014, 59, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chittur, K.K.; Lacefield, W.R. Analysis of bovine serum albumin adsorption on calcium phosphate and titanium surfaces. Biomaterials 1999, 20, 377–384. [Google Scholar] [CrossRef]
- Wassell, D.T.H.; Hall, R.C.; Embery, G. Adsorption of bovine serum albumin onto hydroxyapatite. Biomaterials 1995, 16, 697–702. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Dorozhkina, E.I. The influence of bovine serum albumin on the crystallization of calcium phosphates from a revised simulated body fluid. Colloids Surf. Physicochem. Eng. Asp. 2003, 215, 191–199. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies : Abstract : Nature. Available online: http://www.nature.com/nature/journal/v127/n3211/abs/127741a0.html (accessed on 22 December 2016).
- Lázár, I.; Bereczki, H.F.; Manó, S.; Daróczi, L.; Deák, G.; Fábián, I.; Csernátony, Z. Synthesis and study of new functionalized silica aerogel poly(methyl methacrylate) composites for biomedical use. Polym. Compos. 2015, 36, 348–358. [Google Scholar] [CrossRef]
- Lázár, I.; Fábián, I. A Continuous Extraction and Pumpless Supercritical CO2 Drying System for Laboratory-Scale Aerogel Production. Gels 2016, 2, 26. [Google Scholar] [CrossRef]
- Vallés Lluch, A.; Gallego Ferrer, G.; Monleón Pradas, M. Biomimetic apatite coating on P(EMA-co-HEA)/SiO2 hybrid nanocomposites. Polymer 2009, 50, 2874–2884. [Google Scholar] [CrossRef]
- Clark, V.L.; Kruse, J.A. Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Metabolic & Therapeutic Aspects of Amino Acids in Clinical Nutrition, Second Edition. Available online: https://www.crcpress.com/Metabolic--Therapeutic-Aspects-of-Amino-Acids-in-Clinical-Nutrition-Second/Cynober/p/book/9780849313820 (accessed on 9 November 2017).
- Steele, B.F.; Reynolds, M.S.; Baumann, C.A. Amino acids in the blood and urine of human subjects ingesting different amounts of the same proteins. J. Nutr. 1950, 40, 145–158. [Google Scholar] [PubMed]
- Stein, W.H.; Moore, S. The free amino acids of human blood plasma. J. Biol. Chem. 1954, 211, 915–926. [Google Scholar] [PubMed]
- Li, H.; Li, Z.-X.; Li, H.; Wu, Y.-Z.; Wei, Q. Characterization of plasma sprayed hydroxyapatite/ZrO2 graded coating. Mater. Des. 2009, 30, 3920–3924. [Google Scholar] [CrossRef]
- Aljabo, A.; Abou Neel, E.A.; Knowles, J.C.; Young, A.M. Development of dental composites with reactive fillers that promote precipitation of antibacterial-hydroxyapatite layers. Mater. Sci. Eng. C 2016, 60, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Wang, M. Production and evaluation of biodegradable composites based on PHB–PHV copolymer. Biomaterials 2002, 23, 2631–2639. [Google Scholar] [CrossRef]
- Ni, J.; Wang, M. In vitro evaluation of hydroxyapatite reinforced polyhydroxybutyrate composite. Mater. Sci. Eng. C 2002, 20, 101–109. [Google Scholar] [CrossRef]
- Jaakkola, T.; Rich, J.; Tirri, T.; Närhi, T.; Jokinen, M.; Seppälä, J.; Yli-Urpo, A. In vitro Ca-P precipitation on biodegradable thermoplastic composite of poly(ε-caprolactone-co-dl-lactide) and bioactive glass (S53P4). Biomaterials 2004, 25, 575–581. [Google Scholar] [CrossRef]
- Barrie, P.J. NMR Applications to Porous Solids. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Oxford, MA, USA, 1995; Volume 30, pp. 37–95. [Google Scholar]
- Mekmene, O.; Quillard, S.; Rouillon, T.; Bouler, J.-M.; Piot, M.; Gaucheron, F. Effects of pH and Ca/P molar ratio on the quantity and crystalline structure of calcium phosphates obtained from aqueous solutions. Dairy Sci. Technol. 2009, 89, 301–316. [Google Scholar] [CrossRef]
- Szabó, B.A.; Kiss, L.; Manó, S.; Jónás, Z.; Lázár, I.; Fábián, I.; Dezső, B.; Csernátony, Z. The examination of aerogel composite artificial bone substitutes in animal models. Biomech. Hung. 2013, 52–63. [Google Scholar] [CrossRef]
- Kuttor, A.; Szalóki, M.; Rente, T.; Kerényi, F.; Bakó, J.; Fábián, I.; Lázár, I.; Jenei, A.; Hegedüs, C. Preparation and application of highly porous aerogel-based bioactive materials in dentistry. Front. Mater. Sci. 2014, 8, 46–52. [Google Scholar] [CrossRef]
| Obtained Parameters | Samples | Control Sample | ||||
|---|---|---|---|---|---|---|
| Calcination temperatures: | 500 °C | 1000 °C | 1050 °C | 1100 °C | 500 °C | 1000 °C |
| SBET (m2·g−1) | 331 | 76 | 9 | 4 | 540 | 159 |
| d (nm) | 32 | 14 | n/a | n/a | 32 | 24 |
| Vtotal (cm3·g−1) | 1.5807 | 0.321 | 0.0329 | 0.0113 | 1.6274 | 0.865 |
| Vmacropore (cm3·g−1) | 0.1159 | 0.007 | 0.0022 | 0.0029 | 0.2066 | 0.01 |
| Vmeso-and micropore (cm3·g−1) | 1.4648 | 0.314 | 0.0307 | 0.0084 | 1.4208 | 0.855 |
| Components | Blood Plasma (mmol·L−1) | SBF ** (mmol·L−1) | SBF1 (mmol·L−1) | SBF2 (mmol·L−1) | SBF3 (mmol·L−1) | SBF4 (mmol·L−1) |
|---|---|---|---|---|---|---|
| Ca2+ | 2.5 | 2.5 | 2.50 | 1.26 | 5.01 | 5.02 |
| HPO42− | 1 | 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Na+ | 142 | 142 | 141.98 | 142.02 | 142.02 | 142.03 |
| Cl− | 103 | 148.5 | 152.83 | 150.60 | 154.55 | 133.46 |
| Mg2+ | 1.5 | 1.5 | 1.50 | 1.50 | 1.51 | 1.50 |
| K+ | 5 | 5 | 5.01 | 5.02 | 4.99 | 5.02 |
| SO42− | 0.5 | 0.5 | 0.50 | 0.51 | 0.50 | 0.50 |
| HCO3−/CO32− | 27 | 4.2 | 4.19 | 4.19 | 4.20 | 27.00 |
| TRIS | - | 5 | 5.00 | 5.00 | 5.00 | 5.00 |
| Kanamycin | - | - | 0.21 | 0.21 | 0.21 | 0.21 |
| Gentamicin | - | - | 0.11 | 0.10 | 0.10 | 0.11 |
| Glutamic acid * | 0.05–0.30 | - | - | - | 0.19 | 0.19 |
| Alanine | 0.31–0.38 | - | - | - | 0.36 | 0.36 |
| Glycine | 0.11–0.29 | - | - | - | 0.24 | 0.24 |
| Proline | 0.17–0.21 | - | - | - | 0.21 | 0.21 |
| Leucine | 0.11–0.16 | - | - | - | 0.12 | 0.12 |
| Lysine | 0.13–0.18 | - | - | - | 0.17 | 0.17 |
| Serine | 0.088–0.094 | - | - | - | 0.09 | 0.09 |
| Valine | 0.19–0.24 | - | - | - | 0.22 | 0.22 |
| Serum albumin (g·L−1) | 35–50 | - | - | - | 40 | 40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Győri, E.; Fábián, I.; Lázár, I. Effect of the Chemical Composition of Simulated Body Fluids on Aerogel-Based Bioactive Composites. J. Compos. Sci. 2017, 1, 15. https://doi.org/10.3390/jcs1020015
Győri E, Fábián I, Lázár I. Effect of the Chemical Composition of Simulated Body Fluids on Aerogel-Based Bioactive Composites. Journal of Composites Science. 2017; 1(2):15. https://doi.org/10.3390/jcs1020015
Chicago/Turabian StyleGyőri, Enikő, István Fábián, and István Lázár. 2017. "Effect of the Chemical Composition of Simulated Body Fluids on Aerogel-Based Bioactive Composites" Journal of Composites Science 1, no. 2: 15. https://doi.org/10.3390/jcs1020015





